Autumn Application of Synthetic Auxin Herbicide for Weed Control in Cereals in Poland and Germany
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schils, R.; Olesen, J.E.; Kersebaum, K.-C.; Rijk, B.; Oberforster, M.; Kalyada, V.; Khitrykau, M.; Gobin, A.; Kirchev, H.; Manolova, V.; et al. Cereal yield gaps across Europe. Eur. J. Agron. 2018, 101, 109–120. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Moss, S.R.; Storkey, J.; Cussans, J.W.; Perryman, S.A.M.; Hewitt, M.V. The Broadbalk long-term experiment at Rothamsted: What has it told us about weeds? Weed Sci. 2004, 52, 864–873. [Google Scholar] [CrossRef]
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Délye, C.; Jasieniuk, M.; Le Corre, V. Deciphering the evolution of herbicide resistance in weeds. Trends Genet. 2013, 29, 649–658. [Google Scholar] [CrossRef]
- Mallory-Smith, C.; Retzinger, J. Revised classification of herbicides by site of action for weed resistance. Weed Technol. 2003, 17, 605–619. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Boger, P. Target sites for herbicides: Entering the 21st century. Pest Manag. Sci. 2002, 58, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Jasinskas, A.; Steponavičius, D.; Šniauka, P.; Zinkevičius, R. Weed Control by Chemical and Mechanical Means. In Weed Biology and Control; Pilipavičius, V., Ed.; IntechOpen: London, UK, 2015; Available online: https://www.intechopen.com/chapters/48176 (accessed on 10 October 2022). [CrossRef]
- Rahman, M.M. Potential environmental impacts of herbicides used in agriculture. J. Agric. For. Meteorol. Res. 2020, 3, 266–269. [Google Scholar]
- Andrew, I.K.S.; Storkey, J.; Sparkes, D.L. A review of the potential for competitive cereal cultivars as a tool in integrated weed management. Weed Res. 2015, 55, 239–248. [Google Scholar] [CrossRef]
- Tottman, D.R.; Ingram, G.H.; Lock, A.A.; Makepeace, R.J.; Orson, J.; Smith, J.; Wilson, B.J. Weed control in Cereals. In Weed Control Handbook: Principles, 7th ed.; Roberts, H.A., Ed.; Blackwell Scientific Publications: Boston, MA, USA; Melbourne, VIC, Australia, 1982; pp. 268–291. [Google Scholar]
- Korav, S.; Dhaka, A.K.; Singh, R.; Premaradhya, N.; Reddy, G.C. A study on crop weed competition in field crops. J. Pharmacogn. Phytochem. 2018, 7, 3235–3240. [Google Scholar]
