Occurrence and Mechanism of Papaver rhoeas ALS Inhibitors Resistance in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of P. rhoeas Seeds
2.2. Biological Tests in Glasshouses
2.2.1. Discriminate Dose Experiments
2.2.2. Whole-Plant Dose-Response Bioassays
2.3. Molecular Analysis of the ALS Gene
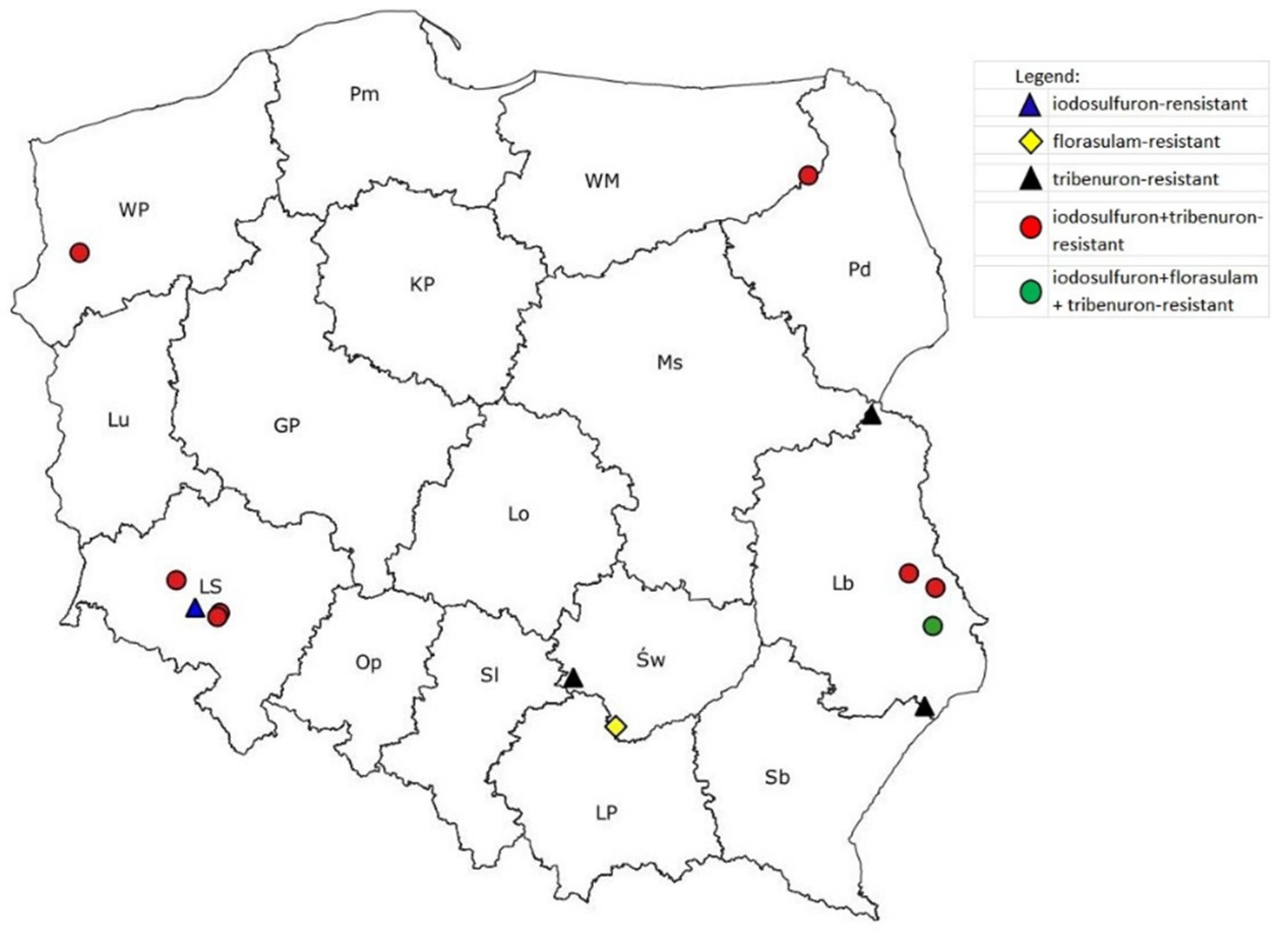
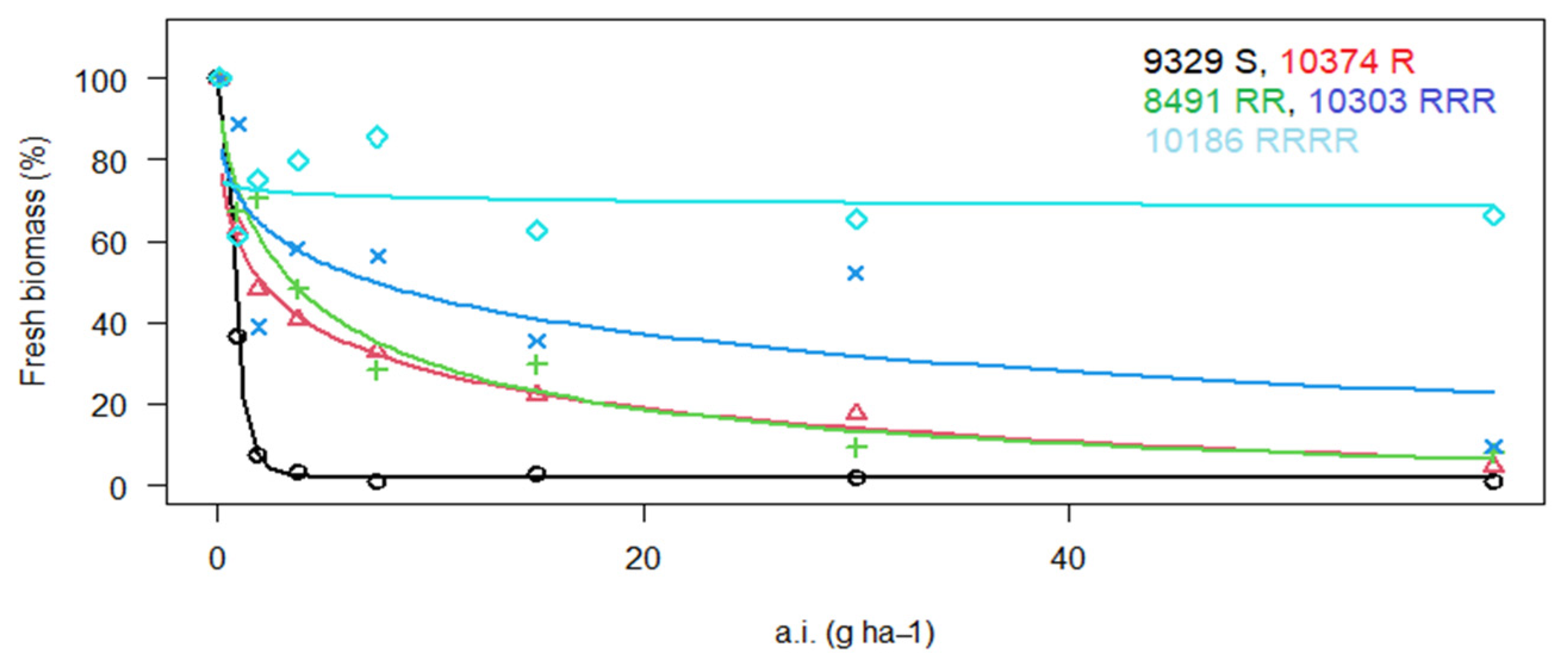
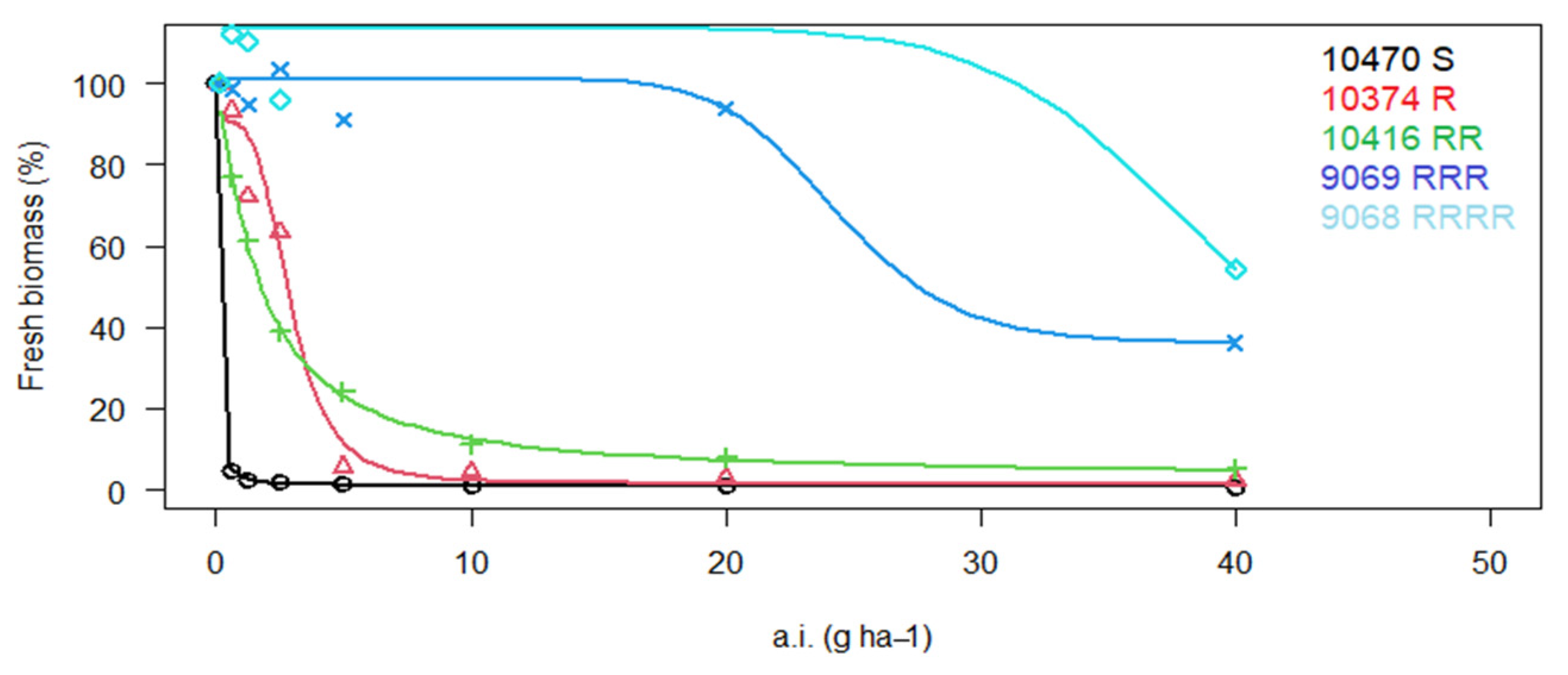
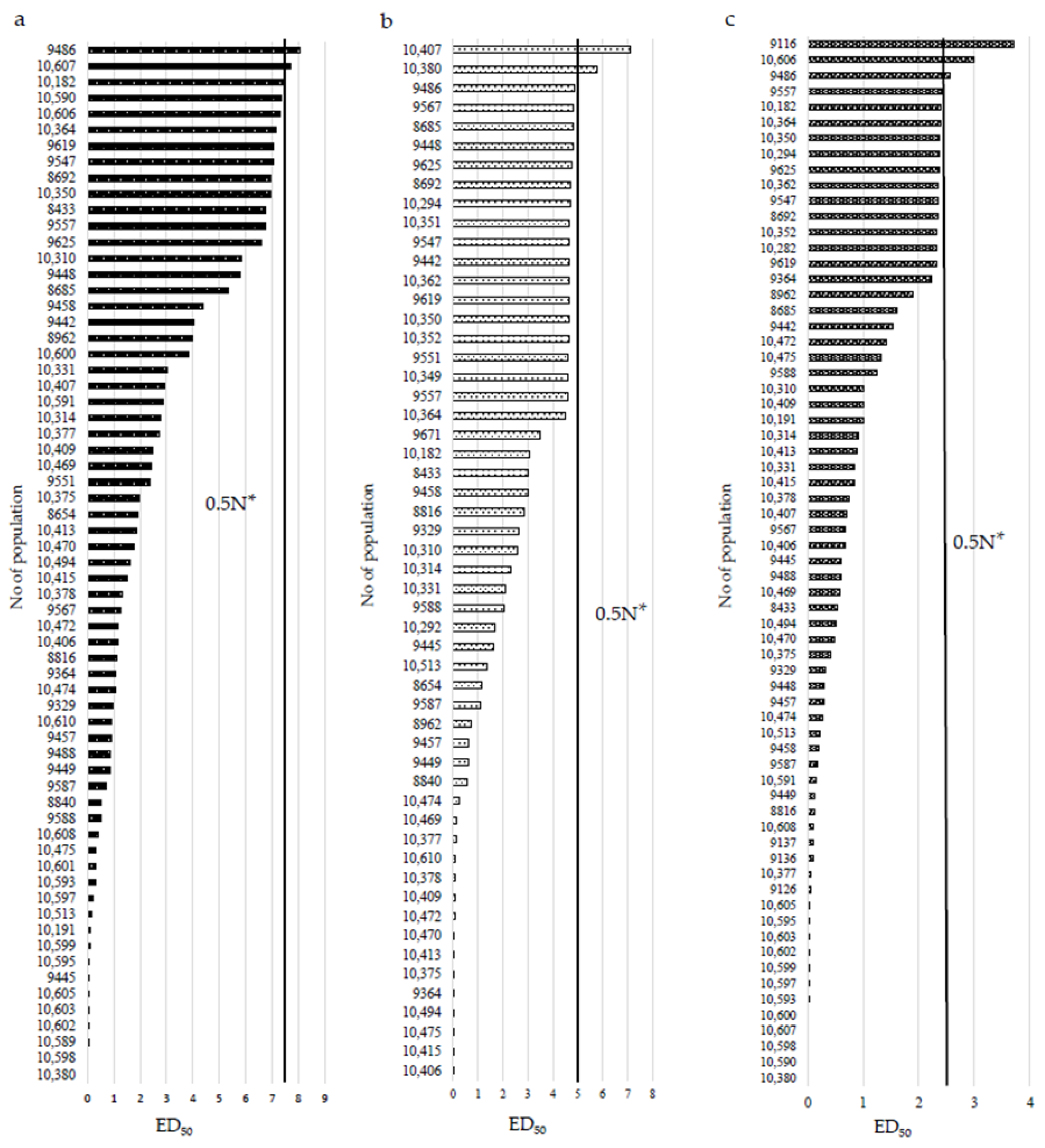
3. Results
3.1. Reaction of P. rhoeas Populations to ALS Inhibitors
3.2. Molecular Analysis of the ALS Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heap, I. International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 20 March 2022).
