Responses of Soybean to Selected Abiotic Stresses—Photoperiod, Temperature and Water
Abstract
1. Introduction
2. Light Requirements
3. Thermal Stress
4. Water Stress
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Sun, J.; Mooney, H.; Wu, W.; Tang, H.; Tong, Y.; Xu, Z.; Huang, B.; Cheng, Y.; Yang, X.; Wei, D.; et al. Importing food damages domestic environment: Evidence from global soybean trade. Proc. Natl. Acad. Sci. USA 2018, 115, 5415–5419. [Google Scholar] [CrossRef] [PubMed]
- Kotecki, A.; Lewandowska, S. Studia nad Uprawą soi Zwyczajnej (Glycine max (L.) Merrill) w Południowo-Zachodniej Polsce/Studies on the Cultivation of Soybean (Glycine max (L.) Merrill) in South-Western Poland; Wyd. UP Wrocław: Wrocław, Poland, 2020; p. 226. (In Polish) [Google Scholar]
- Święcicki, W.; Chudy, M.; Żuk-Gołaszewska, K. Rośliny strączkowe w projektach badawczych Unii Europejskiej. Zesz. Probl. Post. Nauk Rol. 2007, 522, 55–65. (In Polish) [Google Scholar]
- Bellaloui, N.; Bruns, H.A.; Abbas, H.K.; Mengistu, A.; Fisher, D.K.; Reddy, K.N. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- SoyStats 2021. Available online: http://www.soystats.com (accessed on 15 July 2021).
- Szpunar-Krok, E.; Wondołowska-Grabowska, A.; Bobrecka-Jamro, D.; Jańczak-Pieniążek, M.; Kotecki, A.; Kozak, M. Effect of nitrogen fertilisation and inoculation with Bradyrhizobium japonicum on the fatty acid profile of soybean (Glycine max (L.) Merrill) seeds. Agronomy 2021, 11, 941. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Wondołowska-Grabowska, A. Quality evaluation indices for soybean oil in relation to cultivar, application of N fertiliser and seed inoculation with Bradyrhizobium japonicum. Foods 2022, 11, 762. [Google Scholar] [CrossRef] [PubMed]
- Borawska, J.; Darewicz, M.; Iwaniak, A.; Minkiewicz, P. Biologically active peptides from food proteins as factors preventing diet-related diseases/Biologicznie aktywne peptydy pochodzące z białek żywności jako czynniki prewencji wybranych chorób dietozależnych. Bromat. Chem. Toksykol. 2014, 47, 230–236. Available online: https://www.ptfarm.pl/download/?file=File%2FBromatologia%2F2014%2FBR+2-2014+s_+230-236.pdf (accessed on 2 August 2022). (In Polish).
- Martyniuk, S. Naukowe i praktyczne aspekty symbiozy roślin strączkowych z bakteriami brodawkowymi/Scientific and practical aspects of legumes symbiosis with root-nodule bacteria. Pol. J. Agron. 2012, 9, 17–22. Available online: https://iung.pl/PJA/wydane/9/PJA9_3.pdf (accessed on 2 August 2022). (in Polish).
- Kocira, A.; Staniak, M.; Tomaszewska, M.; Kornas, R.; Cymerman, J.; Panasiewicz, K.; Lipińska, H. legume cover crops as one of the elements of strategic weed management and soil quality improvement. A Review. Agriculture 2020, 10, 394. [Google Scholar] [CrossRef]
- Bethlenfalvay, J.G.; Franson, L.R.; Brown, S.M. Nutrition of mycorrhizal soybean evaluated by the diagnosis and recommendation integrated system (DRIS). Agron. J. 1990, 82, 302–304. [Google Scholar] [CrossRef]
- Śliwa, J.; Zając, T.; Oleksy, A.; Klimek-Kopyra, A.; Lorenc-Kozik, A.; Kulig, B. Comparison of the development and productivity of soybean (Glycine max (L.) Merr.) cultivated in western Poland. Acta Sci. Pol. Sec. Agricultura 2015, 14, 81–95. [Google Scholar]
- Badaruddin, M.; Meyer, D.W. Grain legume effects on soil nitrogen, grain yield, and nitrogen nutrition of wheat. Crop Sci. 1994, 34, 1304–1309. [Google Scholar] [CrossRef]
- FAOSTAT. 2022. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 July 2022).
- STATISTA. 2022. Available online: https://www.statista.com/search/?q=soybean (accessed on 15 July 2022).
- Ritchie, H.; Roser, M. Soy. Published Online at OurWorldInData.org. 2021. Available online: https://ourworldindata.org/soy (accessed on 21 December 2022).
- EUROStat. 2022. Available online: http://ec.europa.eu/eurostat/de (accessed on 20 July 2022).
- Kuepper, B.; Stravens, M. Mapping the European Soy Supply Chain—Embedded Soy in Animal Products Consumed in the EU27+UK; Profundo: Amsterdam, The Netherlands, 2022; Available online: https://wwfeu.awsassets.panda.org/downloads/2021_106_european_soy_supply_wnf_2201_final.pdf (accessed on 23 December 2022).
- Gaynor, L.G.; Lawn, R.J.; James, A.T. Agronomic studies on irrigated soybean in southern New South Wales. I. Phenological adaptation of genotypes to sowing date. Crop Pasture Sci. 2011, 62, 1056–1066. [Google Scholar] [CrossRef]
- Câmara, G.M.S.; Sediyama, T.; Dourado-Neto, D.; Bernardes, M.S. Influence of fotoperiod and air temperature on the growth, flowering and maturation of the soybean (Glycine max. L. Merrill). Sci. Agric. 1997, 54, 149–154. Available online: https://www.scienceopen.com/document?vid=f604f4c0-fa07-4018-afaa-ff18d7c9b5aa (accessed on 2 August 2022). [CrossRef]
- Gass, T.; Schori, A.; Fossati, A.; Soldati, A.; Stamp, P. Cold tolerance of soybean (Glycine max. L. Merrill) during the reproductive phase. Eur. J. Agron. 1996, 5, 71–88. [Google Scholar] [CrossRef]
- Gawęda, D.; Nowak, A.; Haliniarz, M.; Woźniak, A. Yield and economic effectiveness of soybean grown under different cropping systems. Int. J. Plant Prod. 2020, 14, 475–485. [Google Scholar] [CrossRef]
- Kühling, I.; Hüsing, B.; Bome, N.; Trautz, D. Soybeans in high latitudes: Effects of Bradyrhizobium inoculation in Northwest Germany and southern West Siberia. Org. Agric. 2018, 8, 159–171. [Google Scholar] [CrossRef]
- Żarski, J.; Kuśmierek-Tomaszewska, R.; Dudek, S.; Krokowski, M.; Kledzik, R. Identifying climatic risk to soybean cultivation in the transitional type of moderate climate in Central Poland. J. Cent. Eur. Agric. 2019, 20, 143–156. [Google Scholar] [CrossRef]
- Karges, K.; Bellingrath-Kimura, S.D.; Watson, C.A.; Stoddard, F.L.; Halwani, M.; Reckling, M. Agro-economic prospects for expanding soybean production beyond its current northerly limit in Europe. Eur. J. Agron. 2022, 133, 126415. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 407, 2365–2379. [Google Scholar] [CrossRef]
- Khan, M.; Jannat, A.; Munir, F.; Fatima, N.; Amir, R. Biochemical and molecular mechanisms of abiotic stress tolerance. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; ISBN 978-981-15-2171-3. Available online: https://link.springer.com/book/10.1007/978-981-15-2172-0 (accessed on 23 December 2022).