- VanGessel, M.J.; Johnson, Q.R.; Scott, B.A. Effect of application timing on winter wheat response to metribuzin. Weed Technol. 2017, 31, 94–99. [Google Scholar] [CrossRef]
- Pilipavičius, V.; Aliukonienė, I.; Romaneckas, K. Chemical weed control in winter wheat (Triticum aestivum L.) crop of early stages of development: I. Crop weediness. J. Food Agric. Environ. 2010, 8, 206–209. [Google Scholar]
- Beckie, H.J. Herbicide-resistant weeds: Management tactics and practices. Weed Technol. 2006, 20, 793–814. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Baucom, R.G. Evolutionary and ecological insights from herbicide resistant weeds: What have we learned about plant adaptation, and what is left to uncover? New Phytol. 2019, 223, 68–82. [Google Scholar] [CrossRef]
- Beckie, H.J.; Harker, K.N. Our top 10 herbicide-resistant weed management practices. Pest Manag. Sci. 2017, 73, 1045–1052. [Google Scholar] [CrossRef]
- Shaw, D.R.; Arnold, J.C. Weed control from herbicide combinations with glyphosate. Weed Technol. 2002, 16, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Hamill, A.S.; Weaver, S.E. Antagonism and synergism between herbicides: Trends from previous studies. Weed Technol. 1995, 9, 86–90. [Google Scholar] [CrossRef]
- Pawlonka, Z.; Rymuza, K.; Starczewski, K.; Bombik, A. Biodiversity of segetal weed communities when chlorsulfuron-based weed control is being used on continuous winter wheat. J. Plant Prot. Res. 2014, 54, 300–305. [Google Scholar] [CrossRef]
- Iglesias-Rios, R.; Mazzoni, R. Measuring diversity: Looking for processes that generate diversity. Nat. Conserv. 2014, 12, 156–161. [Google Scholar] [CrossRef]
- Lakicevic, M.; Srdjevic, B. Measuring biodiversity in forest communities—A role of biodiversity indices. Contemp. Agric. 2018, 67, 65–70. [Google Scholar] [CrossRef]
- Yakob, G.; Fekadu, A. Diversity and Regeneration Status of Woody Species: The Case of Keja Araba and Tula Forests, South West Ethiopia. Open Access Libr. J. 2016, 3, 1. [Google Scholar] [CrossRef]
- Meyer, S.; Wesche, K.; Krause, B.; Leuschner, C. Dramatic losses of specialist arable plants in Central Germany since the 1950s/60s—A cross-regional analysis. Divers. Distrib. 2013, 19, 1175–1187. [Google Scholar] [CrossRef]