- HRAC: Herbicide Resistance Action Committee. Available online: www.hracglobal.com (accessed on 20 March 2022).
- Gressel, J. Evolving understanding of the evolution of herbicide resistance. Pest Manag. Sci. 2009, 65, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Vidotto, F.; Valle, N.D.; Fogliatto, S.; Milan, M.; De Palo, F.; Tabacchi, M.; Ferrero, A. Rapid increase of herbicide resistance in Echinochloa spp. consequent to repeated applications of the same herbicides over time. Arch. Agron Soil Sci. 2021, 67, 620–632. [Google Scholar] [CrossRef]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Hanf, M. Farbatlas Feldflora. Wildkräuter und Unkräuter; Verlag Eugen Ulmer: Stuttgart, Germany, 1990; p. 226. (In German) [Google Scholar]
- Torra, J.; Royo-Esnal, A.; Recasens, J. Management of herbicide-resistant Papaver rhoeas in dry land cereal fields. Agron. Sustain. Dev. 2011, 31, 483–490. [Google Scholar] [CrossRef]
- Mitich, L.W. Corn Poppy (Papaver rhoeas L.). Weed Technol. 2000, 14, 826–829. [Google Scholar] [CrossRef]
- Torra, J.; Recasens, J. Demography of corn poppy (Papaver rhoeas) in relation to emergence time and crop competition. Weed Sci. 2008, 56, 826–833. [Google Scholar] [CrossRef]
- Torra, J.; Gonzalez-Andujar, L.; Recasens, J. Modelling the population dynamics of Papaver rhoeas under various weed management systems in a Mediterranean climate. Weed Res. 2008, 48, 136–146. [Google Scholar] [CrossRef]
- Stankiewicz-Kosyl, M.; Synowiec, A.; Haliniarz, M.; Wenda-Piesik, A.; Domaradzki, K.; Parylak, D.; Wrochna, M.; Pytlarz, E.; Gala-Czekaj, D.; Marczewska-Kolasa, K.; et al. Herbicide Resistance and Management Options of Papaver rhoeas L. and Centaurea cyanus L. in Europe: A Review. Agronomy 2020, 10, 874. [Google Scholar] [CrossRef]
- Cirujeda, A.; Recasens, J.; Taberner, A. Dormancy cycle and viability of buried seeds of Papaver rhoeas. Weed Res. 2006, 46, 327–334. [Google Scholar] [CrossRef]
- Torra, J.; Royo-Esnal, A.; Rey-Caballero, J.; Recasens, J.; Salas, M. Management of herbicide-resistant corn poppy (Papaver rhoeas) under different tillage systems does not change the frequency of resistant plants. Weed Sci. 2018, 66, 764–772. [Google Scholar] [CrossRef]
- Scherner, A.; Melander, B.; Kudsk, P. Vertical distribution and composition of weed seeds within the plough layer after eleven years of contrasting crop rotation and tillage schemes. Soil Tillage Res. 2016, 161, 135–142. [Google Scholar] [CrossRef]
- Cirujeda, A.; Recasens, J.; Torra, J.; Taberner, A. A germination study of herbicide-resistant field poppies in Spain. Agron. Sustain. Dev. 2008, 28, 207–220. [Google Scholar] [CrossRef][Green Version]
- Holm, L.; Doll, J.; Holm, E.; Pancho, J.; Herberger, J. World Weeds: Natural Histories and Distribution; John Wiley: New York, NY, USA, 1997; p. 129. [Google Scholar]
- Calha, I.; Rocha, F.; Ruiz-Santaella, J.P.; Cruz-Hipolito, H. Two decades of herbicide resistance in the Iberian Peninsula. J. Plant Dis. Protect. 2008, 21, 79–84. [Google Scholar]
- Rey-Caballero, J.; Menéndez, J.; Giné-Bordonaba, J.; Salas, M.; Alcántara, R.; Torra, J. Unravelling the resistance mechanisms to 2,4-D (2,4-dichlorophenoxyacetic acid) in corn poppy (Papaver rhoeas). Pestic. Biochem. Phys. 2016, 133, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kati, V.; Scarabel, L.; Thiery-Lanfranchi, D.; Kioleoglou, V.; Liberopoulou, S.; Délye, C. Multiple resistance of Papaver rhoeas L. to 2,4-D and acetolactate synthase inhibitors in four European countries. Weed Res. 2019, 59, 367–376. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Ann. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Torra, J.; Rojano-Delgado, A.M.; Menéndez, J.; Salas, M.; De Prado, R. Cytochrome P450 metabolism-based herbicide resistance to imazamox and 2, 4-D in Papaver rhoeas. Plant Physiol. Biochem. 2021, 160, 51–61. [Google Scholar] [CrossRef]