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- de Melo, B.P.; Carpinetti, P.d.A.; Fraga, O.T.; Rodrigues-Silva, P.L.; Fioresi, V.S.; de Camargos, L.F.; Ferreira, M.F.d.S. Abiotic stresses in plants and their markers: A practice view of plant stress responses and programmed cell death mechanisms. Plants 2022, 11, 1100. [Google Scholar] [CrossRef]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Ablett, G.R.; Pauls, K.P.; Rajcan, I. Environmental Effects on Fatty Acid Levels in Soybean Seed Oil. J. Am. Oil Chem. Soc. 2006, 83, 759–763. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sharma, R.; Sharma, M.K.; Sharma, M.P.; Satpute, G.K.; Garg, S.; Singla-Pareek, S.L.; Pareek, A. Signaling cross talk between biotic and abiotic stress responses in soybean. In Abiotic and Biotic Stresses in Soybean Production; Miransari, M., Ed.; Academic Press: Oxford, UK, 2016; pp. 27–52. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Ma, Y.; Li, S.; Dong, S.; Zu, W. Transcriptome profiling analysis characterized the gene expression patterns responded to combined drought and heat stresses in soybean. Comput. Biol. Chem. 2018, 77, 413–429. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Interactive effect of temperature and water stress on physiological and biochemical processes in soybean. Physiol. Mol. Biol. Plants. 2019, 25, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Haak, D.C.; Fukao, T.; Grene, R.; Hua, Z.; Ivanov, R.; Perrella, G.; Li, S. Multilevel regulation of abiotic stress responses in plants. Front. Plant Sci. 2017, 8, 1564. [Google Scholar] [CrossRef]
- Cooper, R.L. A delayed flowering barrier to higher soybean yields. Field Crop Res. 2003, 82, 27–35. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. Available online: http://ri.agro.uba.ar/files/download/articulo/2013casal1.pdf (accessed on 10 December 2022). [CrossRef] [PubMed]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef] [PubMed]
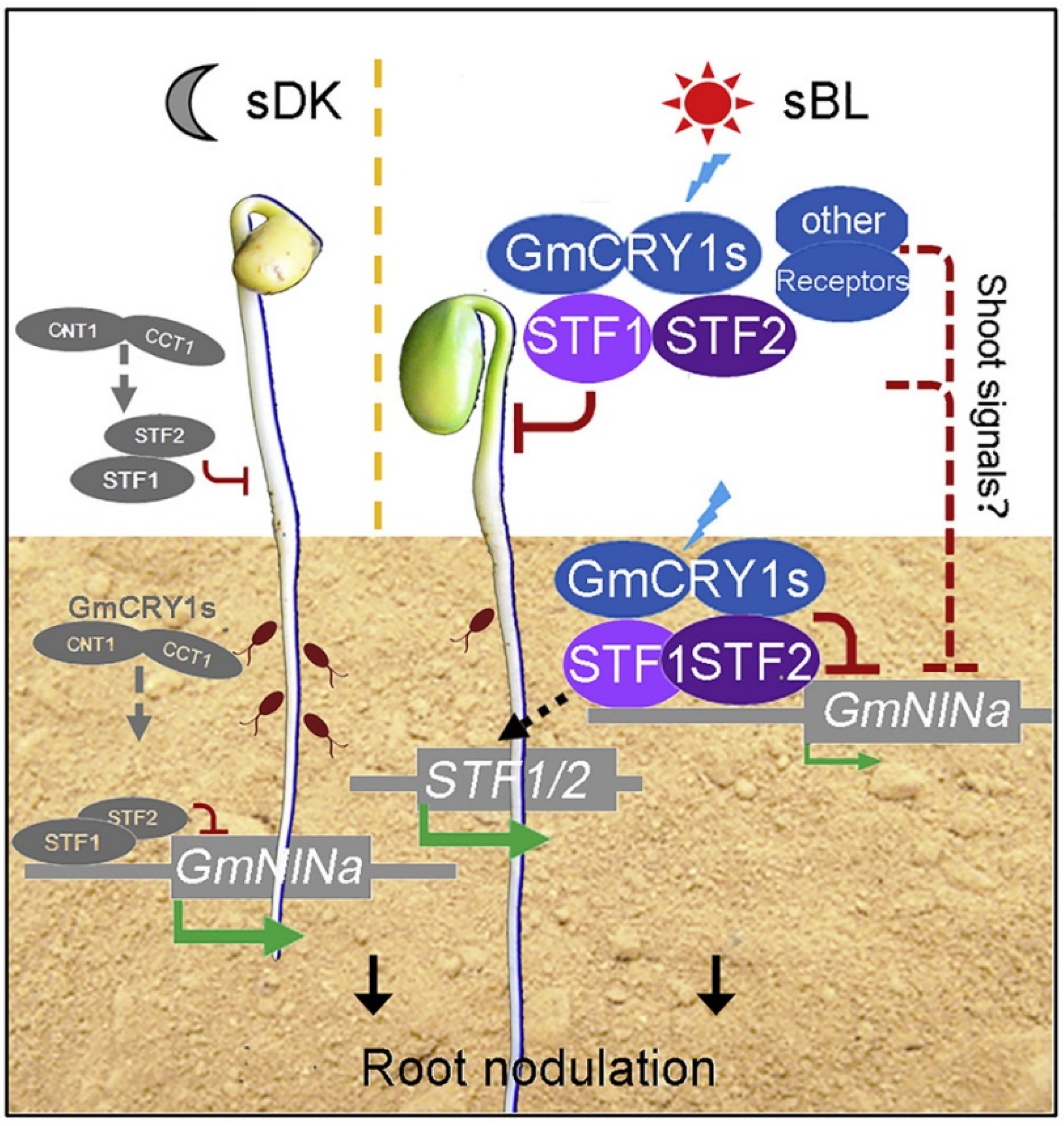
- Ji, H.; Xiao, R.; Lyu, X.; Chen, J.; Zhang, X.; Wang, Z.; Deng, Z.; Wang, Y.; Wang, H.; Li, R.; et al. Differential light-dependent regulation of soybean nodulation by papilionoid—Specific HY5 homologs. Curr. Biol. 2022, 32, 783–795.e5. [Google Scholar] [CrossRef]
- Osterlund, C.M.T.; Hardtke, S.; Wei, N.; Deng, X.W. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 2000, 405, 462–466. [Google Scholar] [CrossRef]
- Heng, Y.; Jiang, Y.; Zhao, X.; Zhou, H.; Wang, X.; Deng, X.W.; Xu, D. BBX4, a phyB-interacting and modulated regulator, directly interacts with PIF3 to fine tune red light-mediated photomorphogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 4429–4430. [Google Scholar] [CrossRef] [PubMed]
- Iqbalm, Z.; Iqbal, M.S.; Hashemmm, A.; Abd_Allah, E.F.; Mi, A. Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions. Front. Plant Sci. 2021, 12, 631810. [Google Scholar] [CrossRef]
- Malik, N.S.A.; Pence, M.K.; Calvert, H.E.; Bauer, W.D.; Kettering, C.F. Rhizobium infection and nodule development in soybean are affected by exposure of the cotyledons to light. Plant Physiol. 1984, 75, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, J.; Peng, Y.; Lyu, X.; Liu, B.; Sun, S.; Wang, X. Light-induced mobile factors from shoots regulate rhizobium-triggered soybean root nodulation. Science 2021, 374, 65–71. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Ang, L.H.; Puente, P.; Deng, X.-W.; Wei, N. Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 1998, 10, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yao, Q.; Gao, X.; Jiang, C.; Harberd, N.P.; Fu, X. Shoot-to-root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr. Biol. 2016, 26, 640–646. [Google Scholar] [CrossRef]
- van Gelderen, K.; Kang, C.; Paalman, R.; Keuskamp, D.; Hayes, S.; Pierik, R. Far-red light detection in the shoot regulates lateral root development through the HY5 transcription factor. Plant Cell 2018, 30, 101–116. [Google Scholar] [CrossRef]
- Garner, W.W.; Allard, H.A. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. J. Agric. Res. 1920, 48, 553–606. [Google Scholar] [CrossRef]
- Garner, W.W.; Allard, H.A. Further studies in photoperiodism. The response of the plant to relative length of day and night. J. Agric. Res. 1923, 23, 871–920. [Google Scholar]
- Zhang, L.; Wang, R.; Hesketh, J.D. Effects of photoperiod on growth and development of soybean floral bud in different maturity. Agron J. 2001, 93, 944–948. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Wu, C.; Sun, S.; Mao, T.; Jiang, B.; Hou, W.; Han, T. Analysis of the independent and interactive photo thermal effects on soybean flowering. J. Integr. Agric. 2015, 14, 622–632. [Google Scholar] [CrossRef]
- Kantolic, A.G.; Slafer, G.A. Development and seed number in indeterminate soybean as affected by timing and duration of exposure to long photoperiods after flowering. Ann. Bot. 2007, 99, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Harada, K.; Abe, J. Genetic and molecular bases of photoperiod responses of flowering in soybean. Breed Sci. 2012, 61, 531–543. [Google Scholar] [CrossRef] [PubMed]
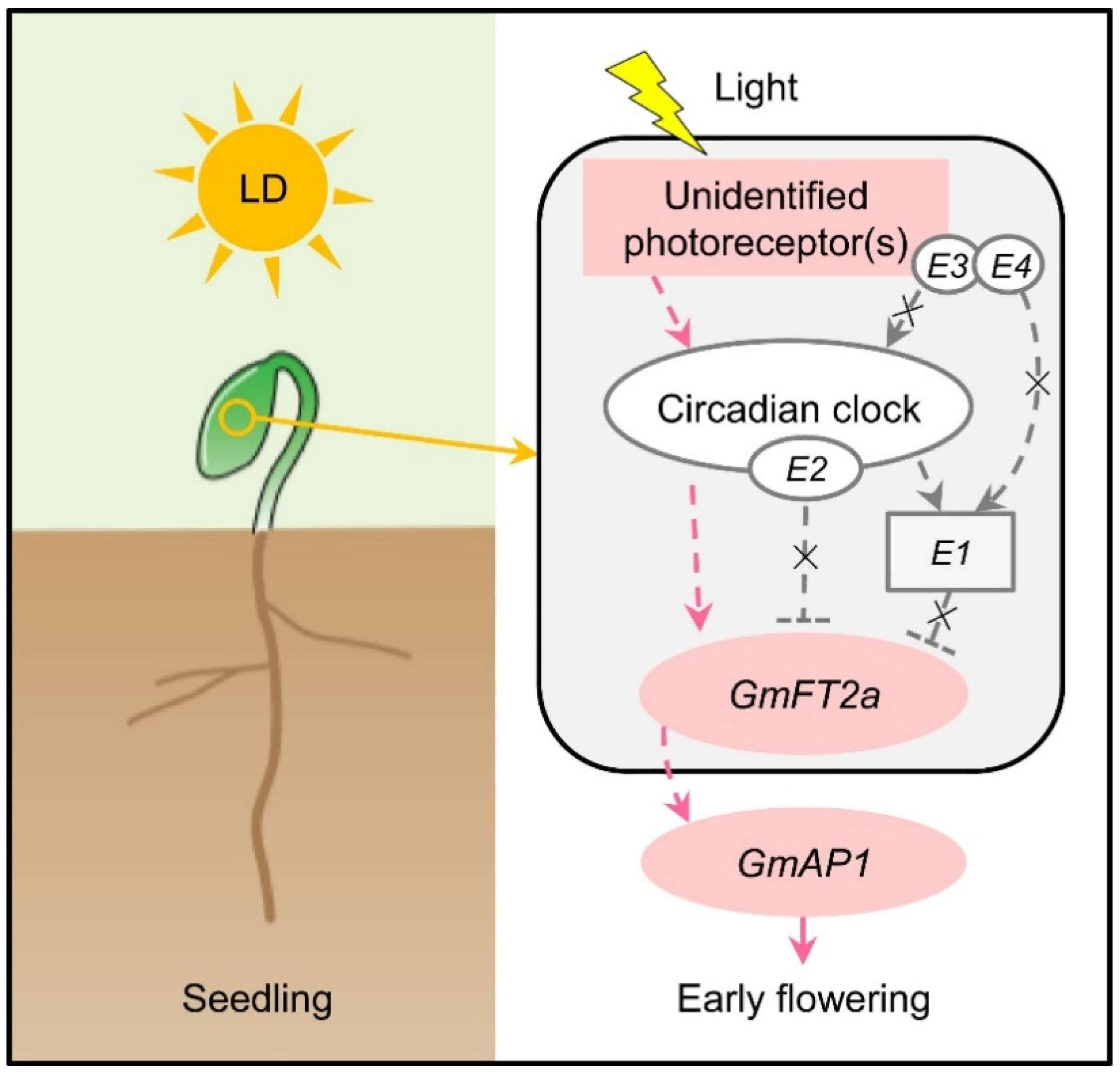
- Xu, M.; Yamagishi, N.; Zhao, C.; Takeshima, R.; Kasai, M.; Watanabe, S.; Kanazawa, A.; Yoshikawa, N.; Liu, B.; Yamada, T.; et al. The soybean-specific maturity gene E1 family of floral repressors controls night-break responses through down-regulation of FLOWERING LOCUS T orthologs. Plant Physiol. 2015, 168, 1735–1746. [Google Scholar] [CrossRef]
- Mao, T.; Li, J.; Wen, Z.; Wu, T.; Wu, C.; Shi, S.; Jiang, B.; Hou, W.; Li, W.; Song, Q.; et al. Association mapping of loci controlling genetic and environmental interaction of soybean flowering time under various photo-thermal conditions. BMC Genom. 2017, 18, 415. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.P.; Herbert, S.J.; Zhang, S.; Rautenkranz, A.A.F.; Litchfield, G.V. Differential response of soybean yield components to the timing of light enrichment. Agron. J. 2000, 92, 1156–1161. [Google Scholar] [CrossRef]
