Abstract
Low temperature is the most common abiotic stress factor in peanut cultivation. Chitooligosaccharide (COS) plays an important role in the low-temperature resistance in plants, however, the role of COS in regulating the cold tolerance in peanuts is not clear. This research investigated the effects of exogenous COS on peanut seedlings in response to low temperatures. The results showed that exogenous COS can significantly alleviate the cooling symptoms of seedlings by reducing the content of malondialdehyde (MDA) and reactive oxygen species (ROS) under simulated low-temperature conditions (8 °C). These reductions may be related to the elevation of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and polyphenoloxidase (PPO) activities and the increased contents of osmotic substances such as soluble sugars (SS), soluble protein (SP), proline (Pro), and total phenols (TP) compared with those in untreated seedlings. Meanwhile, the contents of chlorophyll a and b in a peanut leaf also increased, as well as the net photosynthetic rate (Pn), resulted in an increased plant height, a heavier fresh weight, and an increased dry weight. Notably, the treatment of 100 mg·L−1 COS had maximum gain effects compared with those of other treatments. In summary, this study revealed the usage of COS for chilling stress alleviation, and 100 mg L−1 foliar spraying is recommended.
1. Introduction
Climate change in the natural environment is becoming an influencing factor affecting plant growth and agricultural production [1]. In the context of global warming, extreme weather is no longer a low-probability event, but it is becoming normal [2]. The frequent occurrence of low temperatures, high temperatures, droughts, heavy rainfall, and other disasters around the world greatly endanger agricultural production [3]. Seed germination is a crucial period in the plant life cycle, which is influenced by a variety of external factors. Temperature is essential for seed germination, which not only determines the germination speed, but it also determines whether seeds can germinate [4,5]. Low temperatures will restrict the seed germination process, forming one of the main environmental factors affecting the growth and development, yield, quality, and geographical distribution of plants [6,7,8].
Low temperatures can cause certain stress and damage to plants, which are reflected in many aspects, such as slow growth, wrinkled leaves, cell membrane damage, the reduction of photosynthesis, and so on. The damage of the cell membrane can be characterized by the change in the malondialdehyde content and the sudden increase in the membrane lipid peroxide content, which can also be used as the basis for the identification of plant cold resistance [9]. The plant response to cold stress is a complex process involving multiple metabolic pathways such as membrane fluidity, changes in the antioxidant system enzyme activity and osmoregulatory material content, and even gene regulation [10,11,12,13]. Plants usually activate their internal antioxidant defense mechanisms to protect themselves from cold damage [14,15] and initiate a series of physiological and biochemical reactions, including changes in the content of osmotic substances, mainly the organic small molecules, such as soluble sugars (SS), soluble proteins (SP), proline (Pro), and total phenols (TP), etc., [16], and changes in the antioxidant system, mainly the activities of some protease classes substances, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and polyphenoloxidase (PPO), etc. [9]. However, when the plants still cannot resist low-temperature stress through their own regulation mechanisms, the plant’s growth will be restricted, the senescence process will worsen, and it may even wither and die. In order to improve the cold tolerance of plants, scholars have carried out many studies such as [17], ones using glutathione [18] and DA-6 [19], and [20] to improve the low-temperature tolerances of Nicotiana tabacum L., Zea mays L., Gossypium spp., Amygdalus persica L., and other plants. However, some processing methods are more expensive during the production, and a certain number of toxic residues are used. Their long-term use is not in line with the concept of green production, and it is urgent to develop safer and more effective technologies to improve the cold resistance of plants.
Chitososaccharide (COS) is an environmentally friendly inducer, which has an important role in plant stress resistance [21]. As one of the plant growth regulators, COS has an important role in promoting the seed germination and early growth, enhancing the defense against stress of Nitraria tangutorum [22], Solanum lycopersicum [23], Capsicum annuum [24], Keteleeria evelyniana [25], and Liriodendron hybrid [26]. COS promotes plants to produce allergic reactions and systemic acquired resistance and other defense reactions, induce the expression of anti-stress genes, promote the synthesis of related proteins and nucleic acids, activate the plants’ autoimmune function, and enhance the plants’ resistance to stress [27,28], and increase the content of osmotic material, change the cell plasma membrane permeability, reduce membranous peroxidation, and alleviate the stress toxicity [22], moreover, it can also maintain the leaf high water content and chlorophyll content, enhance the photosynthetic rate, and the nutrient transport rate, and provide more substances and energy [29,30] for the plant to resist adverse stresses.
Peanuts (Arachis hypogaea Linn.) are the most widely grown leguminous oil crop all over the world. In the majority of the peanut-producing areas, the phenomenon of low temperature affecting early sowing spring peanut germination is very common. In northern China, the spring peanut is sown in the middle of April and late April, and a large area of low-temperature rot appears, resulting in a serious production reduction in peanuts [8]. Additionally, in southern China, continuously low temperatures and rainy weather often occur after spring peanut sowing, which leads to peanut rot during early sowing, resulting in adverse consequences such as uneven emergences and a prolonged emergence time, which seriously threatens peanut production [31]. It is of great significance to study the effects of low temperatures on the growth and development of peanut during germination, find out the methods to help peanuts to resist low temperatures, and take measures to improve the cold resistance of peanut production. Currently, a few studies have applied spray shell oligosaccharides to improve the low-temperature resistance in peanut seedlings.
To investigate the functions of exogenous COS on the cold tolerance in peanuts, the determining growth indicators, ROS levels, antioxidant capacity, and the content of cold stress-related osmotic substances were researched. These results preliminarily revealed the mechanism of peanuts to reduce the cold damage under a COS treatment, and it will further provide a reference for improving the cold tolerance of oil crops in production facilities.
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials
The peanut variety ‘Yuhua 22’ was selected in this study. The seeds of peanuts, which were collected from Henan Modern Agriculture research and development base (35.0053° N, 113.7046° E) on September 2019 in Wangcun, Yuanyang County, Xinxiang City, Henan Province of China, are stable strains that were provided by Henan Crop Molecular Breeding Research Institute. Seed germination substrates were sampled from 0–30 cm of soil layers grown from natural communities.
2.1.2. Experimental Agent
Chitooligosaccharide (COS) was purchased from Shanghai Qiming Biotechnology Co., Ltd. (http://m.shkambio.com/, accessed on 1 June 2020), with a relative molecular weight of less than 3000 Da. Additionally, it is an emerging plant growth regulator with the advantages of being safe, efficient, non-toxic, and soluble in water.