- Kudsk, P. Optimising herbicide dose: A straightforward approach to reduce the risk of side effects of herbicides. Environmentalist 2008, 28, 49–55. [Google Scholar] [CrossRef]
- Locke, M.A.; Reddy, K.N.; Zablotowicz, R.M. Weed management in conservation crop production systems. Weed Biol. Manag. 2002, 2, 123–132. [Google Scholar] [CrossRef]
- Płaza, A.; Ceglarek, F.; Królikowska, A.; Próchnicka, M. Działanie następcze wsiewek międzyplonowych i słomy jęczmienia jarego na plonowanie i elementy struktury plonu pszenżyta ozimego. Folia Pomer. Univ. Technol. Stetin. Agric. Aliment. Pisc. Zootech. 2010, 276, 31–38. [Google Scholar]
- Gaweda, D.; Cierpiała, R.; Harasim, E.; Haliniarz, M. Effect of tillage systems on yield, weed infestation and seed quality elements of soybean. Acta Agrophys. 2016, 23, 175–187. [Google Scholar]
- Zegeye, H.; Teketay, D.; Kelbessa, E. Diversity, regeneration status and socio-economic importance of the vegetation in the islands of Lake Ziway, south-central Ethiopia. Flora-Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 483–498. [Google Scholar] [CrossRef]
- Dibaba, A.; Soromessa, T.; Kelbessa, E.; Tilahun, A. Diversity, structure and regeneration status of the woodland and riverine vegetation of Sire Beggo in Gololcha District, Eastern Ethiopia. MEJS 2014, 6, 70–96. [Google Scholar] [CrossRef]
- Brown, H.M. Mode of action, crop selectivity, and soil relations of the sulfonylurea herbicides. Pestic. Sci. 1990, 29, 263–281. [Google Scholar] [CrossRef]
- Tranel, P.; Wright, T.R. Resistance of Weeds to ALS-Inhibiting Herbicides: What Have We Learned? Weed Sci. 2002, 50, 700–712. [Google Scholar] [CrossRef]
- Damalas, C.A. Herbicide tank mixtures: Common interactions. Int. J. Agric. Biol. 2004, 6, 209–212. [Google Scholar]
- Isaacs, M.A.; Hatzios, K.K.; Wilson, H.P.; Toler, J. Halosulfuron and 2,4-d mixtures’ effects on common lambsquarters (Chenopodium album). Weed Technol. 2006, 20, 137–142. [Google Scholar] [CrossRef]
- Morderer, Y.Y.; Sychuk, A.M.; Rodzevych, O.P.; Pavlenko, V.V.; Sarbash, O.M. Effectiveness of auxin-like herbicide halauxifen-methyl mixtures with other herbicides for canada thistle (Cirsium arvense) control in wheat crops. Fiziol. Rastenij Genet. 2018, 50, 508–516. [Google Scholar] [CrossRef]
