Abstract
Strigolactones, a new group of phytohormones, are reported to improve plant tolerance to multiple abiotic stresses. A pot experiment was conducted to investigate the impact of synthetic strigolactone (GR24 at 0.001, 0.01 and 0.1 mg L−1) application on ornamental sunflowers (Helianthus annuus cv. Vincent’s Choice) grown under salt stress (150 mM NaCl). Salt stress was applied after 14 days, and SL was applied 25 days seed sowing. The results showed that amongst various GR24 concentrations, 0.01 mg L−1 proved to be superior, as it enhanced the photosynthetic rate (9.29%), transpiration rate (0.76%), stomatal conductance (77.5%), total soluble protein (0.55%) and K+ (14.63% in roots; 14.87% in shoots) and Ca2+ (12.63% in roots; 11.48% in shoots) contents under control conditions. Similarly, the leaf turgor potential (Ψp), osmotic potential (Ψs) and free proline, glycinebetaine (GB), superoxide dismutase (SOD), catalase (CAT) and peroxide (POD) contents increased by 58.17, 89.95, 159.04, 101.54, 74.42, 175.68 and 53.62%, respectively, under salt stress conditions. The leaf water potential (Ψw) decreased (−0.14%) and the malondialdehyde (MDA) content increased (16.65%) when treated with the 0.001 mg L−1 GR24 level. Meanwhile, hydrogen peroxide (H2O2) and Na+ concentrations in roots and shoots increased by 62.53%, 74.66% and 38.55% under saline conditions with a GR24 level of 0 mg L−1. Regarding the plant biomass, a GR24 level of 0.01 mg L−1 with salt stress greatly decreased the root (−47.27% and −50.45%) and shoot (−44.79% and −59.42%) fresh and dry weights, respectively, compared to control conditions. These results reveal that exogenously applied GR24 might be an effective way to mitigate the perilous impacts of salt stress in ornamental sunflower production. It is suggested that the use of molecular techniques to study different processes in which GR24 could play a vital part in various commercial floricultural crops is extremely imperative and can open novel horizons for future investigations in this exhilarating field of plant hormones.
1. Introduction
Increased soil salinization significantly hampers crop growth and productivity, particularly in arid and semi-arid regions [1,2]. Globally, about 20% of the cultivated land has been reported to be salt-affected [3], which causes more than USD 12.5 billion in yearly losses due to crop productivity losses [4]. Salt-induced crop yield losses are largely attributed to specific ion toxicity, impaired CO2 exchange, reduced photosynthetic performance and other physiological and metabolic consequences [5,6]. The enhanced production of reactive oxygen species (ROS) under salt stress also contributes to the oxidative stress load of crop plants [2]. To cope with salinity stress, plants have evolved different tolerance mechanisms, such as degrading proteins and nucleic acids. Plants also induce lipid peroxidation and the activation of enzymatic and non-enzymatic antioxidant defense systems for ROS removal and the maintenance of redox homeostasis [7,8].
Sunflower (Helianthus annuus L.) is the most vital crop for the production of oilseeds across the globe [3,9]. It is an extremely nutritive crop and a rich source of antioxidants, as well as polyunsaturated fatty acids [10]. Furthermore, the advancement of floriculture has also led to the use of sunflower as an ornamental plant. Thus, the ornamental sunflower is an extremely esteemed specialty cut-flower plant used for vase decorations in pots, for interior decoration and as garden plants due to its various colors and the liveliness of its inflorescence [11]. Flower growers are mainly interested in this crop due to its flower color, flower size, straight and long stem and decreased branching [12]. However, floricultural cut-flower crops are reported to be salt sensitive, and farmers have been apathetic to endangering the flower quality and economic output by growing them under saline soil conditions [13].
Phytohormone biosynthesis and signaling under abiotic stresses are well known to regulate plant growth and development [14,15]. Strigolactones (SLs), carotenoid-derived terpene lactones, were first isolated from a root culture solution of Gossypium hirsutum in the 1960s [16]. Later, in 2008, SLs were recognized as novel hormones that could suppress the generation of branches in higher plants [17]. GR24, a synthetic SL, is involved as a positive regulator in response to salt stress [18]. Under nutrient-deficient conditions, SLs released from the plant roots enhance the development of lateral roots and root hairs, which, in turn, improves nutrient uptake [2]. Simultaneously, SLs translocated to aboveground plant parts stifle lateral bud or branch generation and decrease the inorganic nutrient requirements of the branches [19,20]. Due to the minute concentrations of SLs in different plants, a series of SLs, such as GR5, GR7 and GR24, have been chemically manufactured, among which GR24 had the maximum activity [21]. GR24 application improved chlorophyll a and b contents as well as augmented the transpiration rate and stomatal conductance in tomato under salinity stress [22]. SLs proved to be an imperative signaling molecule required for the synthesis of photosynthetic pigments during salt stress [22]. SLs also significantly release the salt stress suppression by elevating SOD and POD activities in rice [2] and rapeseed [23]. SLs could also lessen salt stress by regulating the antioxidant system in tomato seedlings [22]. The foliar application of GR24 was previously reported to regulate growth responses in Arabidopsis [21] and sunflower [24] exposed to salt stress.
To overcome the negative impacts of salt stress on crop growth and productivity, plant scientists have developed different conventional and contemporary techniques to screen for salt-tolerant and salt-resistant varieties [25]. Previous studies quantified the implementation of tissue culture and other laboratory techniques for the screening of genotypes by germinating cells on severely salty media to pick tolerant cells, which redevelop salt-tolerant plant varieties [26]. Therefore, the current investigation was carried out to explore the impact of GR24 on photosynthesis, water relations, antioxidant enzymatic activities and mineral ion composition in ornamental sunflowers (Helianthus annuus cv. Vincent’s Choice) under salinity stress.
2. Materials and Methods
2.1. Experimental Area, Seed Material and Layout
A pot experiment was conducted in a lath-house of the Old Botanical Research Area (31°24′ N, 72°09′ E, 300 m above average sea level) at the University of Agriculture, Faisalabad, Pakistan, to understand the interaction between selected levels of salinity and SLs. Sunflower seeds of Vincent’s Choice (F1) were purchased from Sunny View Seed Company, Lahore, Pakistan. Pots were arranged according to a two-factor factorial completely randomized design (CRD) with four replications. There were eight pots in each treatment, and each pot contained 10.0 kg of sterilized canal sand. In pots, seeds were sown during the second week of September 2019. After germination, three seedlings of equal size were maintained in each pot until the harvesting of fully opened flowers (64 to 78 days after sowing). Essential nutrients were supplied by Hoagland’s nutrient solution (full strength) equally to each pot [27].