2.2. Experimental Design
2.2.1. Simulated Low-Temperature Growth Conditions
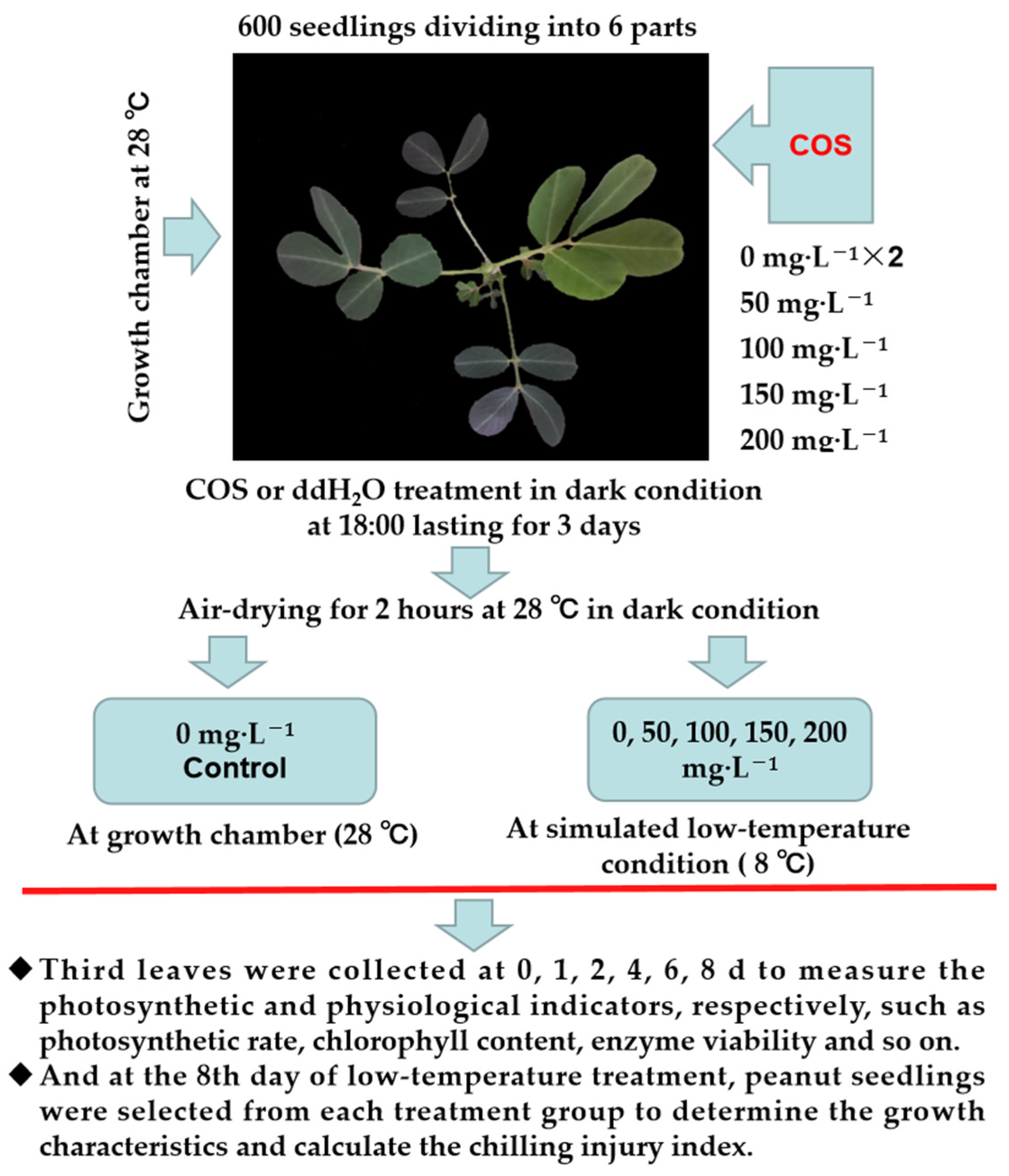
In June 2020, the low-temperature treatment (8 °C) was conducted in a low-temperature-simulated growth room in the College of Agriculture/Peony College, Henan University of Science and Technology, Luoyang (34.6027° N, 112.4149° E), Henan Province, China. A schematic diagram of the simulated low-temperature experimental design is shown in Figure 1. Six hundred peanut seedlings were divided into six aliquots and grown at a temperature of 28 °C and 16 h/8 h (light/dark) until the fourth leaf was fully unfolded. Of them, four seedlings were sprayed with 50, 100, 150, and 200 mg L−1 COS for 3 consecutive days each time until the solution flowed from the leaves. Moreover, the control and 0 mg L−1 COS groups, were sprayed with double-distilled water (ddH2O). After spraying the peanut seedlings with COS or ddH2O, they were air dried for 2 h in the dark. Then, the COS-treated groups (0, 50, 100, 150, and 200 mg·L−1) were subjected to 8 °C, and the control group was subjected to 28 °C. The third leaves down from the top of the seedlings were collected at 0, 2, 4, 6, and 8 days, respectively, and they were used to determine the contents of malondialdehyde (MDA), hydrogen peroxide (H2O2), superoxide anion (O2−), soluble sugars (SS), soluble protein (SP), proline (Pro), total polyphenols (TP), and the activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and polyphenol oxidase (PPO). At the end of the cold injury stress (8 DAT), the growth characteristics were measured, and the cold damage index was calculated to evaluated the degree of injury [32].
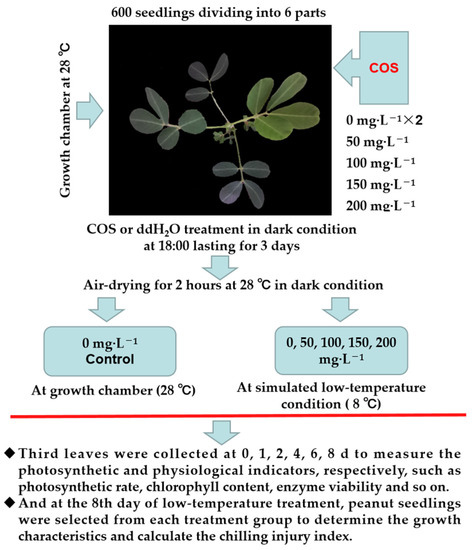
Figure 1.
Schematic representation of the simulated cryogenic experimental design.
2.2.2. Growth Characteristics
On the 8th day of the low-temperature treatment, 5 peanut seedlings were selected from each treatment group to determine the growth characteristics such as the plant height, main root length, fresh weights, and the aboveground and belowground biomasses. The roots of the selected plants were washed with distilled water to remove the soil particles and divided from the stem, and the plant height and root length were measured using a ruler (precision of 0.1 cm). Then, the aboveground part and belowground part of the 5 seedlings were wrapped in 105 °C for 30 min, and then dried to constant weight at 65 °C. The weights were measured using an analytical balance (precision of 0.0001 g), and the results were averaged for the 5 seedlings. Additionally, 15 peanut seedlings were labeled for each treatment to investigate the cold hazard level and to calculate the chilling injury index. The classification standard of the cold damage degree is slightly modified according to Wu et al. [32] as follows, level 0: asymptomatic; level 1: basic normal growth and the new leaves are slightly wrinkled and wilting; level 2: 1/3 leaves are wilting; level 3: 2/3 leaves are wilting; level 4: all of the leaves wilting and deforming; level 5: the whole plant is wilting/dead.
2.2.3. Determination of the MDA and ROS Levels
The MDA level was measured using a malondialdehyde (MDA) content kit according to the manufacturer’s instructions (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China). The concentrations of H2O2 and O2− and the rate of O2− production in the fresh leaves were determined by using a detection kit (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China). Three replicates were set up for each sample.
2.2.4. Determination of Net Photosynthetic Rate and Chlorophyll Levels
The third leaves from the top were selected to determine the net photosynthetic rate at 09:00–11:00, which were measured using a Li-6400 portable photosynthetic meter (Li-6400, LI-COR, Lincoln, NE, USA). For the chlorophyll content determination, 0.1 g of the leaves was placed into centrifuge tubes containing 5 mL of 95% alcohol and incubated at room temperature for 48 h in the dark. The absorbance of the chlorophyll extract was measured using a spectrophotometer (UV-5300, Shimadzu, Kyoto, Japan) at 665 and 649 nm against 95% alcohol. Chlorophyll a, chlorophyll b, and the total chlorophyll content were calculated according to the formula of Zou [33]. Three replicates were set up for each sample.
2.2.5. Determination of Antioxidant Enzymatic Activities
The fresh leaf samples (0.1 g) were ground into 1 mL extracting solution from the detection kit (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China) using a chilled mortar. After centrifugation (8000× g, 10 min) at 4 °C, the supernatant of the enzyme extract was obtained. Next, the SOD, POD, CAT, and PPO activities were determined as described in the manufacturer’s instructions of the detection kit. The analyses of each sample were conducted in triplicate.
2.2.6. Determination of Osmotic Substance Levels
The proline content was determined according to the manufacturer’s instructions (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China). Briefly, 0.1 g of fresh leaves was placed into tubes filled with 1 mL of the extract, then, a 95 °C water bath for 10 min, and then they were centrifuged at 10,000× g (25 °C, 10 min), Additionally, after cooling, the supernatant was used for testing.
2.3. Statistical Analysis
SPSS 22.0 (SPSS Inc., Chicago, IL, USA) was used for analysis of the statistical significance for all of the data, including the LSD method for multiple comparisons and Duncan’s method for the difference significance test (α = 0.05), and the measurement results are expressed as mean ± standard deviation. Photoshop 7.0 (Adobe Systems Inc., San Jose, CA, USA) and Origin 9.1 (OriginLab Software Inc., Northampton, MA, USA) were used to process all of the images.
3. Results
3.1. Changes in Phenotypes of Peanut Affected by Chitooligosaccharide under Simulated Low-Temperature Conditions
Cold stress (8 °C) induced severe injuries in the seedlings, manifesting as leaf chlorosis and wilting. Compared to the control conditions used at 28 °C, the targets such as the plant height, main root length, fresh weight, shoot and lower ground dry weight decreased by 34.42%, 38.44%, 57.03%, 51.64%, and 51.85%, respectively (Table 1), while these injuries were significantly alleviated after the treatment with exogenous chitooligosaccharides, especially at a concentration of 100 mg·L−1. Additionally, at this concentration (100 mg·L−1), the plant height, main root length, fresh weight, shoot and lower ground dry weight increased by 23.12%, 51.20%, 121.51%, 98.31%, and 84.62% compared with those in the only cold stress treatment conditions at 8 °C (0 mg·L−1), respectively.