- Hollaway, K.L.; Hallam, N.D.; Flynn, A.G. Synergistic joint action of MCPA ester and metsulfuron-methyl. Weed Res. 1996, 36, 369–374. [Google Scholar] [CrossRef]
- Curran, W.S.; Wallace, J.M.; Mirsky, S.; Crockett, B. Effectiveness of Herbicides for Control of Hairy Vetch (Vicia villosa) in Winter Wheat. Weed Technol. 2015, 29, 509–518. [Google Scholar] [CrossRef]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest. Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef]
- Silva, D.R.O.D.; Aguiar, A.C.M.D.; Basso, C.J.; Muraro, D.S. Application time affects synthetic auxins herbicides in tank-mixture with paraquat on hairy fleabane control. Rev. Ceres Viçosa 2021, 68, 194–200. [Google Scholar] [CrossRef]
- Lang, J.; Zikmundová, B.; Hájek, J.; Barták, M.; Váczi, P. The Effects of Foliar Application of Phenoxy and Imidazoline Family Herbicides on the Limitation of Primary Photosynthetic Processes in Galega orientalis L. Agronomy 2022, 12, 96. [Google Scholar] [CrossRef]
- Messelhäuser, M.; Saile, M.; Sievernich, B.; Gerhards, R. Effect of cinmethylin against Alopecurus myosuroides Huds. in winter cereals. Plant Soil Environ. 2021, 67, 46–54. [Google Scholar] [CrossRef]
- Pawlonka, Z.; Skrzyczyńska, J.; Ługowska, M. Rozwój Apera spica-venti (L.) Beauv. w pszenżycie ozimym w warunkach różnej uprawy roli i nawożenia. Fragm. Agron. 2010, 27, 94–101. [Google Scholar]
- Le Gouis, J.; Oury, F.-X.; Charmet, G. How changes in climate and agricultural practices influenced wheat production in Western Europe. J. Cereal Sci. 2020, 93, 102960. [Google Scholar] [CrossRef]
- Ruosteenoja, K.; Markkanen, T.; Räisänen, J. Thermal seasons in northern Europe in projected future climate. Int. J. Climatol. 2020, 40, 4444–4462. [Google Scholar] [CrossRef]
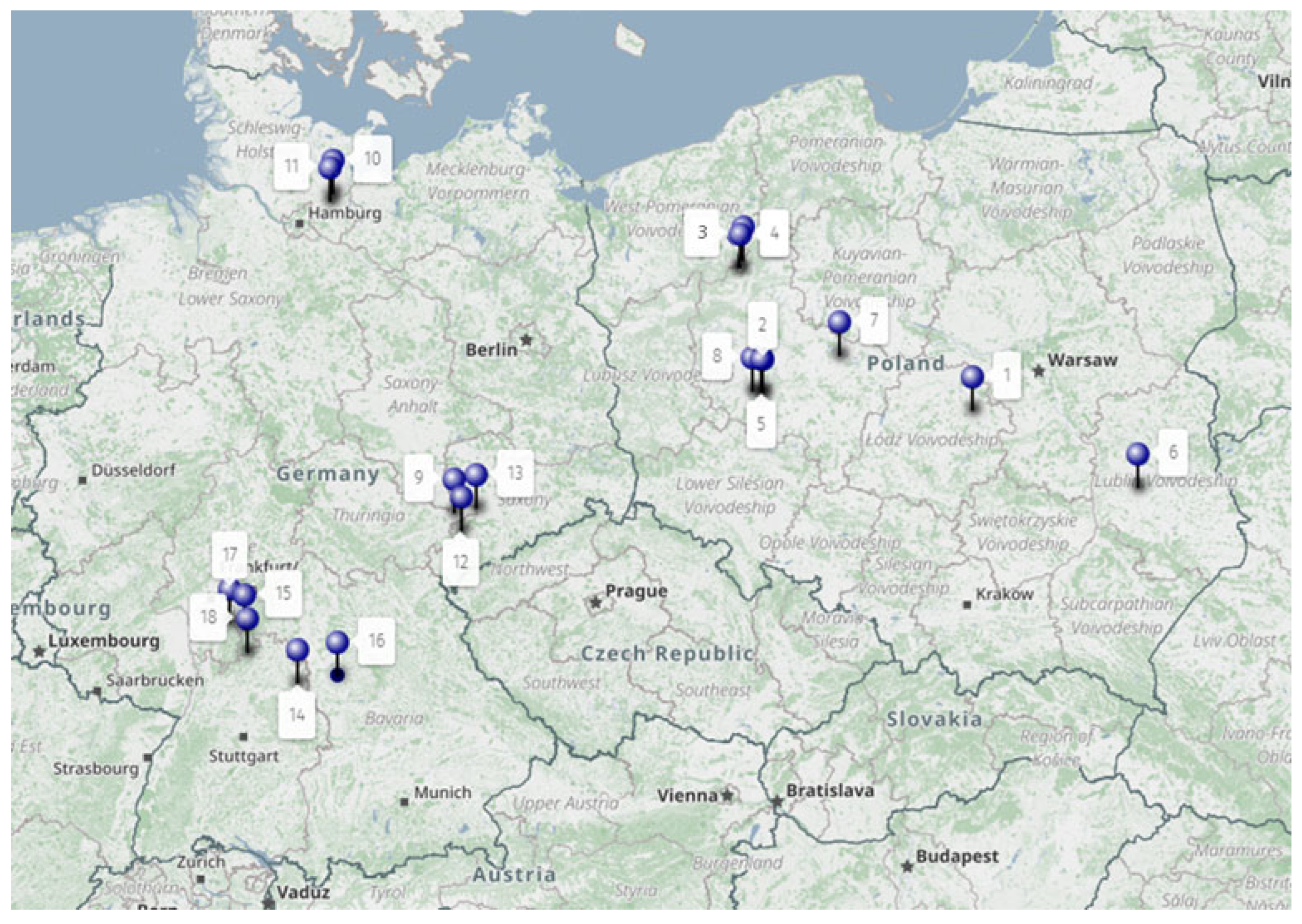

| Trial No. | Country | GPS | Crop and Variety (var.) | Application Date; Crop Stage (BBCH) | Soil | Soil pH | Water Volume at Application (L ha−1) |
|---|---|---|---|---|---|---|---|
| 1 | Poland | 51.87723 N 20.01983 E | Winter wheat var. Muszelka | 15/11/16; 13 | Sandy clay loam | 5.8 | 300 |
| 2 | Poland | 52.03594 N 16.89602 E | Winter wheat var. Hondia | 15/11/16; 15 | Sandy clay | 5.8 | 200 |
| 3 | Poland | 53.15822 N 16.54780 E | Winter wheat var. Ostroga | 21/11/16; 24 | Clay loam | 7.1 | 200 |