2.2. Chemical Materials
Except for GR24, all chemicals were acquired from Sigma, Germany. The chemicals and their CAT numbers are as follows: BAP (Benzyl amino purine, CAT # 13151); NAA (Naphthalene acetic acid, CAT # 80862005); GR24 (obtained from the Department of Organic Chemistry, Radhoud University Nijmegen, The Netherlands); NBT (Nitroblue tetrazolium, CAT # 124823500); Salfosalicylic acid (CAT # 8006910100); 2,4-dichlorophenoxy acetic acid (CAT # D70724); Methionine (CAT # M9500); Toluene (CAT # 244511); Guaiacol (CAT # W253200); and Trichloroacetic acid (CAT # 8223420250). Strigolactone (GR24) was provided by Professor Dr. B. Zwanenburg, Department of Organic Chemistry, Radboud University Nijmegen, Holland.
2.3. Application of Salinity and Strigolactone (GR24)
Two salinity levels, i.e., non-saline and 150 mM NaCl, were applied two weeks after sowing. About 50 mM NaCl was applied thrice at three-day intervals to reach the 150 mM salt level. The potting sand was moistened daily by adding 250 mL of distilled water. Four SL (GR24) concentrations (0, 0.001, 0.01 and 0.1 mg L−1) were foliar-applied (25 days after germination) twice at three-day intervals at 25 mL pot−1 during the vegetative stage of plants.
2.4. Measurement of Photosynthetic Attributes and Water Relations
An infrared gas analyzer (IRGA) (LCA-4, Analytical Development Company, Hoddesdon, UK) was used for the measurement of the photosynthetic rate (A), transpiration rate (E) and stomatal conductance (gs). Water potential (Ψw) was determined with the help of a pressure chamber (Plant Moisture Stress (PMS) Instrument Company, Model 670, Albany, USA). Leaf tissues were frozen (at −80 °C) for two weeks, and leaf Ψw was extracted for the determination of osmotic potential (Ψs) with the help of a Wescor Vapor Pressure Osmometer (Model VAPRO 5520, El Cajon, CA, USA). Leaf turgor potential (Ψp) was calculated as Ψp = Ψw − Ψs.
2.5. Determination of Stress-Related Metabolites (Proline and Malondialdehyde)
Free proline contents in leaves were determined by following the protocol described by Bates et al. [28]. For this purpose, 500 mg leaf samples were extracted using 10 mL of 3% (w/v) sulfosalicylic acid (MP, Biomedicals, Inc., Irvine, CA, USA), and 2.0 mL of crushed filtered samples in a test tube was taken along with 2.0 mL of GAA (glacial acetic acid) and acid ninhydrin. This reaction was undertaken at 100 °C and completed in an ice-filled container. Toluene (4.0 mL) was added, and aliquots were vortexed. The OD of the filtrate was measured at 520 nm, while toluene was utilized as a blank, and proline was calculated.
Malondialdehyde (MDA) was determined by taking the extract of a fresh leaf sample (500 mg) in 5 mL of 1.0% (w/v) TCA (MP Biomedicals, de Kayserberg Illkirch, France). The homogenate was centrifuged at 20,000× g for 15 min (Model Sigma 3K30, Bremen, Germany). The supernatant (500 μL) was reacted with 2 mL of 0.5% TBA (2-thiobarbituric acid) (Sigma-Alderich Chemie GmbH, Steinheim, Germany) in 20% TCA. At 100 °C, the leaf sample was subjected to a shaking water bath for 1 h. Afterwards, the reaction was stopped by cooling the samples in ice and centrifuging them at 1000× g for 10 min, and the OD of the filtrate was recorded at 532 and 600 nm.
2.6. Determination of Glycinebetaine (GB), Total Soluble Protein and Hydrogen Peroxide (H2O2)
Glycinebetaine (GB) was determined by placing 0.5 g of dry leaf material in 10 mL of toluene (0.5%) and keeping it overnight at 4 °C. About 1.0 mL of the filtrate was reacted with 1.0 mL of H2SO4. In a test tube, this extract (0.5 mL) was taken, 200 μL of a solution of KI3 was added, and in a chiller, all contents were cooled. Ice-cooled deionized H2O (2.8 mL) and 1–2 di-chloroethane (5.0 mL) were added. The organic layer absorbance was noted at λ 365 nm using a spectrophotometer. The GB concentration was verified with a curve by following Grieve and Grattan [29]. Furthermore, total soluble protein was determined by taking a fresh leaf sample (500 mg) and extracting it with 50 mM potassium phosphate (10 mL) buffer in an ice bath. The aliquot was centrifuged at 4 °C for 15 min at 10,000× g. The protein content in the extract was determined following Bradford [30]. Hydrogen peroxide (H2O2) was determined by taking 0.5 g of tissues of fresh leaves, which were crushed in a chilled mortar with 5 mL of 0.1% (w/v) trichloroacetic acid (TCA). The mixture was centrifuged at 12,000× g for 15 min. After vortexing, the OD of the blend was noted at 390 nm, and H2O2 was calculated by following the procedure explained by Velikova et al. [31].
2.7. Determination of Antioxidant Enzymatic Activities
Fresh leaf tissues (500 mg) were extracted in 10 mL of phosphate buffer (50 mM; pH 7.8). At 4 °C, the extract was centrifuged at 15,000× g for 10 min. The supernatant was separated and used for the determination of enzyme activities, i.e., superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT). For SOD, the photoreduction inhibition of nitro blue tetrazolium (NBT) was used by following the method described by Giannopolities and Ries [32]. Distilled water (100 μL), NBT (50 μL), methionine (100 μL), phosphate buffer at pH 7.6 (500 μL) and the sample extract (50 μL) were mixed in cuvettes that were kept for 20 min under light. SOD in the irradiated aliquot was read at 560 nm.
The activities of CAT and POD were calculated in accordance with the method described by Chance and Maehly [33]. The CAT reaction mixture (2 mL) containing phosphate buffer (50 mM) having a pH of 7.0 and H2O2 (5.9 mM) was taken, and the reaction was started by dissolving an aliquot (100 μL) of enzyme extract. The reduction in OD was recorded spectrophotometrically every 20 s for 2 min at 240 nm. For the determination of POD, 1.0 mL of the reaction mixture possessed 750 μL of phosphate buffer (50 mM; pH 5.0), 100 μL of H2O2 (40 mM), 100 μL of guaiacol (20 mM) and 100 μL of the extract of the enzyme. The OD of the reaction mixture was recorded every 20 s for 3 min at 470 nm.