Table 1.
Changes in the growth characteristics of peanut seedlings with exogenous chitooligosaccharide under simulated low-temperature treatment.
3.2. Effects of Chitooligosaccharide on Lipid Peroxidation Contents of Peanut Seedlings under Simulated Low-Temperature Conditions
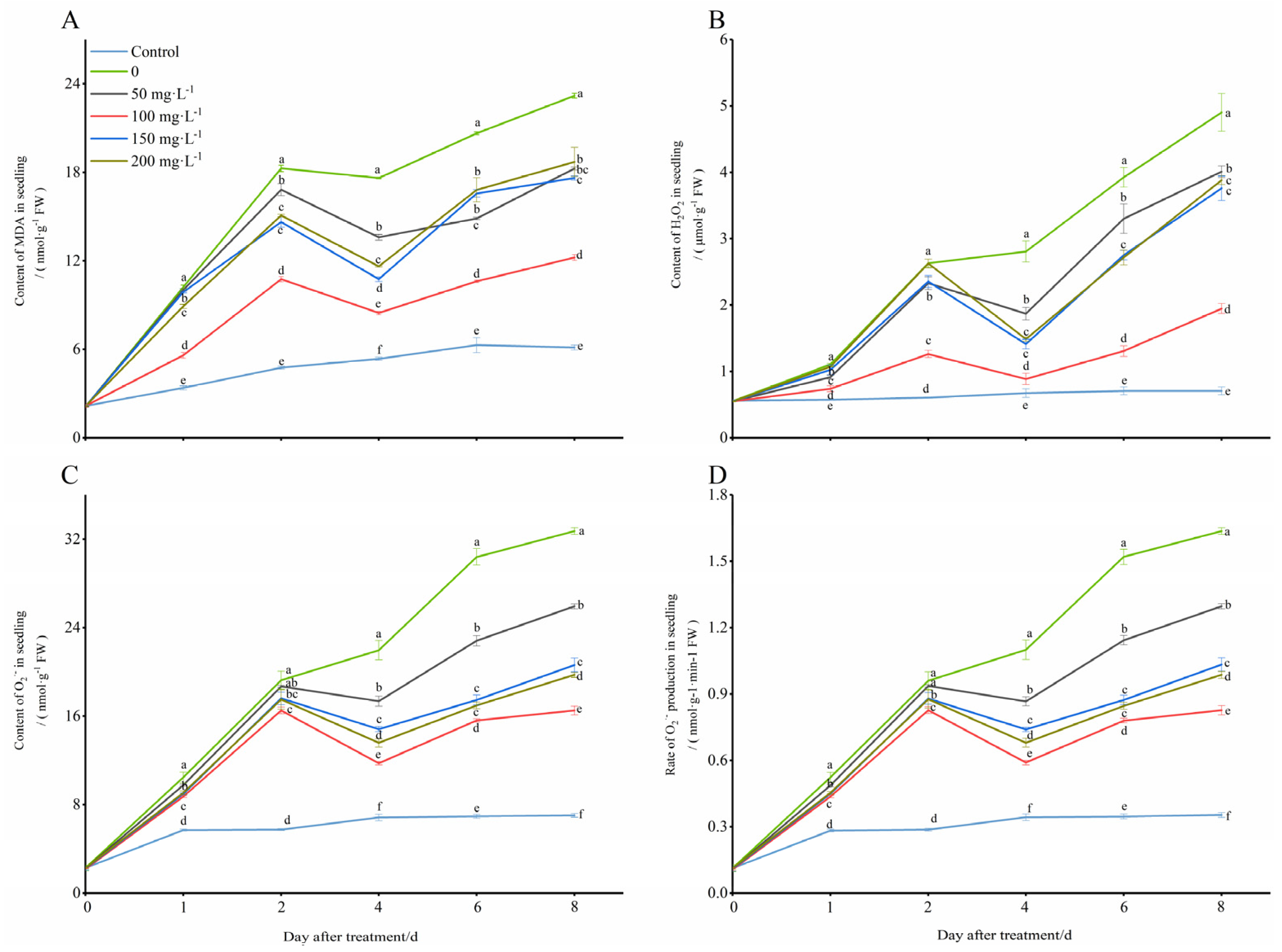
The levels of MDA in the treated seedling leaves were significantly higher than those in the control group (28 °C), and they showed a trend of increasing first, decreasing later, and then increasing again with prolonged exposure to the low temperature (8 °C) (Figure 2A). After the application of exogenous COS, the trend of the MDA content in the seedling leaves remained unchanged, however, the content was significantly decreased, especially in the treatment conditions with a chitooligosaccharides concentration of 100 mg·L−1, which was closest to that of the control compared to those of other concentrations used in the test. At days 1, 2, 4, 6, and 8 of cold stress induction, the MDA contents treated with 100 mg·L−1 chitooligosaccharides were 45.31%, 41.11%, 51.95%, 54.59%, and 47.27% less than those that were only treated with the low temperature (0 mg·L−1), respectively. Next, all of the contents of H2O2, O2−, and the rate of O2− production in the seedlings were increased with prolonged exposure to the cold environment, but the chitooligosaccharides all showed a trend of increasing first, then decreasing, and then increasing again, and this most significantly decreased the contents of H2O2, O2− and the rate of O2− production, especially in the treatment conditions with a chitooligosaccharides concentration of 100 mg·L−1 (Figure 2B–D). Additionally, at days 1, 2, 4, 6, and 8 of cold stress induction, the H2O2 contents decreased by 33.13%, 51.97%, 68.41%, 66.69%, and 60.30%, the O2− contents decreased by 16.57%, 14.19%, 46.51%, 48.61%, and 49.55%, and the rates of O2− production decreased by 16.55%, 13.89%, 46.36%, 48.68%, and 49.49%, respectively. These results indicated that exogenous 100 mg·L−1 oligosaccharides alleviated the ROS formation caused by cold stress and contributed to cold tolerance in the peanut seedlings (Figure 2).
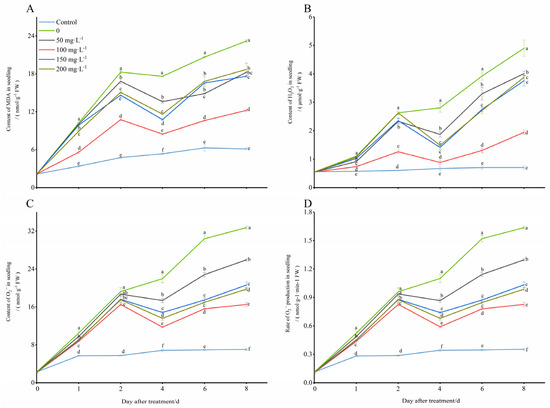
Figure 2.
Changes in lipid peroxidation contents of peanut under low-temperature conditions (8 °C). On each subgraph, different letters in the same column indicate significant differences in the p < 0.05 level. Data in the figure are presented as the mean ± standard deviation (n = 3).