- Torra, J.; Cirujeda, A.; Taberner, A.; Recasens, J. Evaluation of herbicides to manage herbicide-resistant corn poppy (Papaver rhoeas) in winter cereals. Crop Prot. 2010, 29, 731–736. [Google Scholar] [CrossRef]
- Torra, J.; Rojano-Delgado, A.M.; Rey-Caballero, J.; Royo-Esnal, A.; Salas, M.L.; De Prado, R. Enhanced 2,4-D metabolism in two resistant Papaver rhoeas populations from Spain. Front. Plant Sci. 2017, 13, 1584. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Portugal, J.; Vázquez-García, J.G.; Osuna, M.D.; Torra, J.; Lozano-Juste, J.; Gherekhloo, J.; De Prado, R. Tribenuron-methyl metabolism and the rare Pro197Phe double mutation together with 2,4-D metabolism and reduced absorption can evolve in Papaver rhoeas with multiple and cross herbicide resistance to ALS inhibitors and auxin mimics. Pestic. Biochem. Phys. 2022, 188, 105226. [Google Scholar] [CrossRef]
- Duran-Prado, M.; Osuna, M.D.; De Prado, R.; Franco, A.R. Molecular basis of resistance to sulfonylureas in Papaver rhoeas. Pestic. Biochem. Phys. 2004, 79, 10–17. [Google Scholar] [CrossRef]
- Kaloumenos, N.S.; Dordas, C.A.; Diamantidis, G.C.; Eleftherohorinos, I.G. Multiple Pro197 substitutions in the acetolactate synthase of corn poppy (Papaver rhoeas) confer resistance to tribenuron. Weed Sci. 2009, 57, 262–268. [Google Scholar] [CrossRef]
- Marshall, R.; Hull, R.; Moss, S.R. Target site resistance to ALS inhibiting herbicides in Papaver rhoeas and Stellaria media biotypes from the UK. Weed Res. 2010, 50, 621–630. [Google Scholar] [CrossRef]
- Scarabel, L.; Pernin, F.; Déyle, C. Occurrence, genetic control and evolution of non-target-site based resistance to herbicides inhibiting acetolactate synthase (ALS) in the dicot weed Papaver rhoeas. Plant Sci. 2015, 238, 158–169. [Google Scholar] [CrossRef]
- Délye, C.; Pernin, F.; Scarabel, L. Evolution and diversity of the mechanisms endowing resistance to herbicides inhibiting acetolactate-synthase (ALS) in corn poppy (Papaver rhoeas L.). Plant Sci. 2011, 180, 333–342. [Google Scholar] [CrossRef]
- Rey-Caballero, J.; Menéndez, J.; Osuna, M.D.; Salas, M.; Torra, J. Target-site and non-target-site resistance mechanisms to ALS inhibiting herbicides in Papaver rhoeas. Pestic. Biochem. Phys. 2017, 138, 57–65. [Google Scholar] [CrossRef]
- Moss, S.; Ulber, L.; den Hoed, I. A herbicide resistance risk matrix. Crop Prot. 2019, 115, 13–19. [Google Scholar] [CrossRef]
- Diggle, A.J.; Neve, P.B.; Smith, F.P. Herbicides used in combination can reduce the probability of herbicide resistance in finite weed populations. Weed Res. 2003, 43, 371–382. [Google Scholar] [CrossRef]
- Busi, R.; Powles, S.B.; Beckie, H.J.; Renton, M. Rotations and mixtures of soil-applied herbicides delay resistance. Pest Manag. Sci. 2020, 76, 487–496. [Google Scholar] [CrossRef]
- Beckie, H.J.; Harker, K.N. Our top 10 herbicide-resistant weed management practices. Pest Manag. Sci. 2017, 73, 1045–1052. [Google Scholar] [CrossRef]
- Beckie, H.J.; Busi, R.; Lopez-Ruiz, F.J.; Umina, P.A. Herbicide resistance management strategies: How do they compare with those for insecticides, fungicides and antibiotics? Pest Manag. Sci. 2021, 77, 3049–3056. [Google Scholar] [CrossRef] [PubMed]
- Burgos, N.R.; Tranel, P.J.; Streibig, J.C.; Davis, V.M.; Shaner, D.; Norsworthy, J.K.; Ritz, C. Review: Confirmation of resistance to herbicides and evaluation of resistance levels. Weed Sci. 2013, 61, 4–20. [Google Scholar] [CrossRef]
- Panozzo, S.; Scarabel, L.; Collavo, A.; Sattin, M. Protocols for robust herbicide resistance testing in different weed species. J. Vis. Exp. 2015, 101, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R software package for dose-response studies: The concept and data analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 8 January 2022).