2.8. Mineral Ion (Na+, K+ and Ca2+) Quantification
Dry root and shoot samples (0.1 g) were digested in 2 mL of a digestion mixture (HNO3 and HClO4 in a ratio of 5:2) for 25 h. After cooling, 0.5 mL of perchloric acid was added to decolorize the mixture, and a final volume of 50 mL was made using distilled water. Sodium (Na+), potassium (K+) and calcium (Ca2+) ions were quantified using a flame photometer (Jenway PFP 7, Cadmus, Chelmsford, England).
2.9. Statistical Analysis
All recorded data means were analyzed using the LSD test at a 5% probability level using STATISTIX software (version 8.3) and the analysis of variance (ANOVA) technique.
3. Results
3.1. Role of Exogenous GR24 Application in Photosynthetic Attributes and Water Relations of Sunflower under Salt Stress
The analysis of variance shows the mean squares of different physio-biochemical characteristics of ornamental sunflowers with numerous levels of GR24 under control and salinity stress conditions, indicating variable impacts of the treatments on the photosynthetic attributes and water relations of sunflower (Table 1). Furthermore, the imposition of salt stress significantly reduced the photosynthetic rate, transpiration rate and stomatal conductance at all GR24 levels. The highest reduction in the photosynthetic rate (−33.36%) under salt stress was recorded without GR24, while the transpiration rate and stomatal conductance under salt stress were reduced by −18.72% and −77.5%, respectively, at a GR24 concentration of 0.01 mg L−1 compared to control conditions (Figure 1). Statistically highly significant (p < 0.001) results were obtained for Ψw, Ψs and Ψp under salt stress compared to control conditions (Table 1). The application of all GR24 levels significantly enhanced Ψs and Ψp by up to 99.36% and 135.78%, respectively, whereas Ψw was reduced by −71.06%, −79.33% and −60.66% under saline conditions with 0.001, 0.01 and 0.1 mg L−1 GR24 applications, respectively (Figure 2).

Table 1.
Analysis of variance (ANOVA) for different physiological and biochemical characteristics of ornamental sunflower with various GR24 levels (0, 0.001, 0.01 and 0.1 mg L−1) under saline and non-saline conditions.
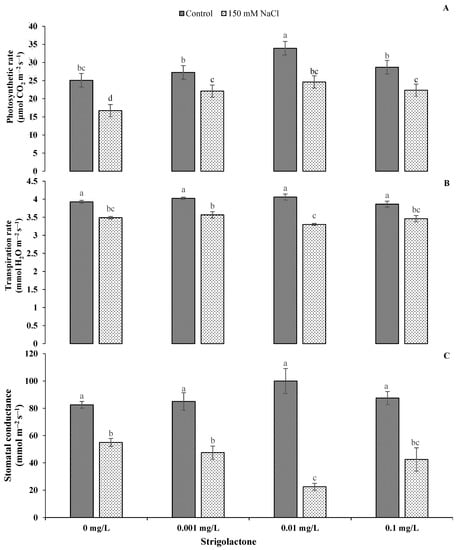
Figure 1.
Impact of GR24 on physiological characteristics, i.e., (A) photosynthetic rate (A), (B) transpiration rate (E) and (C) stomatal conductance (gs) of ornamental sunflower under control and salt stress conditions. Each vertical bar shows the mean of three replicates. Different letters indicate significant differences among treatments at p ≤ 0.01 according to least significant difference test.
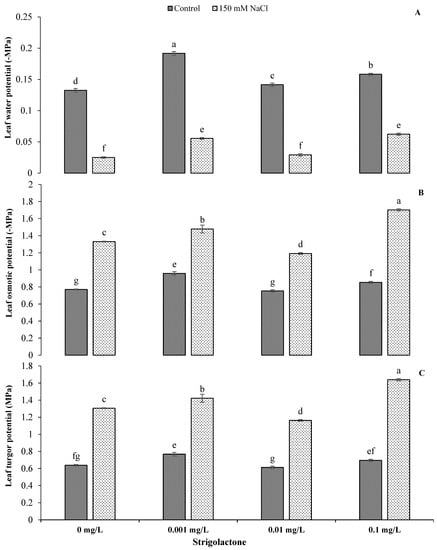
Figure 2.
Impact of GR24 on leaf water potential (A), leaf osmotic potential (B) and leaf turgor potential (C) of ornamental sunflower under control and salt stress conditions. Each vertical bar shows the mean of three replicates. Different letters indicate significant differences among treatments at p ≤ 0.01 according to least significant difference test.
3.2. Effect of Exogenous GR24 Application on Stress-Related Metabolites (Free Proline and MDA) of Sunflower under Salt Stress
Stress-related metabolites showed statistically highly significant (p < 0.01) results under both growing conditions (Table 1). The highest proline content was recorded under saline conditions when GR24 was applied at 0.01 mg L−1, followed by 0.1 mg L −1. There were 159.04% and 173.56% increments in free proline contents under saline conditions, respectively (Figure 3). Parallel outcomes were also obtained for MDA values, which increased with the GR24 level under salt stress as compared to control conditions. The highest MDA content was recorded when the 0.001 mg L−1 GR24 level was applied under salt stress, whereas the minimum value was recorded under control conditions with 0.001 mg/L GR24 (Figure 3).
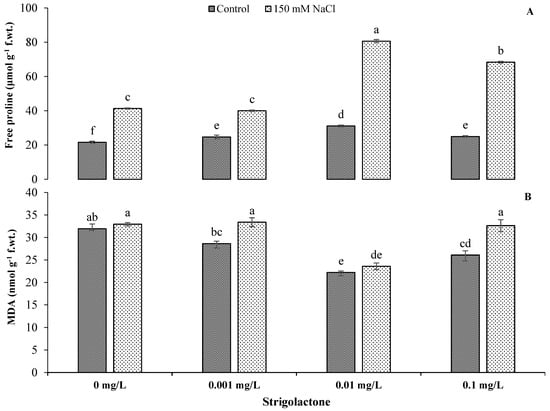
Figure 3.
Impact of GR24 on free proline content (A) and malondialdehyde (B) in ornamental sunflower under control and salt stress conditions. Each vertical bar shows the mean of three replicates. Different letters indicate significant differences among treatments at p ≤ 0.01 according to least significant difference test.