3.3. Effects of Chitooligosaccharides on the Photosynthetic efficiency of Peanut Seedlings under Simulated Low-Temperature Treatment
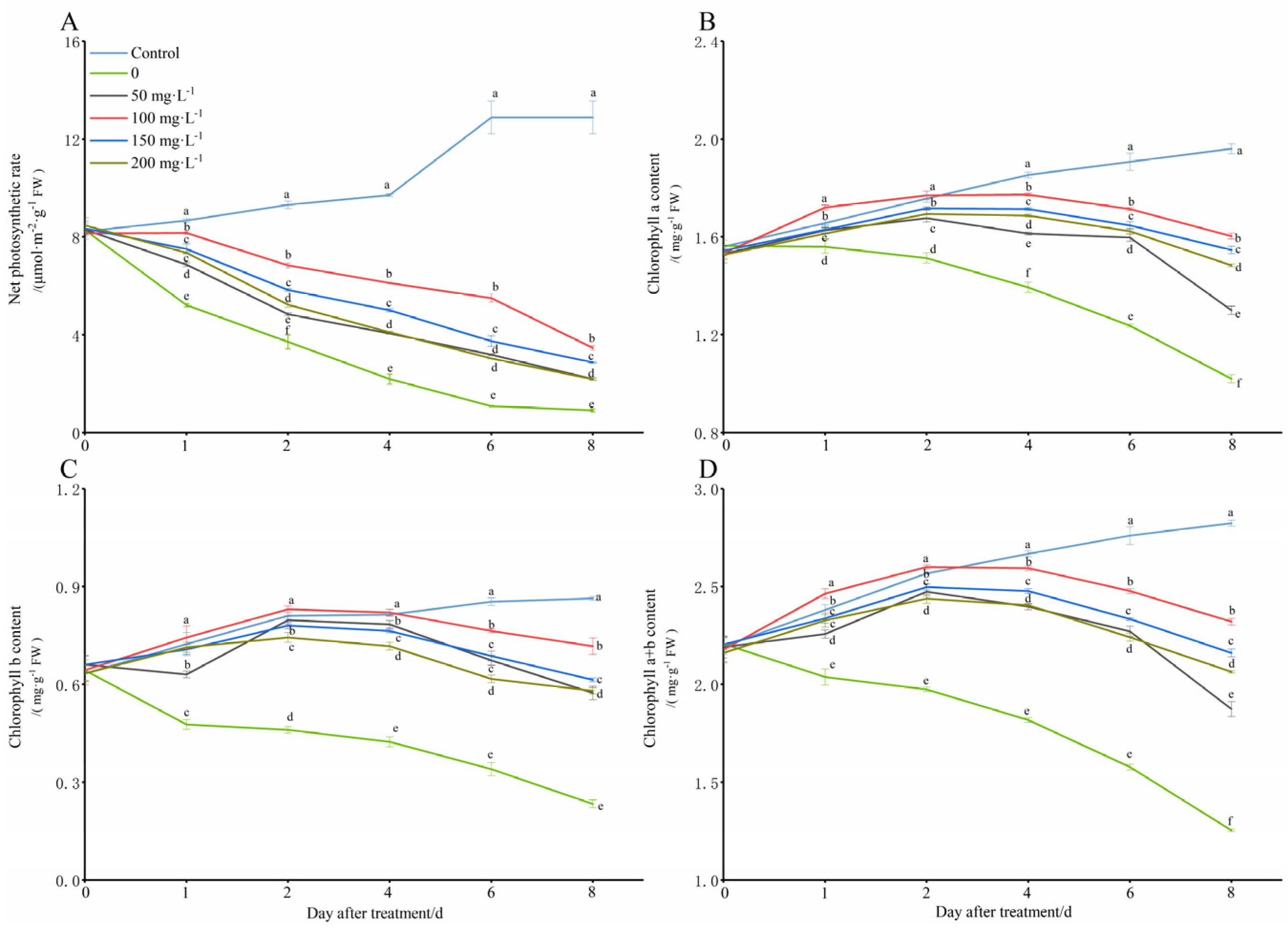
In this study, the net leaf photosynthesis rate and the content of chlorophyll a and b in the peanut seedlings gradually increased under normal growth conditions (28 °C) (Figure 3). However, under the simulated low-temperature conditions (8 °C), the net photosynthetic rate, and the chlorophyll a, chlorophyll b, and total chlorophyll contents were significantly reduced with the extension of the low-temperature stress time, especially by the 8th day, and they were decreased by 92.99%, 47.96%, 72.98%, and 55.61%, respectively, as compared with those of the control. However, the chitooligosaccharides significantly increased the net leaf photosynthesis rate and all of the chlorophyll a, chlorophyll b, and total chlorophyll contents compared with those of the cold stress group alone (0 mg·L−1), especially in the treatment conditions with a chitooligosaccharides concentration of 100 mg·L−1, and on the days 1, 2, 4, 6, and 8 of the cold stress induction, the net photosynthetic rate increased by 56.72%, 84.19%, 179.72%, 408.64%, and 282.67%, the chlorophyll a content increased by 10.26%, 16.96%, 27.27%, 38.54%, and 57.19%, the chlorophyll b content increased by 55.93%, 80.43%, 93.72%, 124.50%, and 207.20%, and the total chlorophyll content increased by 20.95%, 31.76%, 42.75%, 57.08%, and 85.11%, respectively.
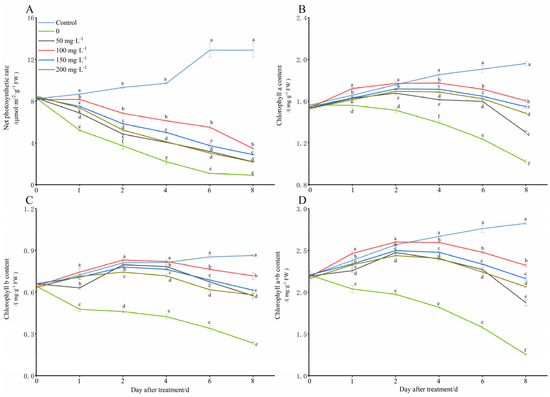
Figure 3.
Change in net photosynthesis rate and chlorophyll a, chlorophyll b and total chlorophyll content with exogenous chitooligosaccharides under low-temperature conditions (8 °C). On each subgraph, different letters in the same column indicate significant differences in the p < 0.05 level. Data in the figure are presented as the mean ± standard deviation (n = 3).
3.4. Effects of Chitooligosaccharide on Antioxidant-Related Enzyme Activities of Peanut Seedlings under Simulated Low-Temperature Conditions
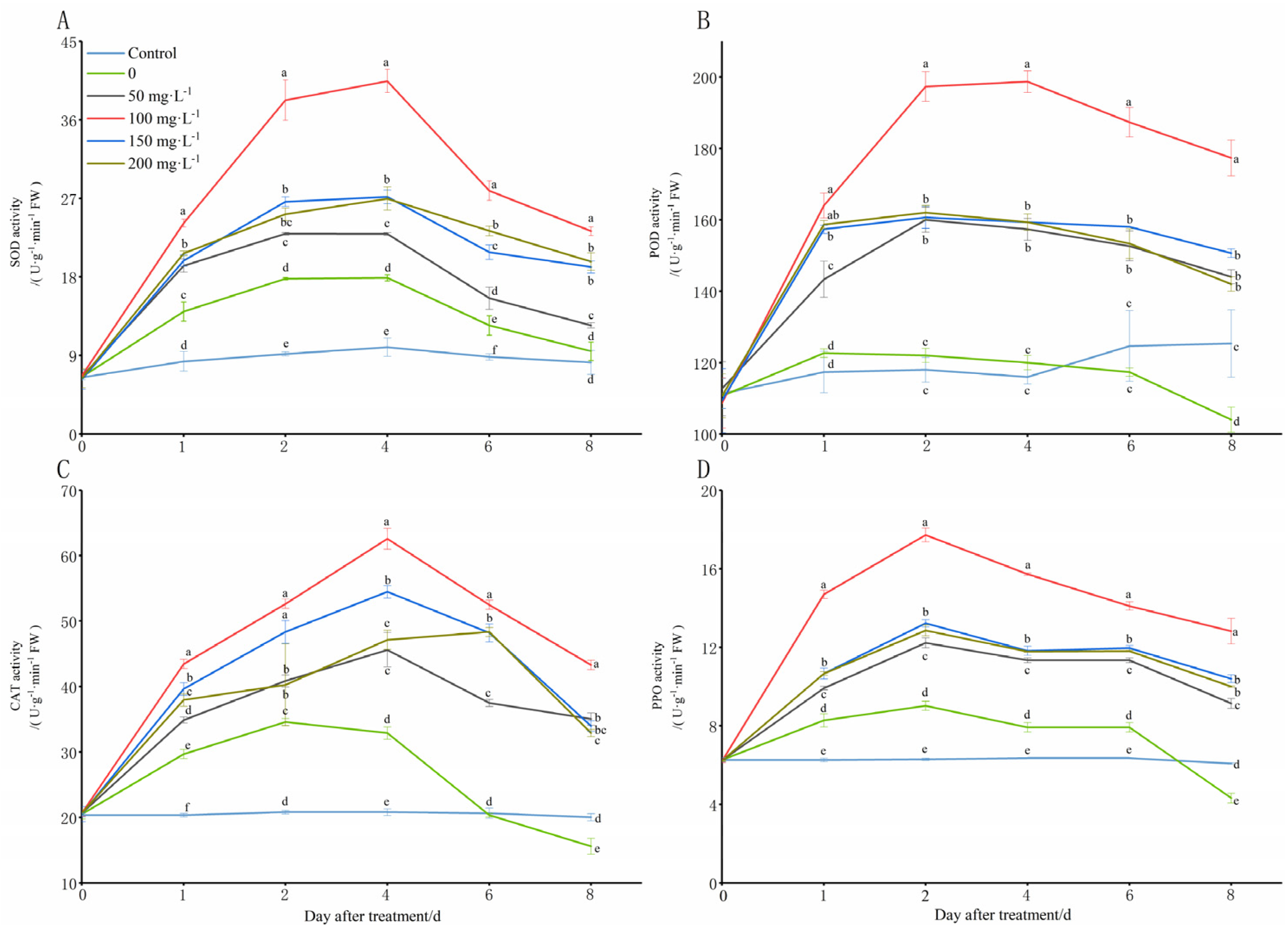
The low temperatures (8 °C) stimulated a rapid increase in the SOD, POD, CAT, and PPO activities at 1 DAT compared to those of the control (28 °C) (Figure 4). However, at 2 DAT, the SOD, CAT, and PPO activities increased slowly, and the POD activity even began to decrease, and it was significantly lower than that of the control after 6 DAT. From 4 DAT of the stress induction, the SOD, CAT, and PPO activities began to decrease rapidly, and the CAT and PPO activity levels were even severely lower than they were in the control after the sixth day of the stress. Under the cold treatments (8 °C) with various concentrations of chitooligosaccharide, the SOD, POD, CAT, and PPO activities exhibited an increase, which was followed by a decrease. Additionally, most of the SOD and POD activities increased rapidly in the first 2 DAT, and they changed slowly over the next two days, and decreased rapidly thereafter. The CAT activities in most of the treatment groups increased rapidly in the first 4 DAT of the cold stress, and then, they