- Polson, D.E. Day-neutrality in soybeans. Crop. Sci. 1972, 7945. [Google Scholar] [CrossRef]
- Yang, W.Y.; Wu, T.T.; Zhang, X.Y.; Song, W.W.; Xu, C.L.; Sun, S.; Hou, W.S.; Jiang, B.J.; Han, T.F.; Wu, C.X. Critical photoperiod measurement of soybean genotype in different maturity groups. Crop. Sci. 2019, 59, 2055–2061. [Google Scholar] [CrossRef]
- Lin, X.; Liu, B.; Weller, J.L.; Abe, J.; Kong, F. Molecular mechanisms for the photoperiodic regulation of flowering in soybean. J. Integr. Plant Biol. 2020, 63, 981–994. [Google Scholar] [CrossRef]
- Chen, X.Z.; Xie, H.; Li, X. Studies on correlation ship of development stages and agronomic traits of summer sowing soybean. Mol. Plant Breed. 2004, 2, 247–252. [Google Scholar] [CrossRef][Green Version]
- Cober, E.R.; Morrison, M.J. Regulation of seed yield and agronomic characters by photoperiod sensitivity and growth habit genes in soybean. Theor. Appl. Genet. 2010, 120, 1005–1012. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Tsegaw, M.; Xu, X.; Qi, Y.; Sapey, E.; Liu, L.; Wu, T.; Sun, S.; Han, T. Principles and practices of the photo-thermal adaptability improvement in soybean. J. Integr. Agric. 2020, 19, 295–310. [Google Scholar] [CrossRef]
- Luo, X.; Yin, M.; He, Y. molecular genetic understanding of photoperiodic regulation of flowering time in Arabidopsis and soybean. Int. J. Mol. Sci. 2022, 23, 466. [Google Scholar] [CrossRef] [PubMed]
- Gai, J.Y.; Wang, Y.S. A study on the varietal eco-regions of soybeans in China. Sci. Agric. Sin. 2001, 34, 139–145. [Google Scholar]
- Seferova, I.V. Soybean in the North-West of the Russian Federation/Macличhьie Kyльtypьi. Hayчho—Texhичeckий бюллetehь Bcepoccийckoгo Hayчhoиccлeдobateльckoгo Иhctиtyta Macличhьix Kyльtyp. Bьin 2016, 3, 101–105. Available online: https://cyberleninka.ru/article/n/soya-v-usloviyah-severo-zapada-rossiyskoy-federatsii (accessed on 10 November 2022).
- Xia, Z.; Watanabe, S.; Yamada, T.; Tsubokura, Y.; Nakashima, H.; Zhai, H.; Anai, T.; Sato, S.; Yamazaki, T.; Lü, S.; et al. Positional cloning and characterization reveal the molecular basis for soybean maturity locus E1 that regulates photoperiodic flowering. Proc. Natl. Acad. Sci. USA 2012, 109, E2155–E2164. [Google Scholar] [CrossRef]
- Watanabe, S.; Xia, Z.; Hideshima, R.; Tsubokura, Y.; Sato, S.; Yamanaka, N.; Takahashi, R.; Anai, T.; Tabata, S.; Kitamura, K.; et al. A map-based cloning strategy employing a residual heterozygous line reveals that the GIGANTEA gene is involved in soybean maturity and flowering. Genetics 2011, 188, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Bernard, R.L. Two major genes for time of flowering and maturity in soybeans. Crop Sci. 1971, 11, 242–244. [Google Scholar] [CrossRef]
- Buzzell, R.I. Inheritance of a soybean flowering response to fluorescent-daylength conditions. Can. J. Genet. Cytol. 1971, 13, 703–707. [Google Scholar] [CrossRef]
- Watanabe, S.; Hideshima, R.; Xia, Z.; Tsubokura, Y.; Sato, S.; Nakamoto, Y.; Yamanaka, N.; Takahashi, R.; Ishimoto, M.; Anai, T.; et al. Map-based cloning of the gene associated with the soybean maturity locus E3. Genetics 2009, 182, 1251–1262. [Google Scholar] [CrossRef]
- Buzzell, R.I.; Voldeng, H.D. Research Notes: Inheritance of insensitivity to long daylength. Soybean Genet. Newsl. 1980, 7, 13. [Google Scholar]
- Liu, B.; Kanazawa, A.; Matsumura, H.; Takahashi, R.; Harada, K.; Abe, J. Genetic redundancy in soybean photoresponses associated with duplication of the phytochrome A gene. Genetics 2008, 180, 995–1007. [Google Scholar] [CrossRef]
- Cober, E.R.; Voldeng, H.D. Low R:FR light quality delays flowering of E7E7 soybean lines. Crop. Sci. 2001, 41, 1823–1826. [Google Scholar] [CrossRef]
- Zhai, H.; Lü, S.; Liang, S.; Wu, H.; Zhang, X.; Liu, B.; Kong, F.; Yuan, X.; Li, J.; Xia, Z. GmFT4, a homolog of FLOWERING LOCUS T, is positively regulated by E1 and functions as a flowering repressor in soybean. PLoS ONE 2014, 9, e89030. [Google Scholar] [CrossRef] [PubMed]
- Samanfar, B.; Molnar, S.J.; Charette, M.; Schoenrock, A.; Dehne, F.; Golshani, A.; Belzile, F.; Cober, E.R. Mapping and identification of a potential candidate gene for a novel maturity locus, E10, in soybean. Theor. Appl. Genet. 2017, 130, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Bonato, E.R.; Vello, N.A. E6, a dominant gene conditioning early flowering and maturity in soybeans. Genet. Mol. Biol. 1999, 22, 229–232. [Google Scholar] [CrossRef]
- Cober, E.R. Long juvenile soybean flowering responses under very short photoperiods. Crop Sci. 2011, 51, 140–145. [Google Scholar] [CrossRef]
- Kong, F.; Liu, B.; Xia, Z.; Sato, S.; Kim, B.M.; Watanabe, S.; Yamada, T.; Tabata, S.; Kanazawa, A.; Harada, K.; et al. Two coordinately regulated homologs of FLOWERING LOCUS T are involved in the control of photoperiodic flowering in soybean. Plant Physiol. 2010, 154, 1220–1231. [Google Scholar] [CrossRef]
- Wang, F.; Nan, H.; Chen, L.; Fang, C.; Zhang, H.; Su, T.; Li, S.; Cheng, Q.; Dong, L.; Liu, B.; et al. A new dominant locus, E11, controls early flowering time and maturity in soybean. Mol. Breed. 2019, 39, 70. [Google Scholar] [CrossRef]
- Ray, J.D.; Hinson, K.; Mankono, J.E.B.; Malo, M.F. Genetic Control of a Long-Juvenile Trait in Soybean. Crop Sci. 1995, 35, 1001. [Google Scholar] [CrossRef]
- Lu, S.; Dong, L.; Fang, C.; Liu, S.; Kong, L.; Cheng, Q.; Chen, L.; Su, T.; Nan, H.; Zhang, D.; et al. Stepwise selection on homeologous PRR genes controlling flowering and maturity during soybean domestication. Nat. Genet. 2020, 52, 428–436. [Google Scholar] [CrossRef]
- Fan, C.; Hu, R.; Zhang, X.; Wang, X.; Zhang, W.; Zhang, Q.; Ma, J.; Fu, Y.F. Conserved CO-FT regulons contribute to the photoperiod flowering control in soybean. BMC Plant Biol. 2014, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Xu, K.; Zhang, X.; Zhu, J.; Lu, M.; Chen, F.; Liu, L.; Xi, Z.-Y.; Bachmair, A.; Chen, Q.; et al. Extensive analysis of GmFTL and GmCOL expression in northern soybean cultivars in field conditions. PLoS ONE 2015, 10, e0136601. [Google Scholar] [CrossRef]