3.3. Impact of GR24 Application on GB, Total Soluble Protein and H2O2 under Salt Stress
Salt stress and GR24 showed a statistically highly significant (p < 0.01) impact on GB, total soluble protein and H2O2 (Table 1). The results showed increments in GB and H2O2 under saline conditions compared to the control with the application of GR24. The highest GB content was noted with the 0.01 mg L−1 GR24 concentration under salinity conditions. There was a 101.54% increase in the GB level compared to control conditions, whereas the lowest GB value was recorded under control conditions without GR24 application (Figure 4). Similar results were also noted for the H2O2 content with GR24 application under salt stress conditions. There were 70.61%, 53.03% and 62.66% increases in the H2O2 level under saline conditions with 0.001, 0.01 and 0.1 mg L−1 GR24 application, respectively (Figure 4). There was a significant drop in the total soluble protein content under salt stress conditions. The application of GR24 slightly improved the soluble protein level, but the highest value was recorded under control conditions. There was a 39.36% reduction in total soluble protein in the control treatment under salt stress (Figure 4).
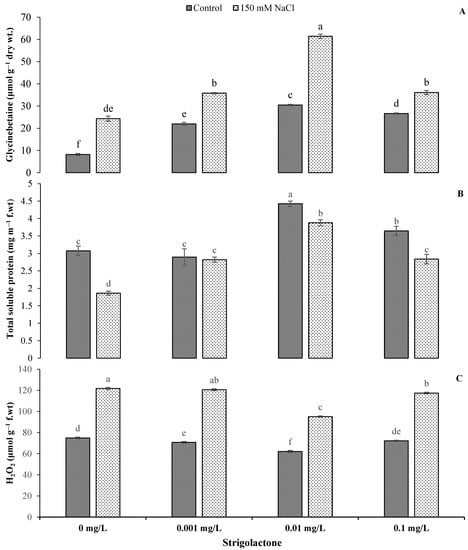
Figure 4.
Impact of GR24 on glycinebetaine (A), total soluble protein (B) and hydrogen peroxide (C) of ornamental sunflower under control and salt stress conditions. Each vertical bar shows the mean of three replicates. Different letters indicate significant differences among treatments at p ≤ 0.01 according to least significant difference test.
3.4. Impact of GR24 on Antioxidant Enzymatic Activities of Sunflower under Salinity Stress
A statistically highly significant (p < 0.01) effect of GR24 and salt stress was recorded for antioxidant enzymatic activities (Table 1). The application of salinity led to a notable enhancement of the activities of SOD, CAT and POD compared to the control treatment. GR24 applied at 0.01 mg L−1 showed the highest antioxidant values compared to other levels with salt stress conditions. There were 74.42%, 53.62% and 175.68% increases in SOD, CAT and POD contents under salt stress, respectively, compared to control treatments at the said GR24 level. The lowest values of these observations were recorded under control conditions without GR24 application (Figure 5).
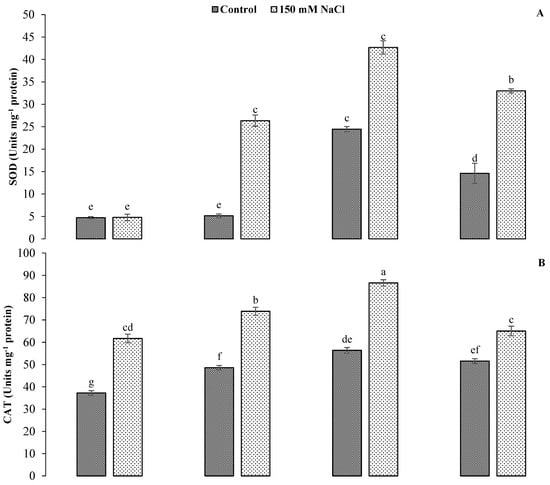
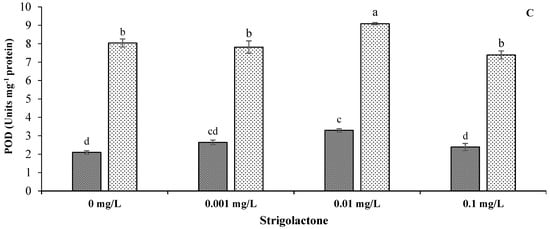
Figure 5.
Impact of GR24 on antioxidant enzymatic activities, i.e., superoxide dismutase (A), catalase (B) and peroxide (C), in ornamental sunflower under control and salt stress conditions. Each vertical bar shows the mean of three replicates. Different letters indicate significant differences among treatments at p ≤ 0.01 according to least significant difference test.
3.5. Impact of GR24 Application on Quantification of Mineral Ions in Roots and Shoots of Ornamental Sunflower under Salt Stress
The application of GR24 showed the elevation of Na+ contents in the roots and shoots of ornamental sunflowers. A reducing trend was recorded in Na+ content due to an increment in the GR24 level. There were 69.57%, 69.23% and 63.28% increases in root Na+ contents and 34.56%, 32.46% and 29.74% increases in shoot Na+ contents with GR24 applications at 0.001, 0.01 and 0.1 mg L−1, respectively, under salinity stress conditions. A decreasing trend was found in K+ and Ca2+ concentrations in both the roots and shoots of ornamental sunflowers under saline conditions. The greatest diminutions in K+ contents in roots (−5.63% and −38.14%) and shoots (−5.63% and −36.29%) were recorded at 0.01 mg L−1, whereas Ca2+ contents were reduced by −2.63% and −30% and −1.9% and 10.95% at the same GR24 level under control and saline conditions, respectively (Figure 6).
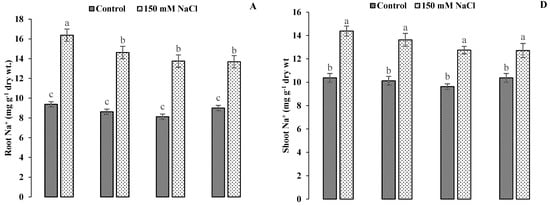
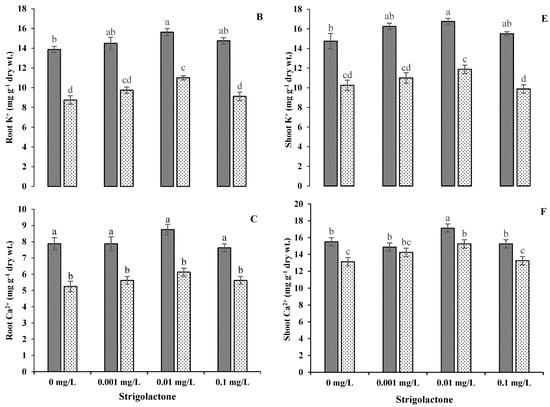
Figure 6.