| 4 | Poland | 53.15891 N 16.54822 E | Winter triticale var. Twingo | 09/11/16; 16 | Sandy loam | 5.3 | 200 |
| 5 | Poland | 52.05919 N 16.75175 E | Winter triticale var. Twingo | 23/11/16; 15 | Sandy loam | 6.2 | 200 |
| 6 | Poland | 51.16363 N 22.47511 E | Winter barley var. Meridian | 26/10/17; 13 | Clay | 5.5 | 200 |
| 7 | Poland | 52.37013 N 18.03480 E | Winter barley var. Zenek | 4/11/17; 22 | Sandy clay | 6.1 | 300 |
| 8 | Poland | 52.05919 N 16.75175 E | Winter barley var. Gloria | 17/11/17; 23 | Sandy clay | 6.1 | 400 |
| 9 | Germany | 50.93083 N 12.29472 E | Winter wheat var. Pamier | 22/11/16; 13 | Silt loam | 6.5 | 200 |
| 10 | Germany | 53.81500 N 10.49416 E | Winter wheat var. Ritmo | 26/10/16; 12 | Loamy sand | 6.3 | 300 |
| 11 | Germany | 53.74694 N 10.46833 E | Winter wheat var. Edgar | 16/11/16; 21 | Loamy sand | 6.3 | 300 |
| 12 | Germany | 50.76138 N 12.41000 E | Winter wheat var. Baranco | 22/11/16; 22 | Silty clay | 6.6 | 300 |
| 13 | Germany | 50.97055 N 12.63555 E | Winter triticale var. Agostino | 22/11/16; 22 | Loam | 6.8 | 200 |
| 14 | Germany | 49.30416 N 9.97555 E | Winter rye var. Protector | 24/11/16; 13 | Silty clay loam | 7.8 | 200 |
| 15 | Germany | 49.84027 N 9.17861 E | Winter rye var. SU Forsetti | 25/10/16; 13 | Silty sand | 6.7 | 300 |
| 16 | Germany | 49.38583 N 10.57222 E | Winter barley var. Malwinta | 22/11/16; 23 | Loamy sand | 5.7 | 200 |
| 17 | Germany | 49.89416 N 8.95388 E | Winter barley var. Sandra | 22/11/16; 13 | Loamy silt | 6.4 | 300 |
| 18 | Germany | 49.60861 N 9.21805 E | Winter barley var. Sandra | 22/11/16; 13 | Loamy sand | 5.6 | 300 |
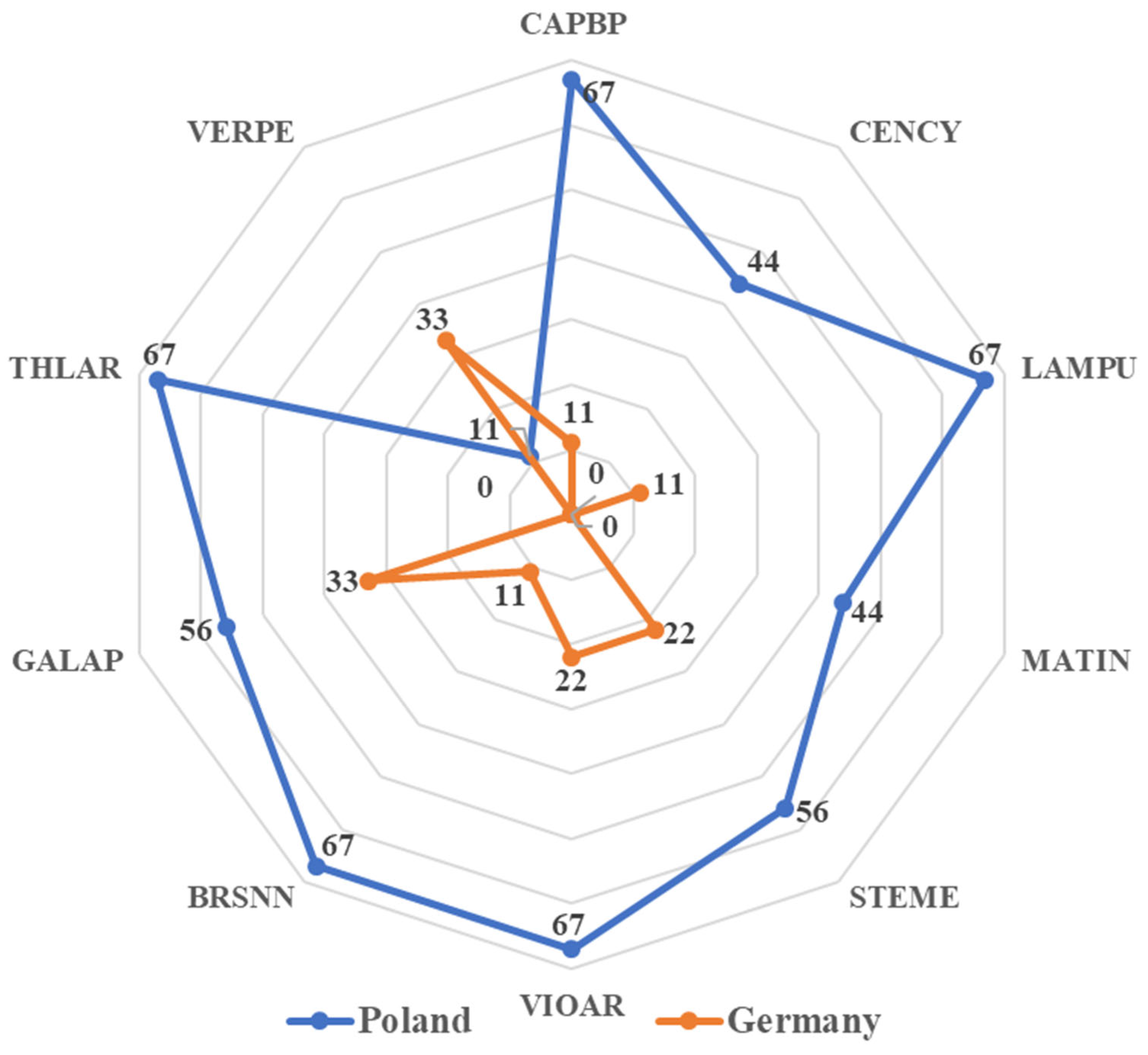
| Trial No. | Country | GPS | Weed Species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAPBP | CENCY | LAMPU | MATIN | STEME | VIOAR | BRSNN | GALAP | THLAR | VERPE | |||
| BBCH | ||||||||||||
| 1 | Poland | 51.87723 N 20.01983 E | 12 | 12 | 12 | 11 | 12 | 11 | - | - | - | - |
| 2 | Poland | 52.03594 N 16.89602 E | 12 | 13 | 13 | - | - | 12 | 13 | 12 | 12 | - |
| 3 | Poland | 53.15822 N 16.54780 E | - | 12 | - | 12 | 12 | 11 | 12 | - | 12 | - |
| 4 | Poland | 53.15891 N 16.54822 E | - | - | - | 11 | 12 | 11 | 12 | - | 12 | - |
| 5 | Poland | 52.05919 N 16.75175 E | 12 | 13 | 13 | - | - | 12 | 23 | - | 12 | - |