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Burgos, N.R. Whole-plant and seed bioassays for resistance confirmation. Weed Sci. 2015, 63, 152–165. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry 1987, 19, 11–15. [Google Scholar]
- Trąba, C.; Ziemińska-Smyk, M. The occurrence of Papaver rhoeas L. in agrocenoses of the buffer zone of the Roztocze National Park compared to other regions of Poland. Acta Agrobot. 2009, 62, 213–230. [Google Scholar] [CrossRef][Green Version]
- Godefroid, S.; Rivie‘re, S.; Waldren, S.; Boretos, N.; Eastwood, R.; Vanderborght, T. To what extent are threatened European plant species conserved in seed banks? Biol. Conserv. 2011, 144, 1494–1498. [Google Scholar] [CrossRef]
- March-Salas, M.; Fitze, P.S. A multi-year experiment shows that lower precipitation predictability encourages plants’ early life stages and enhances population viability. PeerJ 2019, 7, 6443. [Google Scholar] [CrossRef]
- Dąbkowska, T.; Łabza, T.; Krańska, A. Zmiany we florze chwastów segetalnych w latach 1993-2005 zagrożonych na rędzinie brunatnej Wyżyny Miechowskiej. Fragm. Agron. 2007, 3, 55–61. (In Polish) [Google Scholar]
- Fijałkowski, D. Synantropy Roślinne Lubelszczyzny; PWN: Warszawa-Łódź, Poland, 1978. (In Polish) [Google Scholar]
- Wnuk, Z. Zespół Lamio-Veronicetum politae Kornaś 1950 w Polsce. Zesz Nauk AR w Krakowie 1987, 216, 95–133. (In Polish) [Google Scholar]
- Skrzyczyńska, J. Studia nad Florą i Zbiorowiskami Segetalnymi Wysoczyzny Siedleckiej; Rozprawy Naukowe WSR-P w Siedlcach: Siedlce, Poland, 1994. (In Polish) [Google Scholar]
- Kutyna, I.; Młynkowiak, E.; Leśnik, T. Struktura fitosocjologiczna fitocenoz zbóż ozimych na tle warunków glebowych południowo-zachodniej części Niziny Szczecińskiej i terenów do niej przyległych. Fragm. Agron. 2010, 27, 86–102. (In Polish) [Google Scholar]
- Sekutowski, T.R.; Włodek, S.; Biskupski, S.; Sienkiewicz-Cholewa, U. Porównanie odłogu i sąsiadującego pola uprawnego pod względem zasobności w nasiona i rośliny nawłoci (Solidago sp.). Zesz. Nauk. UP Wroc. Rol. C 2012, 584, 99–112. (In Polish) [Google Scholar]
- Staniak, M.; Haliniarz, M.; Kwiecińska-Poppe, E.; Harasim, E.; Wesołowski, M. Diversity of agrocoenoses in the Lublin region, Poland. Acta Agrobot. 2017, 70, 1722. [Google Scholar] [CrossRef][Green Version]
- Adamczewski, K.; Kierzek, R.; Matysiak, K. Biotypes of scentless chamomile Matricaria maritima (L.) ssp. inodora (L.) Dostal and common poppy Papaver rhoeas (L.) resistant to tribenuron methyl, in Poland. J. Plant Prot. Res. 2014, 54, 401–406. [Google Scholar] [CrossRef]
- Petersen, J.; Raffen, H. Evolution der Herbizidresistenz in Alopecurus myosuroides und Apera spica-venti im deutschen Getreideanbau der letzten 15 Jahre. Julius-Kühn-Archiv 2020, 464, 326–332. (In German) [Google Scholar]
- Duggleby, R.G.; McCourt, J.A.; Guddat, L.W. Structure and mechanism of inhibition of plant acetohydroxyacid synthase. Plant Physiol. Biochem. 2008, 46, 309–324. [Google Scholar] [CrossRef]
- Statistical Yearbook of The Republic of Poland; Statistics Poland: Warsaw, Poland, 2021. Available online: https://stat.gov.pl/obszary-tematyczne/roczniki-statystyczne/roczniki-statystyczne/rocznik-statystyczny-rzeczypospolitej-polskiej-2021,2,21.html (accessed on 21 March 2022).