- Dong, L.; Fang, C.; Cheng, Q.; Su, T.; Kou, K.; Kong, L.; Zhang, C.; Li, H.; Hou, Z.; Zhang, Y.; et al. Genetic basis and adaptation trajectory of soybean from its temperate origin to tropics. Nat. Commun. 2021, 12, 5445. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, C.; Yang, Y.; Lv, T.; Su, T.; Chen, L.; Nan, H.; Li, S.; Zhao, X.; Lu, S.; et al. Overcoming the genetic compensation response of soybean florigens to improve adaptation and yield at low latitudes. Curr. Biol. 2021, 31, 3755–3767.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, M.; Xu, C.; Yang, X.; Li, D.; Zhao, X.; Wang, K.; Li, Y.; Zhang, X.; Liu, L.; et al. Natural variation in GmGBP1 promoter affects photoperiod control of flowering time and maturity in soybean. Plant J. 2018, 6, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Song, W.; Wang, L.; Sun, X.; Qi, Y.; Wu, T.; Sun, S.; Jiang, B.; Wu, C.; Hou, W.; et al. Allele combinations of maturity genes E1-E4 affect adaptation of soybean to diverse geographic regions and farming systems in China. PLoS ONE 2020, 15, e0235397. [Google Scholar] [CrossRef]
- Song, Y.H.; Shim, J.S.; Kinmonth-Schultz, H.A.; Imaizumi, T. Photoperiodic flowering: Time measurement mechanisms in leaves. Annu. Rev. Plant Biol. 2015, 66, 441. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Cao, X.; Liu, L.; Jiang, B.; Zhang, C.; Jia, H.; Lyu, X.; Su, Y.; Cai, Y.; et al. Cotyledons facilitate the adaptation of early-maturing soybean varieties to high-latitude long-day environments. Plant Cell Environ. 2021, 44, 2551–2564. [Google Scholar] [CrossRef]
- Huber, S.C.; Rufty, T.W.; Kerr, P.S. Effect of photoperiod on photosynthate partitioning and diurnal rhythms in sucrose phosphate synthase activity in leaves of soybean (Glycine max L. [Merr.]) and tobacco (Nicotiana tabacum L.). Plant Physiol. 1984, 75, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.H.; Yourstone, K.S.; Masaya, P.N.; Zobel, R.W. Photoperiod gene control over partitioning between reproductive and vegetative growth. Theor. Appl. Genet. 1993, 86, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Jarecki, W.; Bobrecka-Jamro, D. Effect of sowing date on the yield and seed quality of soybean (Glycine max (L.) Merr.). J. Elem. 2021, 26, 7–18. [Google Scholar] [CrossRef]
- Morandi, E.N.; Casano, L.M.; Reggiardo, L.M. Post-flowering photoperiodic effect on reproductive efficiency and seed growth in soybean. Field Crop Res. 1988, 18, 227–241. [Google Scholar] [CrossRef]
- Summerfield, R.J.; Roberts, E.H.; Ellis, R.H.; Lawn, R.J. Towards the reliable prediction of time to flowering in six annual crops. I. The development of simple models for fluctuating field environments. Exp. Agric. 1991, 27, 11–31. [Google Scholar] [CrossRef]
- Song, W.; Sun, S.; Ibrahim, S.E.; Xu, Z.; Wu, H.; Hu, X.; Wu, H.; Hu, X.; Jia, H.; Cheng, Y.; et al. Standard cultivar selection and digital quantification for precise classification of maturity groups in soybean. Crop Sci. 2019, 59, 1997–2006. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Buczek, J.; Bobrecka-Jamro, D.; Szpunar-Krok, E.; Tobiasz-Salach, R.; Jarecki, W. Morphophysiology, productivity and quality of soybean (Glycine max (L.) Merr.) cv. Merlin in response to row spacing and seeding systems. Agronomy 2021, 11, 403. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Jan, S.; Abd-Allah, E.F.; Rashid, B.; John, R.; Ahmad, P. Soybean under abiotic stress: Proteomic approach. In Plant-Environment Interaction: Resposes and Approaches to Mitigate Stress; Azooz, M.M., Ahmad, P., Eds.; Wiley-Blackwell: New York, NY, USA, 2016; pp. 28–42. [Google Scholar] [CrossRef]
- Nouri, M.Z.; Toorchi, M.; Komatsu, S. Proteomics approach for identifying abiotic stress responsive proteins in soybean. Soybean Mol. Asp. Breed. 2011, 9, 187–214. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Grimm, S.S.; Jones, J.W.; Boote, K.J.; Hesketh, J.D. Parameter estimation for predicting flowering date of soybean cultivars. Crop Sci. 1993, 33, 137–144. [Google Scholar] [CrossRef]
- Setiyono, T.D.; Weiss, A.; Specht, J.; Bastidas, A.M.; Cassman, K.G.; Dobermann, A. Understanding and modeling the effect of temperature and daylength on soybean phenology under high-yield conditions. Field Crop Res. 2007, 100, 257–271. [Google Scholar] [CrossRef]
- Boote, K.J. Improving soybean cultivars for adaptation to climate change and climate variability. In Crop Adaptation to Climate Change; Wiley-Blackwell: Oxford, UK, 2011; pp. 370–395. [Google Scholar] [CrossRef]
- Parent, B.; Tardieu, F. Temperature responses of developmental processes have not been affected by breeding in different ecological areas for 17 crop species. New Phytol. 2012, 194, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, V.; Shekh, A.; Kumar, M. Growth and yield response of soybean (Glycine max L.) in relation to temperature, photoperiod and sunshine duration at Anand, Gujarat, India. Am. Eur. J. Agron. 2008, 1, 45–50. Available online: http://www.idosi.org/aeja/1(2)08/6.pdf?q=birla-institute-of-technology-mesra-ranchi-835215-india (accessed on 2 August 2022).
- Miransari, M. Soybean Production. In Abiotic and Biotic Stresses in Soybean Production; Miransari, M., Ed.; Academic Press: London, UK, 2015; Available online: https://books.google.pl/books?hl=pl&lr=&id=ILV0BgAAQBAJ&oi=fnd&pg=PP1&dq=Miransari,+M.+Soybean+Production+In:+Miransari,+M.+(Ed.).+Abiotic+and+biotic+stresses+in+soybean+production:+Vol.1,+Vol.+1.+Academic+press,+London,+UK.+2015&ots=2qlhLfAupF&sig=YhEa8nDlLwD-F7pqND9K_EJktPs&redir_esc=y#v=onepage&q=Miransari%2C%20M.%20Soybean%20Production%20In%3A%20Miransari%2C%20M.%20(Ed.).%20Abiotic%20and%20biotic%20stresses%20in%20soybean%20production%3A%20Vol.1%2C%20Vol.%201.%20Academic%20press%2C%20London%2C%20UK.%202015&f=false (accessed on 2 August 2022).