| 6 | Poland | 51.16363 N 22.47511 E | 16 | - | 13 | - | 13 | 13 | - | - | - | - |
| 7 | Poland | 52.37013 N 18.03480 E | 14 | - | 14 | 14 | 14 | - | 15 | - | 15 | 15 |
| 8 | Poland | 52.05919 N 16.75175 E | 12 | - | 12 | - | - | - | 12 | - | 13 | - |
| 9 | Germany | 50.93083 N 12.29472 E | - | - | - | - | - | - | - | 31 | - | - |
| 10 | Germany | 53.81500 N 10.49416 E | 14 | - | - | - | - | 13 | - | - | - | - |
| 11 | Germany | 53.74694 N 10.46833 E | - | - | - | - | - | - | - | 14 | - | - |
| 12 | Germany | 50.76138 N 12.41000 E | - | - | - | - | - | - | - | 31 | - | - |
| 13 | Germany | 50.97055 N 12.63555 E | - | - | 12 | - | 14 | - | - | - | - | - |
| 14 | Germany | 49.30416 N 9.97555 E | - | - | - | - | - | - | - | - | - | 14 |
| 15 | Germany | 49.84027 N 9.17861 E | - | - | - | - | - | 12 | 13 | - | - | - |
| 16 | Germany | 49.38583 N 10.57222 E | - | - | - | - | - | 12 | - | - | - | 12 |
| 17 | Germany | 49.89416 N 8.95388 E | - | - | - | - | - | - | - | 12 | - | 12 |
| 18 | Germany | 49.60861 N 9.21805 E | - | - | - | - | 12 | - | - | - | - | - |
| Trial No. | Country | GPS | Scientific Name | RF (%) | DMg | H′ | D | d |
|---|---|---|---|---|---|---|---|---|
| 1 | Poland | 51.87723 N 20.01983 E | Capsella bursa-pastoris Centaurea cyanus Lamium purpureum Tripleurospermum inodorum Stellaria media Viola arvensis | 18.7 17.5 18.2 13.2 15.0 17.5 | 3.12 | 0.775 | 0.85 | 0.187 |
| 2 | Poland | 52.03594 N 16.89602 E | Brassica napus Capsella bursa-pastoris Centaurea cyanus Galium aparine Lamium purpureum Thlaspi arvense Viola arvensis | 14.1 15.6 10.0 13.2 15.6 13.7 17.9 | 3.48 | 0.839 | 0.87 | 0.179 |
| 3 | Poland | 53.15822 N 16.54780 E | Brassica napus Centaurea cyanus Tripleurospermum inodorum Stellaria media Thlaspi arvense Viola arvensis | 13.6 13.0 13.6 13.6 17.1 29.3 | 3.19 | 0.755 | 0.84 | 0.293 |
| 4 | Poland | 53.15891 N 16.54822 E | Brassica napus Tripleurospermum inodorum Stellaria media Thlaspi arvense Viola arvensis | 21.2 11.2 10.6 12.1 44.9 | 2.33 | 0.620 | 0.73 | 0.449 |
| 5 | Poland | 52.05919 N 16.75175 E | Brassica napus Centaurea cyanus Capsella bursa-pastoris Lamium purpureum Thlaspi arvense Viola arvensis | 15.3 14.2 18.6 16.4 14.2 21.4 | 3.01 | 0.773 | 0.85 | 0.214 |