- Rey-Caballero, J.; Royo-Esnal, A.; Recasens, J.; González, I.; Torra, J. Management options for multiple herbicide–resistant corn poppy (Papaver rhoeas) in Spain. Weed Sci. 2017, 65, 295–304. [Google Scholar] [CrossRef]
- Perotti, W.E.; Larran, A.S.; Palmieri, W.E.; Martinatto, A.K.; Permingeat, H.R. Herbicide resistant weeds: A call to integrate conventional agricultural practices, molecular biology knowledge and new technologies. Plant Sci. 2020, 290, 110255. [Google Scholar] [CrossRef]
- Barber, L.T.; Smith, K.L.; Scott, R.C.; Norsworthy, J.K.; Vangilder, A.M. Zero Tolerance: A Community-Based Program for Glyphosate-Resistant Palmer Amaranth Management; University of Arkansas, Division of Agriculture and Natural Research and Extension FSA2177: Little Rock, AR, USA, 2015; Available online: www.uaex.uada.edu/publications/pdf/FSA2177.pdf (accessed on 4 April 2022).
- Beckie, H.J.; Ashworth, M.B.; Flower, K.C. Herbicide resistance management: Recent developments and trends. Plants 2019, 8, 161. [Google Scholar] [CrossRef] [PubMed]
| Active Substance | Commercial Product | Producer | Content of Active Substancein Commercial Products | Recommended Dose of Commercial Product | Field Dose (1N) of Active Substance |
|---|---|---|---|---|---|
| Tribenuron methyl | Lumer 50 WG | ADAMA, PL | 500 g kg−1 (50%) | 30 g ha−1 | 15 g ha−1 |
| Florasulam | Saracen 050 SC | Cheminova, PL | 50 g L−1 (4.81%) | 0.1 L ha−1 | 5 g ha−1 |
| Iodosulfuron | Autumn 10 WG | Bayer, PL | 100 g kg−1 (10%) | 100 g ha−1 | 10 g ha−1 |
| Population | Province | Tribenuron-Methyl | Iodosulfuron-Methyl-Na | Florasulam | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RI | ED50 (g ha−1) | R/S | RI | ED50 (g ha−1) | R/S | RI | ED50 (g ha−1) | R/S | ||
| 8491 * | Lb | RR | 47.9 ± 14.4 ** | 6.8 | S | X | X | S | X | X |
| 8727 * | Lb | RR | 50.6 ± 10.2 | 7.2 | S | X | X | S | X | X |
| 8833 | Św | S | X | X | S | X | X | r | 4.7 ± 1.6 | 2.0 |
| 8935 | LS | S | X | X | R | 16.7 ± 1.07 | 3.6 | S | X | X |
| 8961 * | WM | RRRR | 545.6 ± 109.1 | 77.5 | RR | 23.2 ± 5.8 | 5.0 | S | X | X |
| 9068 * | LS | RRR | 201.1 ± 20.1 | 28.6 | RRRR | 398.5 ± 14.2 | 85.4 | S | X | X |
| 9069 * | LS | RRRR | 4089 ± 531.6 | 580.7 | RRR | 283.7 ± 37.4 | 60.8 | S | X | X |
| 9440 | Św | RR | 35.7 ± 5.3 | 5.1 | S | X | X | S | X | X |
| 10,186 | LS | RRRR | 10,212 ± 2246.6 | 1450.2 | RRR | 101.0 ± 16.2 | 21.6 | S | X | X |
| 10,303 * | WP | RRR | 63.9 ± 11.5 | 3.2 | RRR | 136.3 ± 30.0 | 29.2 | S | X | X |
| 10,374 | Lb | R | 16.3 ± 2.3 | 2.3 | R | 23.0 ± 4.4 | 4.9 | S | X | X |
| 10,410 * | Lb | RRR | 164.1 ± 34.5 | 23.3 | r | 10.7 ± 2.0 | 2.3 | S | X | X |
| 10,416 * | Lb | RRR | 180.6 ± 11.7 | 25.7 | RR | 24.3 ± 4.6 | 5.2 | r | 6.0 ± 1.8 | 2.5 |
| 10,612 | LS | RRRR | >480 | >68.2 | r | 9.5 ± 1.22 | 2.0 | S | X | X |
| Ala 122 GCA | Ile 136 ATT | Glu 144 GAA | Pro 197 CCT | Ala 205 GCA | Ile 211 ATT | Asp 376 GAT | Arg 377 CGT | Ile 411 ATC | Gly 417 GTG | Glu 427 GAA | Gly 438 GTG | Met 445 ATG | Leu 453 TTG | Trp 574 TGG | Leu 648 TTG | Ala 653 GCT | Gly 654 GGT | No. of occ.* | Population Wild Type ALS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GCA | ATT | GAA | CCT | GCA | ATT | GAT | CGT | ATC | GTG | GAA | GTG | ATG | TTG | TGG | TTG | GCT | GGT | 20 | 8816 8727 8491 8483 9329 10,188 10,310 10,314 10,331 |
| . . . | . . . | . . . | CST ** | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | 6 | 10,303 |
| . . . | . . . | . . . | CST | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | YTG | . . . | . . . | . . . | . . . | 1 | 10,303 |
| . . . | . . . | . . . | CGT | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | CTG | . . . | . . . | . . . | . . . | 4 | 10,303 |
| . . . | . . . | . . . | CGT | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | AWG | CTG | . . . | . . . | . . . | . . . | 1 | 10,303 |
| . . . | . . . | . . . | CGT | . . . | . . . | . . . | . . . | . . . | RTG | . . . | . . . | AWG | YTG | . . . | . . . | . . . | . . . | 1 | 10,303 |