Impact of GR24 on root Na+ (A), K+ (B) and Ca2+ (C) and shoot (D), K+ (E) and Ca2+ (F) ions of ornamental sunflower under control and salt stress conditions. Each vertical bar shows the mean of three replicates. Different letters indicate significant differences among treatments at p ≤ 0.01 according to least significant difference test.
3.6. Impact of GR24 Application on Plant Biomass of Ornamental Sunflower under Salt Stress
Statistically, salt stress and GR24 showed highly significant (p < 0.01) impacts on root and shoot fresh and dry weights (Table 2). The results showed that the application of salt stress greatly reduced the fresh weights and dry weights of both plant parts (roots and shoots). The greatest fresh and dry weights were recorded when GR24 was applied at 0.01 mg L−1 under control and salt stress conditions. The lowest weights (fresh and dry) of roots and shoots were found under control conditions. There were −47.27% and −50.45% reductions in fresh and dry weights, respectively, in roots with a GR24 level of 0.01 mg L−1 compared to control conditions. A similar trend was also noted in shoots, where there were decreases of −40.79% and −59.42% in fresh and dry weights with the same GR24 concentration. Overall, there were reductions of −56.67% and −54.06% in fresh weight and −47.26% and −49.13% in dry weight with 0.001 and 0.1 mg L−1 GR24 levels in the roots of ornamental sunflowers. In shoots, reductions of −34.14% and −41.18% and −43.45% and −51.28% in fresh and dry weights were observed, respectively, at the same GR24 level compared to control conditions (Figure 7).

Table 2.
Analysis of variance (ANOVA) for different mineral ions and plant biomass characteristics of ornamental sunflower with various GR24 levels (0, 0.001, 0.01 and 0.1 mg L−1) under saline and non-saline conditions.
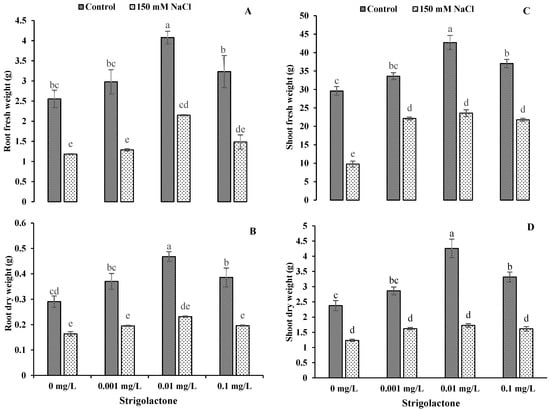
Figure 7.
Impact of GR24 on root fresh weight (A), root dry weight (B), shoot fresh weight (C) and shoot fresh weight (D) of ornamental sunflower under control and salt stress conditions. Each vertical bar shows the mean of three replicates. Different letters indicate significant differences among treatments at p ≤ 0.01 according to least significant difference test.
4. Discussion
The current investigation evaluated the role of GR24 in the physio-biochemical characteristics and biomass of the ornamental sunflower (Helianthus annuus cv. Vincent’s Choice) under salinity stress to identify its most suitable quantity to diminish the drastic impacts of salt stress. It was observed that salinity stress drastically reduced the photosynthetic rate (A), transpiration rate (E) and stomatal conductance (gs). Salinity stress mostly abolishes chlorophyll and decreases photosynthesis by limiting the rate of transpiration and stomatal conductance [18]. Salt stress not only overwhelms photosynthetic activity but also represses the plant’s photosynthetic machinery. Salinity stress also disturbs cell organelles such as the chloroplast. The chloroplast is the site of most photosynthetic processes (PSI and PSII) and reactive oxygen species (ROS) generation [34]. Photosynthesis is the foundation of crop yield and quality in cropping systems [35]. The efficiency of the photosynthetic process is affected by salt stress, such as metabolic process changes or the limitation of stomata to CO2 diffusion [36]. Similar findings were reported by Kausar and Shahbaz [37], who showed a lower photosynthetic rate and stomatal conductance due to salt stress even after GR24 application at different concentrations. These findings were contrary to the results of Zhang et al. [38], who observed increases in stomatal conductance, the rate of transpiration and photosynthesis in cucumber seedlings with exogenous GR24 application during salinity stress. This variation might be due to variations in environmental circumstances among plant species. Ma et al. [23] also noted that GR24 increased all photosynthetic attributes of Brassica napus. This could be due to increased sucrose synthase 2 (SUS2) and the decreased activity of kinases. SUS2 is responsible for the deprivation of sucrose [39]. The application of GR24 treatment enhanced the expression of SUS2 enzyme activity in rice [40].
The present study showed limited water relations due to the limited uptake of water and reduced solute potential. Similar results were also observed by Cha-um et al. [41], who observed negative water potential that limited plant growth and development after GR24 application. This decrease could be because of higher Na+ and Cl− ion accumulation in sunflower leaves. Contrary to the leaf water potential, leaf osmotic and turgor potentials were enhanced in our study under salt stress conditions. Similar findings were also noted by Sarwar and Shahbaz [24] in sunflower after GR24 treatment. The different effects of GR24 on water relation attributes indicate very intricate interactions among GR24 and other hormones [23]. Due to salinity stress, excessive ROS production has been noticed, which possesses lethal effects on the cellular organelles, causing decreased plant growth and development [42,43]. In the present experiment, remarkable increases in proline and MDA were detected. Similar findings were observed in sunflower [24] under salt stress conditions. When using GR24 (especially at 0.01 mg L−1), there was a considerable elevation recorded in the proline content. Exogenously applied GR24 enhanced the free proline concentration in rapeseed, which mitigated the hindering effects of salt stress [23]. MDA is used to gauge the extent of oxidative impairment in stressed plants [44]. The results of the present study also showed higher MDA accumulation due to salt stress, indicating an elevation of lipid peroxidation in ornamental sunflowers. The higher MDA content due to GR24 application in salt stress plants demonstrated the strong positive impact of GR24 on protecting the membrane from stress damage [45]. Increased MDA levels under salt stress also indicated that despite the presence of the antioxidant system mechanism, salt stress might still cause membrane lipid peroxidation in plant leaves [46]. Naveed et al. [47] also argued that higher stress-related metabolites show a defensive impact as well as plant support to alleviate the lethal role of salt stress.