| 6 | Poland | 51.16363 N 22.47511 E | Capsella bursa-pastoris Lamium purpureum Stellaria media Viola arvensis | 22.6 26.3 29.3 21.8 | 2.11 | 0.599 | 0.78 | 0.293 |
| 7 | Poland | 52.37013 N 18.03480 E | Brassica napus Capsella bursa-pastoris Lamium purpureum Stellaria media Tripleurospermum inodorum Thlaspi arvense Veronica persica | 15.3 13.4 13.0 14.3 11.5 15.3 17.2 | 3.49 | 0.842 | 0.87 | 0.172 |
| 8 | Poland | 52.05919 N 16.75175 E | Brassica napus Capsella bursa-pastoris Lamium purpureum Thlaspi arvense | 21.9 29.5 26.0 22.7 | 1.92 | 0.528 | 0.76 | 0.295 |
| 9 | Germany | 50.93083 N 12.29472 E | Galium aparine | 100.0 | 0.0 | 0.0 | 0.0 | 1.0 |
| 10 | Germany | 53.81500 N 10.49416 E | Capsella bursa-pastoris Viola arvensis | 2.8 97.2 | 0.42 | 0.055 | 0.05 | 0.972 |
| 11 | Germany | 53.74694 N 10.46833 E | Galium aparine | 100.0 | 0.0 | 0.0 | 0.0 | 1.0 |
| 12 | Germany | 50.76138 N 12.41000 E | Galium aparine | 100.0 | 0.0 | 0.0 | 0.0 | 1.0 |
| 13 | Germany | 50.97055 N 12.63555 E | Lamium purpureum Stellaria media | 36.4 63.6 | 0.74 | 0.285 | 0.48 | 0.636 |
| 14 | Germany | 49.30416 N 9.97555 E | Veronica persica | 100.0 | 0.0 | 0.0 | 0.0 | 1.0 |
| 15 | Germany | 49.84027 N 9.17861 E | Brassica napus Viola arvensis | 17.9 82.1 | 0.54 | 0.204 | 0.30 | 0.821 |
| 16 | Germany | 49.38583 N 10.57222 E | Veronica persica Viola arvensis | 86.2 13.8 | 0.55 | 0.175 | 0.24 | 0.862 |
| 17 | Germany | 49.89416 N 8.95388 E | Galium aparine Veronica persica | 13.5 86.5 | 0.46 | 0.172 | 0.23 | 0.865 |
| 18 | Germany | 49.60861 N 9.21805 E | Stellaria media | 100.0 | 0.0 | 0.0 | 0.0 | 1.0 |
| No. | Treatment | Dose Per Ha | Weed Species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRSNN | CAPBP | CENCY | GALAP | LAMPU | MATIN | STEME | THLAR | VERPE | VIOAR | |||
| Efficacy (%) | ||||||||||||
| 1st assessment before end of vegetation period (about 24 DAT) | ||||||||||||
| 1 | MCPA+ tribenuron-methyl | 0.8 kg | 31 | 44 | 26 | 1 | 44 | 35 | 41 | 29 | 50 | 42 |
| 2 | MCPA+ tribenuron-methyl | 1.0 kg | 48 | 67 | 38 | 2 | 61 | 47 | 54 | 57 | 61 | 54 |
| 3 | diflufenican+ chlorotoluron | 2.5 L | 45 | 63 | 57 | 2 | 63 | 51 | 58 | 56 | 62 | 67 |
| HSD 0.05 | 9.7 | 8.7 | 8.8 | ns | 9.7 | 2.4 | 3.4 | 10.2 | 5.7 | 5.0 | ||
| 2nd assessment in the spring (about 150 DAT) | ||||||||||||
| 1 | MCPA+tribenuron-methyl | 0.8 kg | 68 | 72 | 67 | 47 | 59 | 70 | 79 | 62 | 74 | 66 |