decreased rapidly, except for the group with a chitooligosaccharide concentration of 200 mg·L−1. Additionally, the PPO activities in most of the treatment groups increased rapidly in the first 2 DAT of the cold stress, and they declined sharply thereafter. After the treatment with different concentrations of exogenous chitooligosaccharide, their SOD, POD, CAT, and PPO activity levels were consistently higher than they were the control group, especially after the treatment with 100 mg·L−1, and the increase effect was significantly higher than it was in the other concentrations. When they were treated with chitooligosaccharide at 100 mg·L−1, the values of SOD and CAT activity reached a maximum on day 4 of the cold stress, and at this time, they were 67.25% and 44.01% higher than those of the group that was not treated with chitooligosaccharide (0 mg·L−1) at low temperatures (8 °C), respectively, and 7.14 and 3.03 times higher than those of the control group (28 °C) (Figure 4A,C). The value of PPO activity reached a maximum on day 2 of the cold stress, and at this time, it was 20.54% higher than that the group that was not treated with chitooligosaccharide at low temperatures (8 °C), and it was 1.21 times higher than that of the control group (Figure 4D). However, the PPO activity was slightly higher under the cold stress on day 4 than it was on day 2, and on days 2 and 4, it was 20.32% and 21.14% higher than that the group that was not treated with chitooligosaccharide (0 mg·L−1) at low temperatures (8 °C), respectively, and it was 1.82 and 1.83 times higher than that of the control group, respectively (Figure 4B).
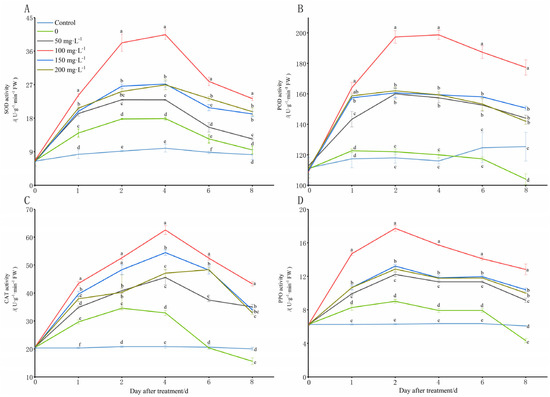
Figure 4.
Changes in lipid peroxidation contents of peanut seedlings under low-temperature conditions (8 °C). On each subgraph, different letters in the same column indicate significant differences in the p < 0.05 level. Data in the figure are presented as the mean ± standard deviation (n = 3).
3.5. Effects of Chitooligosaccharide on Osmotic Substance Contents of Peanut Seedlings under Simulated Low-Temperature Conditions
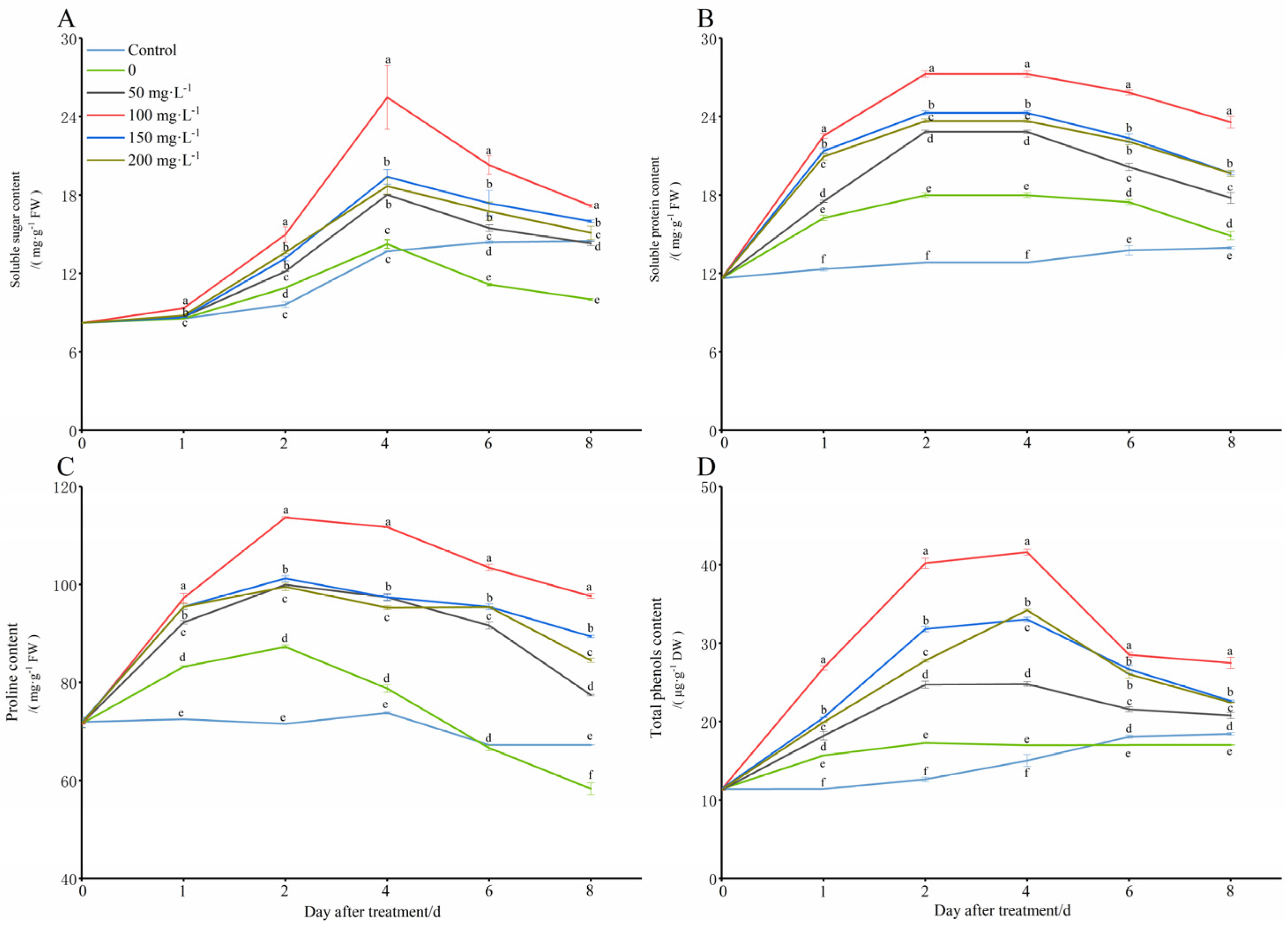
Under the simulated low-temperature conditions (8 °C), the plants would increase the content of soluble sugars (SS), soluble proteins (SP), proline (Pro), and total polyphenols (TP) in vivo in order to reduce the cold damage (Figure 5). However, the contents of soluble sugars, soluble proteins, proline, and total polyphenols began to decrease with the extension of the stress time, and they were even lower than those in the control group (28 °C). The application of different concentration treatments of exogenous chitooligosaccharide can greatly increase those indexes in the peanut seedling leaves, most of which were significantly higher than they were in the only cold treatment group (0 mg·L−1), and some of them were even significantly higher than those in the control group. The treatment group with 100 mg L−1 was significantly more improved than the other groups were, which means it had the best ability to increase the cold tolerance of the seedlings under cold stress.