To manage the deleterious ROS effects in plants, a defensive process is initiated for the tolerance to salts, which includes the compatible production of solutes such as GB [1,48]. In this research, a remarkable elevation of GB was observed under both saline and control conditions with the application of GR24. Under saline conditions, an osmotic modification occurred with the assistance of proteins by decreasing the osmotic potential [49]. The concentration of total soluble protein accumulation in plants differs from species to species [50]. In the present experiment, a reduction in total soluble protein was recorded. Similar results were also found by Zulfiqar et al. [51], where all levels of GR24 reduced the soluble protein content under control and saline conditions. Salt stress causes oxidative injury, particularly due to the enhanced production of H2O2 [52]. These ROS have harmful effects on plant tissues, which results in depressed plant growth [43]. Our results are also in line with this, as an elevation of H2O2 was found under saline conditions. Enhanced levels of H2O2 and others exert a defensive impact and support plants in alleviating perilous abiotic stress impacts [47]. A protective mechanism against ROS is induced in plants by different enzymatic and non-enzymatic antioxidants [53]. In advanced stages, the accrual of ROS tends to inactivate enzymes, degrade nucleic acids and oxidize proteins, which ultimately leads to cell death [54].
Enzymatic activities severely decrease due to stress imposed on plants under external abiotic conditions [2]. Saleem et al. [52] reported an increase in lipid peroxidation among different varieties of potatoes under NaCl exposure. Certainly, metabolite production activates plants to efficiently cope under abiotic stress conditions [55]. Likewise, in the current study, antioxidants (SOD, CAT and POD) were revealed to have boosted activities under salt stress conditions as compared to control conditions, whereas GR24 application enhanced the antioxidant enzymatic activities, especially at the 0.01 mg L−1 concentration. The significant augmentation of CAT, SOD and POD activities indicated that there is a positive regulatory effect on the scavenging of ROS produced by salt stress in plants [23]. This also indicated that GR24 could proficiently diminish superoxide free radicals resulting from saline stress, decreasing the cellular impairment caused by peroxidation by ROS for maintaining the proper development of rice seedlings [2]. As a kind of novel plant hormone, the impact of strigolactone GR24 on resistance to abiotic stressors has become an interesting research topic [56,57].
Tolerance to salinity stress in plants is observed because of ion accretion [53]. Tolerance to salts in plants is directly connected to the Na+/K+ ratio, which depends upon the nature of the plant species [58]. The present study revealed that GR24 increased the Na+ contents in roots and shoots of ornamental sunflowers, whereas K+ and Ca2+ decreased in both plant parts under salinity stress. Parallel results were also reported by Parveen et al. [48], in which elevated Na+ ions in cells lessened the K+ and Ca2+ ions in plant cells. The enhanced Na+ ion levels in tissues under saline stress affect the characteristics of gas exchange, cytosolic enzyme activities and the development of plants [59]. Homeostasis and the attainment of major elements, i.e., K+ and Ca2+, were adversely affected by the accrual of Na+ ions in the cells [24]. These findings confirmed the results of the present research on ornamental sunflowers, as well as previous research on quinoa [60] and wheat [34], under salt stress conditions. GR24 assisted in abating the harsh impacts [36] of salts by hoarding more K+ and Ca2+ ions and minimizing the Na+ ion content. The root is the main plant organ affected by salt stress conditions, and it affects the accumulation of ions and shoot growth [23]. The present results indicated that salt imposition severely decreased the accumulation of root and shoot biomass. The results are in line with the findings of Sarwar and Shahbaz [24] in sunflower and wheat [34]. Ionic toxicity and high osmotic stress along with ROS production could be the main cause of plant biomass reduction [61]. As noted, when GR24 was applied, it could move and transfer from roots into shoots and played a regulatory role in plant responses to salt stress [18,45], and salt stress negatively affects the numerous metabolic processes occurring in plants [3].
5. Conclusions
Strigolactone (GR24) played a valuable part in coping with saline conditions; salt stress reduced the photosynthetic attributes (A, E and gs) and leaf water potential, but GR24 exerted a regulatory impact under salinity. Among various GR24 levels in the current experiment, 0.01 mg L−1 led to significantly elevated photosynthetic characteristics (under control conditions), stress-related metabolites, antioxidant enzyme activities, root and shoot K+ and Ca2+ ions and biomass (fresh and dry weights), while leaf osmotic and turgor potentials were greatest at 0.1 mg L−1. Therefore, the current study offers evidence that exogenous GR24 application may play an important role in mitigating the adverse impacts of salt stress in ornamental sunflowers. Still, there are many gaps in the understanding of GR24 mechanisms and signaling that must be resolved for its sustainable application in agriculture. The diversification of SLs and their downstream signaling processes controlled by the favorable alleles of the genes involved and their identification would be a valuable asset to future breeding operations. A clear understanding of these aspects will open new horizons for plant resistance and improved crop yield.