| 2 | MCPA+tribenuron-methyl | 1.0 kg | 93 | 90 | 83 | 75 | 82 | 89 | 91 | 89 | 83 | 83 |
| 3 | diflufenican+ chlorotoluron | 2.5 L | 95 | 91 | 83 | 81 | 83 | 90 | 97 | 93 | 90 | 87 |
| HSD 0.05 | 8.1 | 9.1 | 3.8 | 6.7 | 7.2 | 6.0 | 4.4 | 8.8 | 5.4 | 5.8 | ||
| No. | Treatment | Dose Per ha | Weed Species | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BRSNN | CAPBP | GALAP | LAMPU | STEME | VERPE | VIOAR | |||
| Efficacy (%) | |||||||||
| 1st assessment before end of vegetation period (about 24 DAT) | |||||||||
| 1 | MCPA+tribenuron-methyl | 0.8 kg | 99 | 33 | 6 | 0 | 15 | 19 | 58 |
| 2 | MCPA+tribenuron-methyl | 1.0 kg | 96 | 45 | 6 | 0 | 50 | 21 | 61 |
| 3 | diflufenican+chlorotoluron | 2.5 L | 99 | 48 | 13 | 0 | 83 | 55 | 71 |
| HSD 0.05 | ns | 8.1 | 6.5 | ns | 23.2 | 15.1 | 11.2 | ||
| 2nd assessment in the spring (about 150 DAT) | |||||||||
| 1 | MCPA+tribenuron-methyl | 0.8 kg | 100 | 100 | 48 | 90 | 98 | 80 | 56 |
| 2 | MCPA+tribenuron-methyl | 1.0 kg | 100 | 100 | 60 | 96 | 99 | 90 | 76 |
| 3 | diflufenican+chlorotoluron | 2.5 L | 100 | 100 | 93 | 100 | 100 | 100 | 99 |
| HSD 0.05 | ns | ns | 18.9 | ns | 1.6 | 7.3 | 17.8 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobiech, Ł.; Joniec, A.; Loryś, B.; Rogulski, J.; Grzanka, M.; Idziak, R. Autumn Application of Synthetic Auxin Herbicide for Weed Control in Cereals in Poland and Germany. Agriculture 2023, 13, 32. https://doi.org/10.3390/agriculture13010032
Sobiech Ł, Joniec A, Loryś B, Rogulski J, Grzanka M, Idziak R. Autumn Application of Synthetic Auxin Herbicide for Weed Control in Cereals in Poland and Germany. Agriculture. 2023; 13(1):32. https://doi.org/10.3390/agriculture13010032
Chicago/Turabian StyleSobiech, Łukasz, Andrzej Joniec, Barbara Loryś, Janusz Rogulski, Monika Grzanka, and Robert Idziak. 2023. "Autumn Application of Synthetic Auxin Herbicide for Weed Control in Cereals in Poland and Germany" Agriculture 13, no. 1: 32. https://doi.org/10.3390/agriculture13010032
APA StyleSobiech, Ł., Joniec, A., Loryś, B., Rogulski, J., Grzanka, M., & Idziak, R. (2023). Autumn Application of Synthetic Auxin Herbicide for Weed Control in Cereals in Poland and Germany. Agriculture, 13(1), 32. https://doi.org/10.3390/agriculture13010032