- Yamaguchi, N.; Yamazaki, H.; Ohnishi, S.; Suzuki, C.; Hagihara, S.; Miyoshi, T.; Senda, M. Method for selection of soybeans tolerant to seed cracking under chilling temperatures. Breed. Sci. 2014, 64, 103–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Staniak, M.; Stępień-Warda, A.; Czopek, K.; Kocira, A.; Baca, E. Seeds quality and quantity of soybean [Glycine max (L.) Merr.] cultivars in response to cold stress. Agronomy 2021, 11, 520. [Google Scholar] [CrossRef]
- Markowski, A. Influence of initial seed moisture and temperature conditions during germination and emergence on seedling survival and yields of soybean (Glycine max L. Merrill). Acta Agrobot. 1982, 35, 43–59. [Google Scholar] [CrossRef][Green Version]
- Janas, K.; Cvirkova, M.; Pałągiewicz, A.; Eder, J. Alterations in phenylpropanoid content in soybean roots during low temperature acclimation. Plant Physiol. Biochem. 2000, 38, 587–593. [Google Scholar] [CrossRef]
- Zhang, F.; Smith, D.L. Effects of low root zone temperature on the early stages of symbiosis establishment between soybean (Glycine max (L.) Merr) and Bradyrhizobium japonicum. J. Exp. Bot. 1994, 45, 1467–1473. [Google Scholar] [CrossRef]
- Duzan, H.M.; Zhou, X.; Souleimanov, A.; Smith, D.L. Perception of Bradyrhizobium japonicum nod factor by soybean [Glycine max (L.) Merr.] root hairs under abiotic stress conditions. J. Exp. Bot. 2004, 55, 2641–2646. [Google Scholar] [CrossRef]
- Egli, D.B.; Cornelius, P.L. A regional analysis of the response of soybean yield to planting date. Agron. J. 2009, 101, 330–335. [Google Scholar] [CrossRef]
- De Bruin, J.L.; Pedersen, P. Soybean seed yield response to planting date and seeding rate in the upper Midwest. Agron. J. 2008, 100, 696–703. [Google Scholar] [CrossRef]
- Marburger, D.A.; Smith, D.L.; Conley, S.P. Revisiting planting date and cultivar effects on soybean sudden death syndrome development and yield loss. Plant Dis. 2016, 100, 2152–2157. [Google Scholar] [CrossRef]
- Gaspar, A.P.; Conley, S.P. Responses of canopy reflectance, light interception, and soybean seed yield to replanting suboptimal stands. Crop Sci. 2015, 55, 377–385. [Google Scholar] [CrossRef]
- Księżak, J.; Bojarszczuk, J. The seed yield of soybean cultivars and their quantity depending on sowing term. Agronomy 2022, 12, 1066. [Google Scholar] [CrossRef]
- Egli, D.B.; Bruening, W.P. Potential of early-maturing soybean cultivars in late plantings. Agron. J. 2000, 92, 532–537. [Google Scholar] [CrossRef]
- Pedersen, P.; Lauer, J.G. Response of soybean yield components to management system and planting date. Agron. J. 2004, 96, 1372–1381. [Google Scholar] [CrossRef]
- Lemichhane, J.R.; Constantina, J.; Schovinga, C.; Mauryb, P.; Debaekea, P.; Aubertota, J.-N.; Dürrc, C. Analysis of soybean germination, emergence, and prediction of a possible northward establishment of the crop under climate change. Eur. J. Agron. 2020, 113, 125972. [Google Scholar] [CrossRef]
- Meyer, D.W.; Badaruddin, M. Frost tolerance of ten seedling legume species at four growth stages. Crop Sci. 2001, 41, 1838–1842. [Google Scholar] [CrossRef]
- Mahieu, P.J.; Brinkman, M.A. Double-cropping soybean after harvesting small grains as forage in the north central USA. J. Prod. Agric. 1990, 3, 385–389. [Google Scholar] [CrossRef]
- Shapiro, B.I.; Brorsen, B.W.; Doster, D.H. Adoption of double-cropping soybeans and wheat. South. J. Agric. Econ. 1992, 24, 33–40. [Google Scholar] [CrossRef]
- Staniak, M.; Czopek, K.; Stępień-Warda, A.; Kocira, A.; Przybyś, M. Cold Stress during Flowering Alters Plant Structure, Yield and Seed Quality of Different Soybean Genotypes. Agronomy 2021, 11, 2059. [Google Scholar] [CrossRef]
- Jähne, F.; Balko, C.; Hahn, V.; Würschum, T.; Leiser, W. Cold stress tolerance of soybeans during flowering: QTL mapping and efficient selection strategies under controlled conditions. Plant Breed. 2019, 138, 708–720. [Google Scholar] [CrossRef]
- Nawracała, J. Efektywność kwitnienia zróżnicowanych genotypów soi (Glycine max (L.) Merrill) w warunkach środowiskowych Wielkopolski/Efficiency of flowering of varied soybean (Glycine max (L.) Merrill) genotypes in the environmental conditions of Wielkopolska. Rośliny Oleiste—Oilseed Crops 2001, 26, 27–44. (In Polish) [Google Scholar]
- Ohnishi, S.; Miyoshi, T.; Shirai, S. Low temperature stress at different flower developmental stages affects pollen development, pollination, and pod set in soybean. Environ. Exp. Bot. 2010, 69, 56–62. [Google Scholar] [CrossRef]
- Kurosaki, H.; Yumoto, S. Effects of low temperature and shading during flowering on the yield components in soybeans. Plant Prod. Sci. 2003, 6, 17–23. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, L.; Han, X. Response of soybean yield to daytime temperature change during seed filling: A long-term field study in Northeast China. Plant Prod. Sci. 2009, 12, 526–532. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Boote, K.J.; Kimnball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef]
- Kurosaki, H.; Yumoto, S.; Matsukawa, I. Pod setting pattern during and after low temperature and the mechanism of cold-weather tolerance at the flowering stage in soybeans. Plant Prod. Sci. 2003, 6, 247–254. [Google Scholar] [CrossRef]
- Hume, D.J.; Jackson, A.K.H. Pod formation in soybeans at low temperatures. Crop Sci. 1981, 21, 933. [Google Scholar] [CrossRef]
- Schor, A.; Fossati, A.; Soldat, A.; Stamp, P. Cold tolerance in soybean (Glycine max L. Merr.) in relation to flowering habit, pod set and compensation for lost reproductive organs. Eur. J. Agron. 1993, 2, 173–178. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hampton, J.G.; Hill, M.J. Soybean development under the cool temperate environment of Canterbury, New Zealand. J. New Seeds 2008, 7, 17–36. [Google Scholar] [CrossRef]
- Rose, G.; Osborne, T.; Greatrex, H.; Wheeler, T. Impact of progressive global warming on the global-scale yield of maize and soybean. Clim. Chang. 2016, 134, 417–428. [Google Scholar] [CrossRef]
- Wamsler, C.; Niven, L.; Beery, T.H.; Bramryd, T.; Ekelund, N.; Jönsson, K.I.; Osmani, A.; Palo, T.; Stålhammar, S. Operationalizing ecosystem-based adaptation: Harnessing ecosystem services to buffer communities against climate change. Ecol. Soc. 2016, 21, 31. [Google Scholar] [CrossRef]
- Poehlman, J.M. Breeding Soybeans. In Breeding Field Crops; Springer: Dordrecht, The Netherlands, 1987. [Google Scholar] [CrossRef]
- Kurasch, A.K.; Hahn, V.; Leiser, W.L.; Vollmann, J.; Schori, A.; Bétrix, C.A.; Mayr, B.; Winkler, J.; Mechtler, K.; Aper, J.; et al. Identification of mega-environments in Europe and effect of allelic variation at maturity E loci on adaptation of European soybean. Plant Cell Environ. 2017, 40, 765–778. [Google Scholar] [CrossRef]
- Zhang, L.X.; Kyei-Boahen, S.; Zhang, J.; Zhang, M.H.; Freeland, T.B.; Watson, C.E.; Liu, X. Modifications of optimum adaptation zones for soybean maturity groups in the USA. Crop Manag. 2007, 6, 1–10. [Google Scholar] [CrossRef]
- Alliprandini, L.F.; Abatti, C.; Bertagnolli, P.F.; Cavassim, J.E.; Gabe, H.L.; Kurek, A.; Matsumoto, M.N.; Rott de Oliveira, M.A.; Pitol, C.; Prado, L.C.; et al. Understanding soybean maturity groups in Brazil: Environment, cultivar classification, and stability. Crop Sci. 2009, 49, 801–808. [Google Scholar] [CrossRef]
- Dardanelli, J.L.; Balzarini, M.; Martínez, M.J.; Cuniberti, M.; Resnik, S.; Ramunda, S.F.; Herrero, R.; Baigorri, H. Soybean maturity groups, environments, and their interaction define mega-environments for seed composition in Argentina. Crop Sci. 2006, 46, 1939–1947. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Jones, C.A. Growth and Mineral Nutrition of Field Crops; CRC Press: Boca Raton, FL, USA, 2010; p. 590. [Google Scholar]
- Kumagai, E.; Sameshima, R. Genotypic differences in soybean yield responses to increasing temperature in a cool climate are related to maturity group. Agric. Forest Meteorol. 2014, 198–199, 265–272. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Gaspar, A.P.; Naeve, S.L.; Conley, S.P. Planting date, maturity, and temperature effects on soybean seed yield and composition. Agron. J. 2017, 109, 2040–2049. [Google Scholar] [CrossRef]
- Ghosh, D.; Xu, J. Abiotic stress responses in plant roots: A proteomics perspective. Front. Plant Sci. 2014, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, N.; Deswal, R. The molecular biology of the low-temperature response in plants. Bioessays 2005, 27, 1048–1059. [Google Scholar] [CrossRef]
- Lee, D.-G.; Ahsan, N.; Lee, S.-H.; Lee, J.J.; Bahk, J.D.; Kang, K.Y.; Lee, B.-H. Chilling stress-induced proteomic changes in rice roots. J. Plant Physiol. 2009, 166, 1–11. [Google Scholar] [CrossRef]
- Dumont, E.; Bahrman, N.; Goulas, E.; Valot, B.; Sellier, H.; Hilbert, J.L.; Vuylsteker, C.; Lejeune-Hénaut, I.; Delbreil, B. A proteomic approach to decipher chilling response from cold acclimation in pea (Pisum sativum L.). Plant Sci. 2011, 180, 86–98. [Google Scholar] [CrossRef]