| . . . | . . . | . . . | CST | . . . | . . . | . . . | . . . | RTC | . . . | . . . | GYG | AWG | . . . | . . . | . . . | . . . | . . . | 1 | 10,303 |
| . . . | . . . | . . . | CST | . . . | . . . | . . . | . . . | . . . | RTG | . . . | . . . | AWG | . . . | . . . | . . . | . . . | . . . | 1 | 10,303 |
| . . . | . . . | . . . | CYT | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | 1 | 8727 |
| . . . | . . . | . . . | TCT | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | 1 | 10,410 |
| . . . | . . . | . . . | YCT | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | 4 | 10,410 10,416 |
| . . . | . . . | . . . | ACT | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | 4 | 9068 9069 |
| . . . | . . . | . . . | MCT | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | 2 | 9068 |
| . . . | . . . | . . . | KCT | . . . | ATY | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | 1 | 8961 |
| . . . | . . . | . . . | SCT | . . . | ATY | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | 2 | 8961 |
| . . . | . . . | . . . | SMT | . . . | ATY | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | 3 | 8961 |
| . . . | . . . | . . . | YCT | . . . | . . . | . . . | . . . | RTC | RTG | . . . | . . . | AWG | . . . | . . . | . . . | . . . | . . . | 1 | 10,416 |
| . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | RTC | RTG | . . . | GYG | AWG | . . . | . . . | . . . | . . . | . . . | 3 | 8491 8483 |
| . . . | ATW | . . . | . . . | . . . | . . . | . . . | . . . | RTC | RTG | . . . | GYG | AWG | . . . | . . . | . . . | . . . | . . . | 1 | 10,314 |
| . . . | . . . | . . . | . . . | . . . | ATY | . . . | . . . | . . . | . . . | . . . | . . . | AWG | . . . | . . . | . . . | . . . | . . . | 1 | 10,310 |
| . . . | . . . | . . . | . . . | . . . | ATY | . . . | . . . | RTC | RTG | . . . | GYG | AWG | . . . | . . . | . . . | . . . | . . . | 1 | 8816 |
| . . . | . . . | . . . | . . . | . . . | ATY | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | 3 | 8816 8727 10314 |
| . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | RTC | . . . | . . . | . . . | AWG | . . . | . . . | . . . | . . . | . . . | 2 | 10,188 10,331 |
| . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | YTG | . . . | . . . | . . . | . . . | 1 | 9329 |
| . . . | ATW | RAA | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | 1 | 9329 |
| . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | . . . | CTG | . . . | . . . | . . . | . . . | 1 | 9329 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stankiewicz-Kosyl, M.; Haliniarz, M.; Wrochna, M.; Obrępalska-Stęplowska, A.; Kuc, P.; Łukasz, J.; Wińska-Krysiak, M.; Wrzesińska-Krupa, B.; Puła, J.; Podsiadło, C.; et al. Occurrence and Mechanism of Papaver rhoeas ALS Inhibitors Resistance in Poland. Agriculture 2023, 13, 82. https://doi.org/10.3390/agriculture13010082
Stankiewicz-Kosyl M, Haliniarz M, Wrochna M, Obrępalska-Stęplowska A, Kuc P, Łukasz J, Wińska-Krysiak M, Wrzesińska-Krupa B, Puła J, Podsiadło C, et al. Occurrence and Mechanism of Papaver rhoeas ALS Inhibitors Resistance in Poland. Agriculture. 2023; 13(1):82. https://doi.org/10.3390/agriculture13010082
Chicago/Turabian StyleStankiewicz-Kosyl, Marta, Małgorzata Haliniarz, Mariola Wrochna, Aleksandra Obrępalska-Stęplowska, Piotr Kuc, Justyna Łukasz, Marzena Wińska-Krysiak, Barbara Wrzesińska-Krupa, Joanna Puła, Cezary Podsiadło, and et al. 2023. "Occurrence and Mechanism of Papaver rhoeas ALS Inhibitors Resistance in Poland" Agriculture 13, no. 1: 82. https://doi.org/10.3390/agriculture13010082
APA StyleStankiewicz-Kosyl, M., Haliniarz, M., Wrochna, M., Obrępalska-Stęplowska, A., Kuc, P., Łukasz, J., Wińska-Krysiak, M., Wrzesińska-Krupa, B., Puła, J., Podsiadło, C., Domaradzki, K., Piekarczyk, M., Bednarczyk, M., & Marcinkowska, K. (2023). Occurrence and Mechanism of Papaver rhoeas ALS Inhibitors Resistance in Poland. Agriculture, 13(1), 82. https://doi.org/10.3390/agriculture13010082