Author Contributions
Conceptualization and methodology, M.A., H.Z., M.A.F., M.S., A.T., M.R.S. and H.G.; software and validation, M.A. and H.Z.; formal analysis, investigation and resources, M.A.,H.Z., M.A.F., A.T., M.S., M.R.S., H.G. and A.J.; data curation, M.A., H.Z., M.S., M.A.F., S.A., S.K., M.S., M.R.S. and H.G.; writing—original draft preparation, writing—review and editing, visualization, supervision and project administration, M.A., H.Z., E.R., R.M., A.J., M.F.S., M.S., M.A.F., A.T., M.S., M.R.S. and H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Su, Q.; Jiang, H.; Cui, J.; He, X.; Wu, Z.; Zhang, Z.; Liu, J.; Zhao, Y. Effects of strigolactone on photosynthetic and physiological characteristics in salt-stressed rice seedlings. Sci. Rep. 2020, 10, 6183. [Google Scholar] [CrossRef] [PubMed]
- Lalarukh, I.; Shahbaz, M. Response of antioxidants and lipid peroxidation to exogenous application of alpha-tocopherol in sunflower (Helianthus annuus L.) under salt stress. Pak. J. Bot. 2020, 52, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.C.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil. 2021, 461, 219–244. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, C.; Xue, Y.; Liu, X.; Chen, S.; Song, C.P.; Yang, Y.; Guo, Y. Calcium-activated 14-3-3 proteins as a molecular switch in salt stress tolerance. Nat. Commun. 2019, 10, 1199. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance Mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Hong, C.Y.; Chao, Y.Y.; Yang, M.Y.; Cheng, S.Y.; Cho, S.C.; Kao, C.H. NaCl-induced expression of glutathione reductase in roots of rice (Oryza sativa L.) seedlings is mediated through hydrogen peroxide but not abscisic acid. Plant Soil. 2009, 320, 103–115. [Google Scholar] [CrossRef]
- Grieve, C.M.; Poss, J.A. Response of ornamental sunflower cultivars ‘sunbeam’ and ‘moonbright’ to irrigation with saline wastewaters. J. Plant Nut. 2010, 11, 1579–1592. [Google Scholar] [CrossRef]
- Bosnjak, D.; Rodić, V.; Karapandžin, J. Possibilities for the improvement of the soybean production in Serbia. Contemp. Agric. 2013, 62, 266–275. [Google Scholar]
- Brito, C.L.L.; Matsumoto, S.N.; Santos, J.L.; Gonçalves, D.N.; Ribeiro, A.F.F. Effect of paclobutrazol on the development of ornamental sunflower plants. J. Agric. Sci. 2016, 39, 153–160. [Google Scholar] [CrossRef]
- Mladenovic, E.; Cvejic, S.; Čukanovic, J.; Žeravica, G.; Jocic, S. Evaluation of sunflower genotypes for ornamental use. Contemp. Agric. 2016, 65, 39–43. [Google Scholar] [CrossRef][Green Version]
- Menzel, C.; González-Martínez, C.; Chiralt, A.; Vilaplana, F. Antioxidant starch films containing sunflower hull extracts. Carbohydr. Polym. 2019, 214, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Koltai, H.; Beveridge, C.A. Diverse roles of strigolactones in plant development. Mol. Plant 2013, 6, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Zwack, P.J.; Rashotte, A.M. Interactions between cytokinin signaling and abiotic stress response. J. Exp. Bot. 2015, 66, 4863–4871. [Google Scholar] [CrossRef]
- Cook, C.E.; Whichard, L.P.; Monroe, W.E.; Egley, G.H.; Coggon, P.; Luhan, P.A.; McPhail, A.T. Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea). J. Am. Chem. Soc. 1972, 94, 6198–6199. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Magome, H.; Kamiya, Y.; Shirasu, K.; Kyozuka, J.; Yamaguchi, S. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Xi, X.; Ma, C.; Sun, Z.; Yang, X.; Li, X.; Tian, Y.; Wang, C. Exogenous Strigolactones alleviate KCl stress by regulating photosynthesis, ROS migration and ion transport in Malus hupehensis Rehd. Plant Physiol. Biochem. 2021, 159, 113–122. [Google Scholar] [CrossRef]
- Rani, K.; Zwanenburg, B.; Sugimoto, Y.; Yoneyama, K.; Bouwmeester, H.J. Biosynthetic considerations could assist the structure elucidation of host plant produced rhizosphere signaling compounds (strigolactones) for arbuscular mycorrhizal fungi and parasitic plants. Plant Physiol. Bioch. 2008, 46, 617–626. [Google Scholar] [CrossRef]
- Kohlen, W.; Tatsiana, C.; Liu, Q.; Bours, R.; Domagalska, M.A.; Beguerie, S.; Verstappen, F.; Leyser, O.; Bouwmeester, H.; Ruyter-Spira, C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011, 155, 974–987. [Google Scholar] [CrossRef]
- Koltai, H.; Prandi, C. Strigolactones: Biosynthesis, synthesis and functions in plant growth and stress responses. In Phytohormones: A Window to Metabolism, Signaling and Biotechnological Applications; Springer: New York, NY, USA, 2014; pp. 265–288. [Google Scholar]
- Liu, H.; Li, C.; Yan, M.; Zhao, Z.; Huang, P.; Wei, L.; Wu, X.; Wang, C.; Liao, W. Strigolactone is involved in nitric oxide-enhanced the salt resistance in tomato seedlings. J. Plant Res. 2022, 135, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Hu, C.; Wan, L.; Hu, Q.; Xiong, J.; Zhang, C. Strigolactones improve plant growth, photosynthesis and alleviate oxidative stress under salinity in rapeseed (Brassica napus L.) by regulating gene expression. Front. Plant Sci. 2017, 8, 1671. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, Y.; Shahbaz, M. Modulation in growth, photosynthetic pigments, gas exchange attributes and inorganic ions in sunflower (Helianthus annuus l.) by strigolactones (GR24) achene priming under saline conditions. Pak. J. Bot. 2020, 52, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tsago, Y.; Andargie, M.; Takele, A. In vitro screening for drought tolerance in different sorghum (Sorghum bicolor (L.) Moench) varieties. J. Stress Physiol. Biochem. 2013, 9, 72–83. [Google Scholar]
- Al-Jibouri, A.M.J.; Altahan, S.F.; Al-Anii, T.A. Evaluation of three sunflower (Helianthus annuus L.) hybrids for salt tolerance in vitro. JLS 2011, 5, 1037–1041. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil; California Agricultural Experiment Station Circular; University of California: Los Angeles, CA, USA, 1950; Volume 347, pp. 1–32. [Google Scholar]
- Bates, L.E.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annu. Rev. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective roles of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A. Assay of catalase and peroxidase. Methods Enzymol. 1955, 2, 764–817. [Google Scholar]