- Mckhann, H.I.; Gery, C.; Berard, A.; Leveque, S.; Zuther, E.; Hincha, D.K.; De Mita, S.; Brunel, D.; Téoulé, E. Natural variation in CBF gene sequence, gene expression and freezing tolerance in the Versailles core collection of Arabidopsis thaliana. BMC Plant Biol. 2008, 8, 105. [Google Scholar] [CrossRef]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef]
- Hossain, Z.; Khatoon, A.Z.; Komatsu, S. Soybean proteomics for unraveling abiotic stress response mechanism. J. Proteome Res. 2013, 12, 4670–4684. [Google Scholar] [CrossRef] [PubMed]
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef]
- Gibson, L.R.; Mullen, R.E. Influence of day and night temperature on soybean seed yield. Crop Sci. 1996, 36, 98–104. [Google Scholar] [CrossRef]
- Wheeler, T.R.; Craufurd, P.Q.; Ellis, R.H.; Porter, J.R.; Prasad, P.V.V. Temperature variability and the yield of annual crops. Agric. Ecosyst. Environ. 2000, 82, 159–167. [Google Scholar] [CrossRef]
- Miransari, M. Handling soybean (Glycine_max L.) under stress. In Crop Improvement; Hakeem, K., Ahmad, P., Ozturk, M., Eds.; Springer: Boston, MA, USA, 2013; pp. 421–439. [Google Scholar] [CrossRef]
- Schlenker, W.; Roberts, M.J. Estimating the Impact of Climate Change on Crop Yields: The Importance of Nonlinear Temperature Effects; Working Paper; National Bureau of Economic Research: Cambridge, MA, USA, 2008; p. 13799. [Google Scholar] [CrossRef]
- Dornbos, D.L.; Mullen, R.E. Influence of stress during soybean seed fill on seed weight, germination, and seedling growth rate. Can. J. Plant Sci. 1991, 71, 373–383. [Google Scholar] [CrossRef]
- Ren, S.; Bilyeu, K.D.; Beuselinck, P.R. Composition, vigor, and proteome of mature soybean seeds developed under high temperature. Crop Sci. 2009, 49, 1010–1022. [Google Scholar] [CrossRef]
- Mustafa, H.S.B.; Hasan, E.; Hassan, M.; Sarwar, S.; Qayyum, A.; Mahmood, T. Influence of Climatic Conditions on Chemical Configuration of Seeds in Safflower, Soybean, Linseed and Sesame. Nat. Sci. 2016, 14, 125–140. [Google Scholar] [CrossRef]
- Hinson, K.; Hartwig, E.E. Soybean Production in the Tropics; FAO Plant Production and Protection Paper; FAO: Roma, Italy, 1982; Volume 4, pp. 2–12. [Google Scholar]
- Keigley, P.J.; Mullen, R.E. Changes in soybean seed quality from high temperature during seed fill and maturation. Crop Sci. 1986, 26, 1212–1216. [Google Scholar] [CrossRef]
- Spears, J.F.; TeKrony, D.M.; Egli, D.B. Temperature during seed filling and soybean seed germination and vigour. Seed Sci. Technol. 1997, 25, 233–244. [Google Scholar]
- Egli, D.B.; TeKrony, D.M.; Heitholt, J.J.; Rupe, J. Air temperature during seed filling and soybean seed germination and vigor. Crop Sci. 2005, 45, 1329–1335. [Google Scholar] [CrossRef]
- Salem, M.A.; Kakani, V.G.; Koti, S.; Reddy, K.R. Pollen-based screening of soy- bean genotypes for high temperatures. Crop Sci. 2007, 47, 219–231. [Google Scholar] [CrossRef]
- Charng, Y.Y.; Liu, H.C.; Liu, N.Y.; Chi, W.T.; Wang, C.N.; Chang, S.H.; Wang, T.T. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007, 143, 251–262. [Google Scholar] [CrossRef]
- Yoshida, T.; Ohama, N.; Nakajima, J.; Kidokoro, S.; Mizoi, J.; Nakashima, K.; Maruyama, K.; Kim, J.-M.; Seki, M.; Todaka, D.; et al. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol. Genet. Genom. 2011, 286, 321–332. [Google Scholar] [CrossRef]
- Thomas, J.M.G.; Boote, K.J.; Allen, L.H.; Gallo-Meagher, M.; Davis, J.M. Elevated temperature and carbon dioxide effects on soybean seed composition and transcript abundance. Crop Sci. 2003, 43, 1548–1557. [Google Scholar] [CrossRef]
- Wilson, R.F. Seed composition. In Soybeans: Improvement, Production, and Uses; Boerma, H.R., Specht, J.E., Eds.; The American Society of America, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2004; pp. 621–677. [Google Scholar]
- Yaklich, R.W.; Vinyard, B.T. A method to estimate soybean seed protein and oil concentration before harvest. J. Am. Oil Chem. Soc. 2004, 81, 1021–1027. [Google Scholar] [CrossRef]
- Dornbos, D.L.; Mullen, R.E. Soybean seed protein and oil contents and fatty acid composition adjustments by drought and temperature. J. Am. Oil. Chem. Soc. 1992, 69, 228–231. [Google Scholar] [CrossRef]
- Alsajri, F.A.; Wijewardana, C.; Irby, J.T.; Bellaloui, N.; Krutz, L.J.; Golden, B.; Gao, W.; Reddy, R.K. Developing functional relationships between temperature and soybean yield and seed quality. Agron. J. 2020, 112, 194–204. [Google Scholar] [CrossRef]
- Khan, A.Z.; Shah, P.; Khan, H.; Nigar, S.; Perveen, S.; Shah, M.K.; Amanullah; Khalil, S.K.; Munir, S.; Zubair, M. Seed quality and vigor of soybean cultivars as influenced by canopy temperature. Pak. J. Bot. 2011, 43, 643–648. Available online: http://www.pakbs.org/pjbot/PDFs/43(1)/PJB43(1)643.pdf (accessed on 2 August 2022).
- Wolf, R.B.; Cavins, J.F.; Kleiman, R.; Black, L.T. Effect of temperature on soybean seed constituents: Oil, protein, moisture, fatty acids, amino acid, and sugars. J. Am. Oil Chem. Soc. 1982, 59, 230–232. [Google Scholar] [CrossRef]
- Nakagawa, A.C.S.; Ario, N.; Tomita, Y.; Tanaka, S.; Murayama, N.; Mizuta, C.; Iwaya-Inoue, M.; Ishibashi, Y. High temperature during soybean seed development differentially alters lipid and protein metabolism. Plant Prod. Sci. 2020, 23, 504–512. [Google Scholar] [CrossRef]
- Rotundo, J.L.; Miller-Garvin, J.E.; Naeve, S.L. Regional and temporal variation in soybean seed protein and oil across the United States. Crop Sci. 2016, 56, 797–808. [Google Scholar] [CrossRef]
- Kołodziej, J.; Pisulewska, E. Wpływ czynników meteorologicznych na plon nasion i tłuszczu oraz zawartość tłuszczu w nasionach dwóch odmian soi. Rośliny Oleiste-Oilseed Crops 2000, 21, 759–773. Available online: https://yadda.icm.edu.pl/agro/element/bwmeta1.element.agro-article-f4b4538a-b39c-455e-a468-52ca8b44ea05 (accessed on 2 August 2022). (In Polish).
- Rotundo, J.L.; Westgate, M.E. Meta-analysis of environmental effects on soybean seed composition. Field Crops Res. 2009, 110, 147–156. [Google Scholar] [CrossRef]
- Rinker, K.; Nelson, R.; Specht, J.; Sleper, D.; Cary, R.; Cianzio, S.; Casteel, S.; Conley, S.; Chen, P.; Davis, V.; et al. Genetic improvement of soybean in maturity groups II, III, and IV. Crop Sci. 2014, 54, 1–14. [Google Scholar] [CrossRef]
- Patil, G.; Mian, R.; Vuong, T.; Pantalone, V.; Song, Q.; Chen, P.; Shannon, J.; Carter, T.C.; Nguyen, H.T. Molecular mapping and genomics of soybean seed protein: A review and perspective for the future. Theor. Appl. Genet. 2017, 130, 1975–1991. [Google Scholar] [CrossRef] [PubMed]
- Starck, Z. Wpływ warunków stresowych na kondycję wytwarzania i dystrybucji fotoasymilatów/Effect of stress conditions on coordination of photosynthetic production and resources allocation. Zesz. Probl. Post. Nauk Rol. 2010, 62, 9–26. [Google Scholar]
- Górski, T.; Kozyra, J.; Doroszewski, A. Field crop losses in Poland due to extreme weather conditions: Case studies. In The Influence of Extreme Phenomena on the Natural Environment and Human Living Conditions; Liszewski, S., Ed.; ŁTN: Łodź, Poland, 2008; pp. 35–49. [Google Scholar]
- Gerten, D.; Rost, S. Climate Change Impacts on Agricultural Water Stress and Impact Mitigation Potential; Background note to the World Development Report 2010; World Bank: Washington, DC, USA, 2009; Available online: https://publications.pik-potsdam.de/pubman/item/item_16181 (accessed on 10 December 2022).
- Karaczun, Z.; Kozyra, J. Wpływ Zmiany Klimatu na Bezpieczeństwo Żywnościowe Polski; Wydawnictwo SGGW: Warszawa, Poland, 2020; p. 120. Available online: http://zgpke.pl/wp-content/uploads/2020/11/Raport_Klimat_bezpieczenstwo_zywienowe_Karaczun_20.03.pdf (accessed on 2 August 2022). (In Polish)
- Souza, G.M.; Catuchi, T.A.; Bertolli, S.C.; Soratto, R.P. Soybean under water deficit: Physiological and yield responses. In A Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships; INTECH: London, UK, 2013; pp. 273–298. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Mohapatra, P.K.; Fujita, K. Differences in the responses of stem diameter and pod thickness to drought stress during the grain filling stage in soybean plants. Acta Physiol Plant. 2009, 31, 271–277. [Google Scholar] [CrossRef]
- Catuchi, T.A.; Guidorizzi, F.V.C.; Guidorizi, K.A.; Barbosa, A.M.; Souza, G.M. Respostas fisiológicas de cultivares de soja à adubação potássica sob diferentes regimes hídricos. Pesq. Agropec. Bras. 2012, 47, 519–527. [Google Scholar] [CrossRef]
- Da Mota, F.S. Soya bean and weather. World Meteorological Organization (WMO). Tech. Not. 1978, 498, 64. [Google Scholar]
- Michałek, S.; Borowski, E. Seed Germination and Seedling Growth of the Polish Soybean (Glycine max (L.) Merr.) Cultivars in Drought Conditions/Kiełkowanie Nasion i Wzrost Siewek Krajowych Odmian soi (Glycine max (L.) Merr.) w Warunkach suszy. 2002, 223/224, 195–201. Available online: https://www.researchgate.net/publication/303043373_Seed_germination_and_seedling_growth_of_the_soybean_Glycine_max_L_Merr_cultivars_in_drought_conditions (accessed on 2 August 2022). (In Polish).