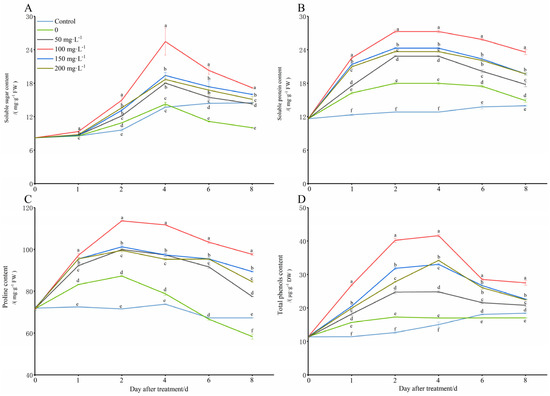
Figure 5.
Changes in osmotic substance contents of peanut seedlings under low-temperature conditions (8 °C). On each subgraph, different letters in the same column indicate significant differences in the p < 0.05 level. Data in the figure are presented as the mean ± standard deviation (n = 3).
4. Discussion
Peanuts originate from tropical regions, which are sensitive to temperature. When the temperature is lower than 10 °C for a long time, it can easily lead to peanut rot, slow emergence, weak seedling potential, and in serious cases, it will result in dead seedlings, and eventually, affect peanut production [34]. The cold damage index can directly reflect the degree of plant damage by low temperature, and it has been widely used in the determination of plant low-temperature resistances [35]. In this study, the low temperature (8 °C) led to limited peanut seedling growth, leaf death and wilting, and an increased cold damage index, which significantly decreased the plant height, primary root length, fresh weight, and the aboveground weight and underground dry weight compared with those of the peanut seedlings grown in a normal environment (28 °C) (Table 1). However, spraying them with chitorioligosaccharide significantly reduced the cold injury of the seedlings, and the reduction effect was better under the appropriate concentration of exogenous chitorioligosaccharide (100 mg L−1). This result is consistent with that of Zhao et al. [36], who also studied the process of chitooligosaccharide to alleviate salt stress during wheat seed germination and seedling growth, and they also found that the mitigation effect showed different trends with the increase in the chitooligosaccharide concentration, with 100 mg·L−1 leading to the best effect. Additionally, the improvement of the peanut growth with the addition of exogenous chitooligosaccharide may be associated with the increased chlorophyll production and photosynthesis rates under low-temperature stress [37,38]. In this research, exogenous chitooligosaccharide increased the contents of chlorophyll a and chlorophyll b in the leaves of peanut seedlings under cold stress, accordingly, promoting the net photosynthetic rate, and thus increasing the accumulation of the biomass. This is in agreement with the findings of Guo et al. [37] and Li et al. [38].
With the increasing scale of peanut production, more and more attention has been paid to the research of low-temperature resistance in recent years [39,40]. Under normal conditions, ROS are in dynamic equilibrium, and they remain at low levels without causing damage to the plant [41]. When the plants are subjected to stress and/or enter senescence, the balance of the ROS is disrupted, leading to an increase in ROS content, leading to membrane peroxidation, leading to increased MDA, hydrogen peroxide, and O2− levels [42,43]. The results of this experiment showed that the contents of MDA, H2O2, and O2− in the peanut seedlings in normal habitats were at low levels, and they increased sharply with the cold stress, which is consistent with the results of Yu, Y. [44] and Chang, W. [45]. Spraying them with exogenous chitooligosaccharide can significantly reduce the contents of MDA, H2O2, and O2− in the peanut leaves and effectively inhibit membrane lipid peroxidation, thus reducing the destruction of the cell membrane structure by low temperature and reducing the seedling damage. The study found that the content was lowest when 100 mg·L−1 chitooligosaccharide was applied under the low-temperature stress (8 °C) (Figure 2), which had the best effect on protecting peanut seedlings.
The resistance of plants to reactive oxygen stress is closely related to the scavenging ability of reactive oxygen species, and the seeds initiate their own metabolic defense mechanisms to regulate adaptation when they encounter low-temperature stress. The antioxidant enzymes of SOD, POD, CAT, and PPO are the main reactive oxygen species scavengers in the plant’s body. Their coordination can maintain the reactive oxygen species at a normal level, so as to prevent the plants from being harmed. Wang et al. [34] found that the activities of SOD, CAT, and POD in the peanut seedlings treated with low temperature showed different changes. The SOD and CAT activities of the peanut seedlings were higher than those in the normal temperature growth treatment, and the overall POD activity showed a trend of first decreasing, then rising, and then decreasing. Similar changes in the activities were also observed in the study by Dong et al. [46]. In this study, the low-temperature stress activated the antioxidant protective enzyme system in the peanuts, and the activities of SOD, POD, CAT, and PPO were enhanced to alleviate the damage caused by the stress, which showed a trend of increasing first, and then decreasing, and the research results were similar to the previous results. Exogenous chitooligosaccharide significantly enhanced the activities of SOD, POD, CAT, and PPO, and they slowed the decrease in the related enzyme activity under cold stress (Figure 4). Furthermore, the damage to the peanuts under low-temperature stress was alleviated to some extent. Additionally, the result was best with chitooligosaccharide at 100 mg L−1.
In the face of adversity environment at low temperatures, the content of osmotic substances in plants will also change significantly. Wang et al. [34] found that proline accumulates in peanuts in low-temperature conditions. At the same time, the content of soluble protein is significantly increased, which may be due to the expression of induced proteins in peanut in low-temperature condition or the release of soluble protein from the membrane or other bound forms, producing an emergency response to resist the impact of the bad external environment. Similar results were obtained in the study by Li et al. [47]. Osmoregulatory substances act synergistically to protect the structural integrity of the cell membrane, thereby alleviating or resisting adversity injuries [48]. Under a certain degree of cold stress, peanut seedlings resist stress by increasing the contents of soluble sugar (SS), soluble protein (SP), Proline (Pro), and total polyphenols (TP). Under a certain degree of low-temperature stress, peanut seedlings increase the content of SS, SP, Pro, and TP in the leaves to resist the stress caused by cold damage. However, with the aggravation of the stress degree, plant self-regulation alone has been unable to resist the cold damage stress, perhaps causing irreparable losses, and so the contents of SS, SP, Pro, and TP in the seedling leaves were gradually decreased (Figure 5). We can effectively improve the peanut seedling leaves’ SS, SP, Pro, and TP contents by spraying them with exogenous chitooligosaccharide. It has been investigated that the cold tolerance of peanut seedlings was improved in the intracellular environment in a stable state, and protoplasm dehydration during cold stress was avoided because the leaf cells accumulated more osmotic substances, reducing the cell penetration, and increasing the cell water absorption capacity to maintain the turgor pressure [49]. Our results showed that the contents of SS, SP, and Pro of the peanut seedlings treated with 100 mg L−1 chitooligosaccharide were significantly higher than those of the other concentration groups. These findings indicated that 100 mg L−1 chitooligosaccharide was most beneficial to improve the ability of the seedlings to resist cold stress.