- Kausar, F.; Shahbaz, M. Influence of strigolactone (GR24) as a seed treatment on growth, gas exchange and chlorophyll fluorescence of wheat under saline conditions. Int. J. Agric. Biol. 2017, 19, 321–327. [Google Scholar] [CrossRef]
- Zhou, X.; Tan, Z.; Zhou, Y.; Guo, S.; Sang, T.; Wang, Y.; Shu, S. Physiological mechanism of strigolactone enhancing tolerance to low light stress in cucumber seedlings. BMC Plant Biol. 2022, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, G.H.; Akhtar, J.; Anwar-Ul-Haq, M.; Ali, S.; Chen, Z.; Malik, W. Exogenous potassium differentially mitigates salt stress in tolerant and sensitive maize hybrids. Pak. J. Bot. 2014, 46, 135–146. [Google Scholar]
- Kausar, F.; Shahbaz, M. Interactive effect of foliar application of nitric oxide (NO) and salinity on wheat (Triticum aestivum L.). Pak. J. Bot. 2013, 45, 67–73. [Google Scholar]
- Zhang, X.; Zhang, L.; Ma, C.; Su, M.; Wang, J.; Zheng, S.; Zhang, T. Exogenous strigolactones alleviate the photosynthetic inhibition and oxidative damage of cucumber seedlings under salt stress. Sci. Hortic. 2022, 297, 110962. [Google Scholar] [CrossRef]
- Perveen, S.; Iqbal, M.; Parveen, A.; Akram, M.S.; Shahbaz, M.; Akber, S.; Mehboob, A. Exogenous triacontanol mediated increase in phenolics, proline, activity of nitrate reductase and shoot k+ confers salt tolerance in maize (Zea mays L.). Braz. J. Bot. 2017, 40, 1–11. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, L.; Zheng, J.; Wang, R. Identification of differentially expressed proteins and phosphorylated proteins in rice seedlings in response to strigolactone treatment. PLoS ONE 2014, 9, e93947. [Google Scholar] [CrossRef]
- Cha-um, S.; Ashraf, M.; Kirdmanee, C. Screening upland rice (Oryza sativa L. ssp. indica) genotypes for salt-tolerance using multivariate cluster analysis. Afr. J. Biotechnol. 2010, 9, 4731–4740. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and Antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rizwan, M.; Kamran, M.; Ibrahim, A.A.M.; Khan, Z.; Bamagoos, A.A.; Alharby, H.F.; Hakeem, K.R. Individual and combined application of EDTA and citric acid assisted phytoextraction of copper using jute (Corchorus capsularis L.) seedlings. Environ. Technol. Innov. 2020, 19, 100895. [Google Scholar] [CrossRef]
- Li, L.; Xia, W.; Li, H.; Zeng, H.; Wei, B.; Han, S.; Yin, C. Salinity inhibits rice seed germination by reducing α-amylase activity via decreased bioactive gibberellin content. Front. Plant Sci. 2018, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Khosla, A.; Nelson, D.C. Strigolactones, super hormones in the fight against Striga. Curr. Opin. Plant Biol. 2016, 33, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Z.; Jiang, X.; Qin, Y. Effects of salt stress on photosynthetic fluorescence characteristics, antioxidant system, and osmoregulation of Coreopsis tinctoria Nutt. Hortscience 2021, 56, 1066–1072. [Google Scholar] [CrossRef]
- Naveed, S.; Mustafa, A.; Niamat, B.; Ahmad, Z.; Yaseen, M.; Kamran, M.; Rafique, M.; Ahmar, S.; Chen, J.T. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability 2020, 12, 846. [Google Scholar] [CrossRef]
- Parveen, A.; Hamzah, S.M.; Kamran, M.; Haider, M.Z.; Chen, J.T.; Hur, G.; Javed, M.T.; Azeem, M. Effect of citric acid on growth, ecophysiology, chloroplast ultrastructure, and phytoremediation potential of jute (Corchorus capsularis l.) seedlings exposed to copper stress. Biomolecules 2020, 10, 592. [Google Scholar] [CrossRef]
- Mohamed, A.N.; Ismail, M.R. Changes in organic and inorganic solutes of in vitro tomato cultivars under NaCl stress. Aust. J. Crop Sci. 2011, 5, 939–944. [Google Scholar]
- Hu, Y.; Xia, S.; Su, Y.; Wang, H.; Luo, W.; Xiao, L. Brassinolide increases potato root growth in vitro in a dose-dependent way and alleviates salinity stress. Biomed Res. Int. 2016, 2016, 8231873. [Google Scholar] [CrossRef]
- Zulfiqar, H.; Shahbaz, M.; Ahsan, M.; Nafees, M.; Nadeem, H.; Akram, M.; Maqsood, A.; Ahmar, S.; Kamran, M.; Alamri, S.; et al. Strigolactone (GR24) induced salinity tolerance in sunflower (Helianthus annuus L.) by ameliorating morpho-physiological and biochemical attributes under in-vitro conditions. J. Plant Growth Regul. 2021, 40, 2079–2091. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rana, M.S.; Rizwan, M.; Kamran, M.; Imran, K.; Riaz, M.; Soliman, M.H.; Elkelish, E.; et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Shi, Q.; Song, X.; Liu, Z.; Wang, Y.; Fu, J.; Su, C.; Xia, X.; Song, E.; Song, Y. Quinones derived from polychlorinated biphenyls induce ROS-dependent autophagy by evoking an Autophagic flux and inhibition of mTOR/p70S6k. Chem. Res. Toxicol. 2016, 29, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Mustafa, A.; Anjum, R.M.A.; Wang, B.; Liu, L. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vera, R.; Garcia, J.M.; Lopez-Raez, J.A. Do strigolactones contribute to plant defence. Mol. Plant Pathol. 2014, 15, 211–216. [Google Scholar] [CrossRef]
- Marzec, M. Strigolactones as part of the plant defence system. Trends Plant Sci. 2016, 21, 900–903. [Google Scholar] [CrossRef]
- Koren, D.; Resnick, N.; Gati, E.M.; Belausov, E.; Weininger, S.; Kapulnik, Y.; Koltai, H. Strigolactone signaling in the endodermis is sufficient to restore root responses and involves SHORT HYPOCOTYL 2 (SHY2) activity. New Phytol. 2013, 198, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Liu, W.; Jing, Q.; Cao, W. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ. Exp. Bot. 2009, 67, 222–227. [Google Scholar] [CrossRef]
- Quintero, J.M.; Fournier, J.M.; Benlloch, M.; Rodríguez-Navarro, A. Na+ accumulation in root symplast of sunflower plants exposed to moderate salinity is transpiration-dependent. J. Plant Physiol. 2008, 165, 1248–1254. [Google Scholar] [CrossRef]
- Eisa, S.; Hussain, S.; Geissler, N.; Koyro, H.W. Effect of NaCl salinity on water relations, photosynthesis and chemical composition of quinoa (Chenopodium quinoa Willd.) as a potential cash crop halophyte. Aust. J. Crop Sci. 2012, 6, 357–368. [Google Scholar]
- Mujahid, N.; Muhammad, S.; Aysha, K.; Muhammad, A.W. Modulations Induced by seed priming of strigolactone (GR24) in morpho physiological and biochemical attributes of ajwain (Trachyspermum ammi L.) under salt stress. J. Plant Growth Regul. 2022, 1–14. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).