- Hafeez, Y.; Iqbal, S.; Jabeen, K.; Shahzad, S.; Jahan, S.; Rasul, F. Effect of biochar application on seed germination and seedling growth of Glycine max (L.) Merr. under drought stress. Pak. J. Bot. 2017, 49, 7–13. Available online: https://www.cabdirect.org/cabdirect/abstract/20173269117 (accessed on 2 August 2022).
- Desclaux, D.; Huynh, T.T.; Roumet, P. Identification of soybean plant characteristics that indicate the timing of drought stress. Crop Sci. 2000, 40, 716–722. [Google Scholar] [CrossRef]
- Staniak, M.; Stępień, A.; Czopek, K. Reakcja soi zwyczajnej (Glycine max (L.) Merr.) na wybrane stresy abiotyczne. Studia i Raporty IUNG-PIB 2018, 57, 51–62. Available online: https://iung.pl/sir/zeszyt57_5.pdf (accessed on 2 August 2022). (In Polish).
- Akýnci, S.; Lösel, D.M. Plant water-stress response mechanisms. In Water Stress; Rahman, I.M.M., Hasegawa, H., Eds.; InTech: London, UK, 2012; pp. 15–42. Available online: https://studylib.net/doc/18748898/plant-water-stress-response-mechanisms (accessed on 2 August 2022).
- Borowska, M.; Prusiński, J. Effect of soybean cultivars sowing dates on seed yield and its correlation with yield parameters. Plant Soil Environ. 2021, 67, 360–366. [Google Scholar] [CrossRef]
- Mandić, V.; Krnjaja, V.; Tomić, Z.; Bijelić, Z.; Simić, A.; Đorđević, S.; Stanojković, A.; Gogić, M. Effect of water stress on soybean production. In Proceedings of the 4th International Congress New Perspectives an Challenges of Sustainable Livestock Production, Belgrade, Serbia, 7–9 October 2015; Available online: http://r.istocar.bg.ac.rs/handle/123456789/602 (accessed on 2 August 2022).
- Sadeghipour, O.; Abbasi, S. Soybean response to drought and seed inoculation. World Appl. Sci. J. 2012, 17, 55–56. [Google Scholar]
- Sionit, N.; Kramer, P.J. Effect of water stress during different stages of growth of soybean. Agron. J. 1977, 69, 274–278. [Google Scholar] [CrossRef]
- de Souza, P.I.; Egli, D.B.; Bruening, W.P. Water stress during seed filling and leaf senescence in soybean. Agron. J. 1997, 89, 807–812. [Google Scholar] [CrossRef]
- Michałek, S.; Borowski, E. Plonowanie oraz zawartość tłuszczu, kwasów tłuszczowych i białka w nasionach krajowych odmian soi w warunkach suszy/Yielding, oil, fatty acids and protein content in the seeds of Polish soybean cultivars under drought conditions. Acta Agroph. 2006, 8, 459–471. (In Polish) [Google Scholar]
- Eck, H.V.; Mathers, A.C.; Musick, J.Y. Plant water stress at various growth stages and growth and yield of soybeans. Field Crops Res. 1987, 17, 716–722. [Google Scholar] [CrossRef]
- Frieler, K.; Schauberger, B.; Arneth, A.; Balkovič, J.; Chryssanthacopoulos, J.; Deryng, D.; Elliott, J.; Folberth, C.; Khabarov, N.; Müller, C.; et al. Understanding the weather signal in national crop-yield variability. Earth’s Future 2017, 5, 605–616. [Google Scholar] [CrossRef] [PubMed]
- di Mauro, G.; Borrás, L.; Rugeroni, P.; Rotundo, J.L. Exploring soybean management options for environments with contrasting water availability. J. Agron. Crop Sci. 2019, 205, 274–282. [Google Scholar] [CrossRef]
- Kobraee, S.; Shamsi, K. Effect of drought stress on dry matter accumulation and morphological traits in soybean. Int. J. Biosci. 2012, 10, 73–79. [Google Scholar]
- Ergo, V.V.; Lascano, R.; Vega, C.R.C.; Parola, R.; Carrera, C.S. Heat and water stressed field-grown soybean: A multivariate study on the relationship between physiological-biochemical traits and yield. Environ. Exp. Bot. 2017, 148, 1–11. [Google Scholar] [CrossRef]
- Napoles, M.C.; Guevara, E.; Montero, F.; Rossi, A.; Ferreira, A. Role of Bradyrhizobium japonicum induced by genistein on soybean stressed by water deficit. Span. J. Agric. Res. 2009, 7, 665–671. [Google Scholar] [CrossRef]
- Kunert, K.J.; Vorster, B.J.; Fenta, B.A.; Kibido, T.; Dionisio, G.; Foyer, C.H. Drought stress responses in soybean roots and nodules. Front. Plant Sci. 2016, 7, 1015. [Google Scholar] [CrossRef]
- Korsak-Adamowicz, M.; Starczewski, J.; Dopka, D. Oddziaływanie niektórych zabiegów agrotechnicznych na brodawkowanie soi. Fragm. Agron. 2007, 3, 232–237. (In Polish) [Google Scholar]
- Arrese-Igor, C.; González, E.; Marino, D.; Ladrera, R.; Larrainzar, E.; Gil-Quintana, E. Physiological response of legume nodules to drought. Plant Stress 2011, 5, 24–31. [Google Scholar]
- Collier, R.; Tegeder, M. Soybean ureide transporters play a critical role in nodule development, function and nitrogen export. Plant J. 2012, 72, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Purcell, L.C.; Serraj, R.; Sinclair, T.R.; De, A. Soybean N2 fixation estimates, ureide concentration, and yield responses to drought. Crop Sci. 2004, 44, 484–492. [Google Scholar] [CrossRef]
- Oya, T.; Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B.; Tobita, S.; Ito, O. Drought tolerance characteristics of Brazilian soybean cultivars—Evaluation and characterization of drought tolerance of various Brazilian soybean cultivars in the field. Plant. Prod. Sci. 2004, 7, 129–137. [Google Scholar] [CrossRef]
- Maleki, A.; Naderi, A.; Naseri, R.; Fathi, A.; Bahamin, S.; Maleki, R. Physiological performance of soybean cultivars under drought stress. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 38–44. [Google Scholar]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Xie, F. Effect of drought stress at reproductive stages on growth and nitrogen metabolism in soybean. Agronomy 2020, 10, 302. [Google Scholar] [CrossRef]
- Dayoub, E.; Lamichhane, J.R.; Schoving, C.; Debaeke, P.; and Maury, P. Early-stage phenotyping of root traits provides insights into the drought tolerance level of soybean cultivars. Agronomy 2021, 11, 188. [Google Scholar] [CrossRef]
- Buezo, J.; Sanz-Saez, A.; Moran, J.F.; Soba, D.; Aranjuelo, I.; Esteban, R. Drought tolerance response of high-yielding soybean varieties to mild drought: Physiological and photochemical adjustments. Physiol. Plant. 2019, 166, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.S.; Au-Yeung, W.K.; Yung, Y.L.; Li, M.W.; Wen, C.Q.; Liu, X.; Lam, H.M. Drought stress and tolerance in soybean. In A Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships; Board, J.E., Ed.; IntechOpen: London, UK, 2013; pp. 209–237. [Google Scholar] [CrossRef]
- Wijewardana, C.; Reddy, K.R.; Bellaloui, N. Soybean seed physiology, quality, and chemical composition under soil moisture stress. Food Chem. 2019, 278, 92–100. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Lotfi, R. Influence of Water Stress and Pod Position on Oil and Protein Accumulation in Soybean Grains. Inter. J. Agron. Plant Prod. 2013, 4, 2341–2345. [Google Scholar]
- Fecak, P.; Sarikova, D.; Cerny, I. Influence of tillage system and starting fertilization on seed yield and quality of soybean Glycine max (L.) Merrill. Plant Soil Environ. 2010, 56, 105–110. [Google Scholar] [CrossRef]
- Lorenc-Kozik, A.M.; Pisulewska, E.; Gondek, K. Wpływ warunków pogodowych na skład chemiczny trzech odmian soi. Ecol. Chem. Eng. 2011, 18, 1079–1085. (In Polish) [Google Scholar]
- Vollmann, J.; Fritz, C.N.; Wagentristl, H.; Ruckenbauer, P. Environmental and genetic variation of soybean seed protein content under Central European growing conditions. J. Sci. Food Agric. 2000, 80, 1300–1306. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Solanki, S.; Hussain, S.M. Influence of growing environment on the biochemicall composition and physical characteristics of soybean seeds. J. Food Comp. Anal. 2006, 19, 188–195. [Google Scholar] [CrossRef]
- Toorchi, M.; Yukawa, K.; Nouri, M.Z.; Komatsu, S. Proteomics approach for identifying osmotic-stress-related proteins in soybean roots. Peptides 2009, 30, 2108–2117. [Google Scholar] [CrossRef]
- Alam, I.; Sharmin, S.A.; Kim, K.H.; Yang, J.K.; Choi, M.S.; Lee, B.H. Proteome analysis of soybean roots subjected to short-term drought stress. Plant Soil 2010, 333, 491–505. [Google Scholar] [CrossRef]