5. Conclusions
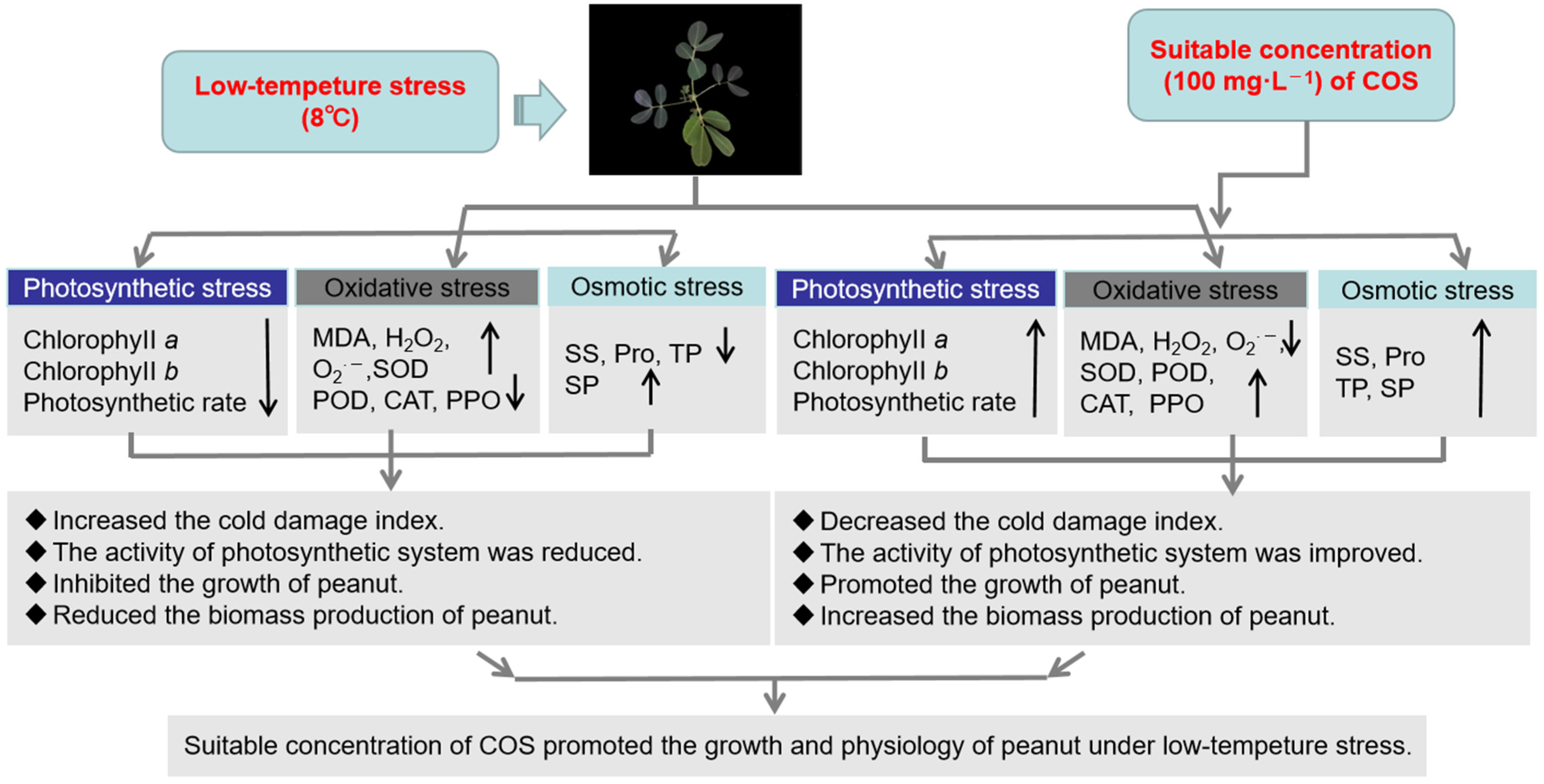
In our research, the cold resistance of peanuts under low-temperature stress (8 °C) was investigated. We found that the appropriate concentration of COS could effectively alleviate the symptoms of cold damage. The MDA, H2O2, and O2− contents increase was inhibited, and SOD, POD, CAT, and PPO activities were induced. Additionally, this increased the accumulation of the osmoregulatory substances SS, SP, Pro, and TP, thus reducing the oxidative damage caused by cold stress. At the same time, the contents of chlorophyll a and chlorophyll b in the plant leaves also increased, as well as the plant net photosynthetic rate. Further, the plants have an increased plant height, they are heavier, and they have increased fresh weights and dry weights. Overall, the 100 mg·L−1 chitooligosaccharide content had maximum gain effects (Figure 6) compared to those which occurred in the other treatments, which will provide an important reference for the future research of peanuts under low-temperature stress.
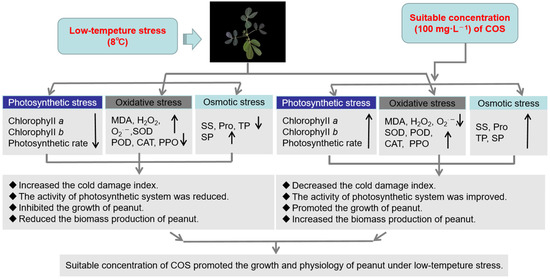
Figure 6.
Summary changes of physiological characteristics in peanut at low temperature (8 °C).
Author Contributions
X.S., Z.S., X.X. and X.Z. conceived the study and drafted the manuscript. X.S., Y.Z., S.H., Y.Y. and R.Z. discussed the writing plan X.S., R.Z., Y.W., H.X. and M.Z. performed the experiments. X.S., Z.S., X.X., Y.Z. and X.Z. obtained the experimental materials. X.S. and H.X. analyzed the data. All authors have read, reviewed, and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Key Research Project of the Shennong Laboratory (SN01-2022-03), China Agriculture Research System (CARS-13), Henan Provincial Agriculture Research System, China (S2012-5), Major Science and Technology Projects of Henan Province (201300111000), PhD Start-up Fund of Henan University of Science and Technology (13480079) and Henan Provincial R&D Projects of Inter-regional Cooperation for Local Scientific and Technological Development Guided by Central Government (YDZX20214100004191).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Huang, C.; Sun, Y.; Yang, W.; Zhang, S. Effect of spring low temperature and frost damage to the growth and yield of maize. J. Agric. Catastrophol. 2013, 3, 53–56. [Google Scholar]
- Ma, Z.; Hao, W.; Zheng, X.; Wang, L.; Xi, Y.; Hou, J. Effect of low temperature and freezing injury on whole growth period of sweet waxy corn in Wuzhai County of Shanxi Province and defensive measures. Agric. Eng. 2022, 12, 139–143. [Google Scholar]
- Xu, Y. Effect of low temperature and late freezing disaster on walnut growth in Chengxian county. J. Agric. Catastrophol. 2012, 2, 44–46. [Google Scholar]
- Mei, Y.Q.; Song, S.Q. Response to temperature stress of reactive oxygen species scavenging enzymes in the crosstolerance of barley seed germination. Zhejiang Univ. Sci. Biomed. 2010, 11, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jian, C.; Zhang, T.; Ma, C.; Shan, S. Effect of temperature on seed vigor of different peanut varieties. J. Peanut 2012, 41, 21–25. [Google Scholar]
- Pearce, R.S. Plant freezing and damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, B.; Zheng, Y.; Sha, J.; Li, A.; Sun, X. Effects of temperature on peanut emergence, seedling growth and flowering. J. Peanut 2003, 32, 7–11. [Google Scholar]
- Tang, Y.; Wang, C.; Gao, H.; Feng, T.; Zhang, J.; Yu, S. Low temperature resistance and its correlation with quality traits during peanut seed imbibition. J. Nucl. Agric. 2011, 25, 436–442. [Google Scholar]
- Pan, R. Plant Physiology; Higher Education Press: Beijing, China, 2008. [Google Scholar]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Yan, W.; Li, J.; Wang, J.; Li, Z. Exogenous H2O2 improves the chilling tolerance of manilagrass and mascarenegrass by activating the antioxidative system. Plant Growth Regul. 2010, 61, 195–204. [Google Scholar]
- Aghdam, M.S.; Moradi, M.; Razavi, F.; Rabiei, V. Exogenous phenylalanine application promotes chilling tolerance in tomato fruits during cold storage by ensuring supply of NADPH for activation of ROS scavenging systems. Sci. Hortic. 2019, 246, 818–825. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Sharafi, Y.; Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Razavi, F.; Fard, J.R.; Farmani, B. Melatonin treatment promotes endogenous melatonin accumulation and triggers gaba shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 2019, 254, 222–227. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.L.; Hou, J.; Luo, R.R.; Wang, R.D.; Hu, J.B.; Huang, S. Advances on mechanism of cucurbit crops in response to lowtemperature stress. Acta Hortic. Sin. 2022, 49, 1382–1394. [Google Scholar]
- Mohammadrezakhani, S.; Rezanejad, F.; Hajilou, J. Effect of putrescine and proline on proflies of GABA, antioxidant activities in leaves of three Citrus species in response to low temperature stress. J. Plant Biochem. Biotechnol. 2021, 30, 545–553. [Google Scholar] [CrossRef]
- Pan, M.; Yin, Y.; Shen, F.; Luo, B.; Tang, X.; He, H.; Chen, D. Effect of calcalate ate on physiological indicators of tobacco seedlings under cold stress. J. Southwest Agric. 2016, 29, 288–293. [Google Scholar]
- Li, L. Mitigation effect of exogenous glutathione on maize seedlings under cold stress. Master’s Thesis, Heilongjiang University, Harbin, China, 2022. [Google Scholar]
- Xu, M. Effect of DA-6 on seed germination and photosynthetic characteristics of cotton seedlings. Master’s Thesis, Shihezi University, Shihezi, China, 2021. [Google Scholar]
- Cao, S.; Song, C.; Shao, J.; Bian, K.; Chen, W.; Yang, Z. Exogenous melatonin treatment increases chilling tolerance andinduces defense response in harvested peach fruit during cold storage. J. Agric. Food Chem. 2016, 64, 5215–5222. [Google Scholar] [CrossRef]
- He, Y.Q.; Bose, S.K.; Wang, M.Y.; Liu, T.; Wang, W.; Lu, H.; Yin, H. Effects of chitosan oligosaccharides postharvest treatment on the quality and ripening related gene expression of cultivated strawberry fruits. J. Berry Res. 2019, 9, 11–25. [Google Scholar] [CrossRef]
- Shi, X.; Yang, Y.; Hou, X.; Xue, X.; Li, M.; Duan, M.; Huo, J.; Zen, F. Effect of exogenous chitooligosaccharides on drought resistance of Tanggut white thorn. Jiangsu Agric. Sci. 2020, 48, 172–177. [Google Scholar]
- Gu, L. Effect of chitooligosaccharides on tomato seed germination. J. Southwest Agric. 2014, 27, 1233–1236. [Google Scholar]
- Lu, J.; Sun, D.; Zhang, C.; Qi, S.; Li, H. Effect of chitooligosaccharides on pepper seed germination and seedling antioxidant enzyme activity. Wild Plant Resour. China 2012, 31, 12–16. [Google Scholar]
- Jiang, R.; Li, L.; Li, Y.; Wang, S.; Liu, C.; Huang, X.; He, J. Effect of IAA, GA3 and chitooligosaccharide species on germination of Yunnan seeds. West. For. Sci. 2021, 50, 110–116+123. [Google Scholar]
- Ali, A.; Zhang, J.J.; Zhou, M.M.; Chen, T.; Liaqat, S.; ur Rehman, S.; Hayat, S.; Shi, J.; Chen, J. Chitosan oligosaccharides stimulate the efficacy of somatic embryogenesis in different genotypes of the Liriodendron hybrid. Forests 2021, 12, 557. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Ou, L.; Ji, D.; Liu, T.; Lan, R.; Li, X.; Jin, L. Response to the cold stress signaling of the tea plant (Camellia sinensis) elicited by chitosan oligosaccharide. Agronomy 2020, 10, 915. [Google Scholar] [CrossRef]
- Jia, X.C.; Qin, H.Q.; Bose, S.K.; Liu, T.; He, J.; Xie, S.; Ye, M.; Yin, H. Proteomics analysis reveals the defense priming effect of chitosan oligosaccharides in Arabidopsis-Pst DC3000 interaction. Plant Physiol. Biochem. 2020, 149, 301–312. [Google Scholar] [CrossRef]
- Liu, J.; Ou, X.; Wang, J. Effect of exogenous H2O2 on leaf physiological characteristics of naked oat seedlings under drought stres. Agric. Res. Arid Areas 2019, 37, 146–153. [Google Scholar]
- Cheplick, S.; Sarkar, D.; Bhowmik, P.C.; Shetty, K. Improved resilience and metabolic response of transplanted blackberry plugs using chitosan oligosaccharide elicitor treatment. Can. J. Plant Sci. 2018, 98, 717–731. [Google Scholar] [CrossRef]
- Song, L. Jiangxi Oil Crop Industry Development Report; Jiangxi Science and Technology Publishing House: Nanchang, China, 2011; pp. 63–64. [Google Scholar]
- Wu, X.; Yang, X.; Cha, D.; Zhu, Z.; Xu, S. Physiological mechanism of low-temperature tolerance in eggplant seedlings. Shanghai Agric. J. 2013, 29, 45–49. [Google Scholar]
- Zou, Q. Experimental Guidance of Plant Physiology; China Agriculture Press: Beijing, China, 2003. [Google Scholar]
- Wang, N.; Chu, Y. Effect of low temperature on the activity of osmoregulators and protective enzymes in peanut seedlings. Anhui Agric. Sci. 2007, 35, 9154–9156. [Google Scholar]
- Peng, W.; Guo, X. Effect of cold injury stress on the physiology and structure of watermelon seedlings. Chin. Melon Veg. 2017, 30, 8–12. [Google Scholar]
- Zhao, X.; Liang, T.; Zhang, H. Effects of chitooligosaccharide on seed germination, seedling growth and osmotic substances in wheat under PEG stress. Seeds 2020, 39, 91–95. [Google Scholar]
- Guo, W.H.; Zhao, X.M.; Du, Y.G. Effects of oligochitosan on the growth and photosynthesis and physiological index related to photosynthesis of tobacco seedlings. Plant Physiol. Commun. 2008, 1155–1157. [Google Scholar]
- Li, Y.; Zhao, X.M.; Xia, X.Y. Effects of oligochitosan on photosynthetic parameter of Brassica napus seedlings under drought stress. Acta Agron. Sin. 2008, 34, 326–329. [Google Scholar] [CrossRef]
- Prasad, P.; Boote, K.; Thomas, J.; Allen, L.H., Jr.; Gorbet, D.W. Influence of soil temperature on seedling emergence and early growth of peanut cultivars in field conditions. J. Agron. Crop Sci. 2006, 192, 168–177. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, F.; Hussain, H.A.; Nie, L. Physiological and biochemical mechanisms of seed priming-induced chilling tolerance in rice cultivars. Front. Plant Sci. 2016, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Reiter, R.J.; Chan, Z. Phytomelatonin: A universal abiotic stress regulator. J. Exp. Bot. 2018, 69, 963–974. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Zheng, J.; Dong, Y.; Liu, Q.; Yang, X.; Wei, C.H.; Zhang, Y.; Ma, F.W.; Zhang, X. Local melatonin application induces cold tolerance in distant organs of Citrullus lanatus L. Via long distance transport. Sci. Rep. 2017, 7, 40858. [Google Scholar]
- Du, B.; Luo, H.W.; He, L.X.; Zhang, L.; Liu, Y.; Mo, Z.; Pan, S.; Tian, H.; Duan, M.; Tang, X. Rice seed priming with sodium selenate: Effects on germination, seedling growth, and biochemical attributes. Sci. Rep. 2019, 9, 3631–3642. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Zhao, X.; Xu, T.; Jiang, Y.; Liu, T.; Zhou, K. H2O2 Regulation of peanut seed germination under low temperature stress. Chin. J. Chin. Oil Crops 2020, 42, 860–868. [Google Scholar]
- Chang, B.; Zhong, P.; Liu, J.; Tang, Z.; Gao, Y.; Yu, H.; Guo, W. Effect of cold stress and gibberellin on peanut seed germination and seedling physiological responses. J. Crop Sci. 2019, 45, 118–130. [Google Scholar]
- Dong, D.; Li, Y.; Jiang, L.; Liang, H.; Huang, J. Long-acting brassinosteroid TS303 and Acpropyl dihydrojasmonate enhances cold resistance in peanut. Guangxi Plants 2008, 28, 675–680. [Google Scholar]
- Li, C.; Ying, C.; Lin, Y.; Ye, H.; Zheng, X.; Xu, G. Effect of chilling stress the RPP, soluble protein and protective enzyme of Arachis. Duranensis leafs. China Grass Ind. Dev. Forum. 2008, 302–305. [Google Scholar]
- Liang, Y.; Chen, Q.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.H.; Wu, Y.; Zeng, J.Z.; Zeng, Q.M. Chill-induced inhibition of photosynthesis was alleviated by 24-epibrassinolide pretreatment in cucumber during chilling and subsequent recovery. Photosynthetica 2010, 48, 537–544. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).