- Mohammadi, P.P.; Moieni, A.; Hiraga, S.; Komatsu, S. Organ-specific proteomic analysis of drought-stressed soybean seedlings. J. Proteom. 2012, 75, 1906–1923. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, W.; Zhao, Q.; Zhou, X.; Jiang, L.; Ma, S.; Liu, X.; Li, Y.; Zhang, C.; Fan, Y.; et al. Analysis of weighted co-regulatory networks in maize provides insights into new genes and regulatory mechanisms related to inositol phosphate metabolism. BMC Genom. 2016, 17, 129. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Bonthala, V.S.; Khandelwal, R.; Jaishankar, J.; Shweta, S.; Nawaz, K.; Prasad, M. Global analysis of WRKY transcription factor superfamily in Setaria identifies potential candidates involved in abiotic stress signaling. Front. Plant Sci. 2015, 6, 910. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Bai, X.; Zhu, D.; Li, Y.; Ji, W.; Cai, H.; Wu, J.; Liu, B.; Zhu, Y. GsZFP1, a new Cys2/His2-type zinc-finger protein, is a positive regulator of plant tolerance to cold and drought stress. Planta 2011, 235, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Striker, G.G. Flooding stress on plants: Anatomical, morphological and physiological responses. In Flooding Stress on Plants: Anatomical, Morphological and Responses; Mworia, J.K., Ed.; InTechOpen: London, UK, 2012; pp. 3–28. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Y.; Chen, P.; Zhang, F.; Li, J.; Yan, F.; Dong, Y.; Feng, B. How does the waterlogging regime affect crop yield? A global meta-analysis. Front. Plant Sci. 2021, 12, 634898. [Google Scholar] [CrossRef] [PubMed]
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of winter crops at early and late stages: Impacts on leaf physiology, growth and yield. Front. Plant Sci. 2018, 9, 1863. [Google Scholar] [CrossRef]
- Sairam, R.K.; Dharmar, K.; Chinnusamy, V.; Meena, R.C. Waterlogging-induced increase in sugar mobilization, fermentation, and related gene expression in the roots of mung bean (Vigna radiata). J. Plant Physiol. 2009, 166, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.; Bailey-Serres, J. Flooding tolerance: O2 sensing and survival strategies. Curr. Opin. Plant Biol. 2013, 16, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.; VanToai, T.; Fausey, N.; Beuerlein, J.; Parkinson, R.; Soboyejo, A. Evaluating On-Farm Flooding Impacts on Soybean. Crop Sci. 2001, 41, 93–100. [Google Scholar] [CrossRef]
- Linkemer, G.; Board, J.E.; Musgrave, M.E. Waterlogging effect on growth and yield components of late-planted soybean. Crop Sci. 1998, 38, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.D.; DeAngulo, J.; Daniels, M.B.; Wood, L.S. Flood duration effects on soybean growth and yield. Agron. J. 1989, 81, 631–636. [Google Scholar] [CrossRef]
- Oosterhuis, D.M.; Scott, H.D.; Hampton, R.E.; Wullschleger, S.D. Physiological responses of two soybean (Glycine max (L.) Merr.) cultivars to short-term flooding. Env. Exp. Bot. 1990, 30, 85–92. [Google Scholar] [CrossRef]
- Board, J.E. Waterlogging effects on plant nutrient concentrations in soybean. J. Plant Nutr. 2008, 31, 828–838. [Google Scholar] [CrossRef]
- Ara, R.; Mannan, M.A.; Khaliq, Q.A.; Uddin Miah, M.M. Waterlogging tolerance of soybean. Bangladesh Agron. J. 2015, 18, 105–109. [Google Scholar] [CrossRef]
- Jackson, M.B.; Ram, P.C. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Ann. Bot. 2003, 91, 227–241. [Google Scholar] [CrossRef]
- Nanjo, Y.; Skultety, L.; Ashraf, Y.; Komatsu, S. Comparative proteomic analysis of early-stage soybean seedlings responses to flooding by using gel and gel-free techniques. J. Proteome Res. 2010, 9, 3989–4002. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.J.; Oyanagi, A.; Komatsu, S. Cell wall proteome of wheat roots under flooding stress using gel-based and LCMS/MS-based proteomics approaches. Biochim. Biophys. Acta 2010, 1804, 124–136. [Google Scholar] [CrossRef]
- Shi, F.; Yamamoto, R.; Shimamura, S.; Hiraga, S.; Nakayama, N.; Nakamura, T.; Yukawa, K.; Hachinohe, M.; Matsumoto, H.; Komatsu, S. Cytosolic ascorbate peroxidase 2 (cAPX 2) is involved in the soybean response to flooding. Phytochemistry 2008, 69, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Hiraga, S.; Yanagawa, Y. Proteomics techniques for the development of flood tolerant crops. J. Proteome Res. 2012, 11, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Sugimoto, T.; Hoshino, T.; Nanjo, Y.; Furukawa, K. Identification of flooding stress responsible cascades in root and hypocotyl of soybean using proteome analysis. Amino Acids 2010, 38, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Xiong, L. Genetic mechanisms conferring adaptation to submergence and drought in rice: Simple or complex? Curr. Opin. Plant Biol. 2013, 16, 196–204. [Google Scholar] [CrossRef]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Roland, P.C.; Mackill, D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef]
- Tamang, B.G.; Fukao, T. Plant adaptation to multiple stresses during submergence and following desubmergence. Int. J. Mol. Sci. 2015, 16, 30164–30180. [Google Scholar] [CrossRef]
- Pucciariello, C.; Perata, P. New insights into reactive oxygen species and nitric oxide signalling under low oxygen in plants. Plant Cell Environ. 2017, 40, 473–482. [Google Scholar] [CrossRef]
- Holman, T.J.; Jones, P.D.; Russell, L.; Medhurst, A.; Ubeda Tomás, S.; Talloji, P.; Marquez, J.; Schmuths, H.; Tung, S.-A.; Taylor, I.; et al. The N-end rule pathway promotes seed germination and establishment through removal of ABA sensitivity in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 4549–4554. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.J.; Md Isa, N.; Movahedi, M.; Lozano-Juste, J.; Mendiondo, G.M.; Berckhan, S.; Marín-de la Rosa, N.; Vicente Conde, J.; Sousa Correia, C.; Pearce, S.P.; et al. Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Mol. Cell 2014, 53, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Graciet, E.; Walter, F.; Maoileidigh, D.O.; Pollmann, S.; Meyerowitz, E.M.; Varshavsky, A.; Wellmer, F. The N-end rule pathway controls multiple functions during Arabidopsis shoot and leaf development. Proc. Natl. Acad. Sci. USA 2009, 106, 13618–13623. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Berckhan, S.; Rooney, D.J.; Gibbs, D.J.; Vicente Conde, J.; Sousa Correia, C.; Bassel, G.W.; Marín-de la Rosa, N.; León, J.; Alabadí, D.; et al. Oxygen sensing coordinates photomorphogenesis to facilitate seedling survival. Curr. Biol. 2015, 25, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, D.T.; Dias, D.C.F.; Medeiros, A.D.; Ribeiro, J.P.O.; Silva, F.L.; Silva, L.J. Weathering deterioration in pre-harvest of soybean seeds: Physiological, physical, and morpho-anatomical changes. Sci. Agric. 2021, 78, e20200166. [Google Scholar] [CrossRef]

| Number of Days with Chilling Stress | Root Lenght (cm) | Dry Mass (mg·g−1) | ||
|---|---|---|---|---|
| 25 °C | 10 °C | 25 °C | 10 °C | |
| 0 | 3.7 ± 0.4 | 3.7 ± 0.4 | 78.0 ± 7.0 | 78.0 ± 7.0 |
| 1 | 5.0 ± 0.4 | 3.9 ± 0.7 | 79.0 ± 6.0 | 76.5 ± 10.5 |
| 2 | 6.6 ± 3.3 | 4.0 ± 0.5 | 81.0 ± 8.0 | 79.5 ± 6.5 |
| 3 | 7.4 ± 0.4 | 4.1 ± 0.9 | 80.0 ± 10.2 | 79.0 ± 7.8 |
| 4 | 8.4 ± 1.8 | 4.1 ± 0.4 | 76.0 ± 5.6 | 79.5 ± 10.1 |
| Freezing Temperature (°C) | Development Stage | |||
|---|---|---|---|---|
| Hypocotyl Arch (1 Week after Planting) | Fully Expanded Cotyledons (2 Weeks after Planting) | First Trifoliolate Emerged (3 Weeks after Planting) | Second Trifoliolate Emerged (4 Weeks after Planting) | |
| −2 | 100 | 94 | 94 | 87 |
| −4 | 81 | 56 | 63 | 44 |
| −6 | 75 | 19 | 0 | 13 |
| −8 | 0 | 0 | 0 | 0 |
| Features | Natural Conditions (Average 24/17 °C) | 7-Day Cold Stress (17/13 °C) | NIR (p = 0.05) Tukey Test |
|---|---|---|---|
| Plant height (cm) | 79.6 | 74.8 | 2.57 |
| Number of nodes | 11.0 | 10.2 | 0.34 |
| Stem dry mass (g) | 5.35 | 4.56 | 0.51 |
| Number of pods per plant | 18.0 | 14.9 | 1.59 |
| Weight of pods per plant (g) | 12.0 | 9.3 | 0.96 |
| Number of seeds per pod | 2.36 | 2.27 | 0.055 |
| Number of seeds per plant | 41.7 | 32.5 | 3.13 |
| Weight of seeds per plant (g) | 8.17 | 6.61 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staniak, M.; Szpunar-Krok, E.; Kocira, A. Responses of Soybean to Selected Abiotic Stresses—Photoperiod, Temperature and Water. Agriculture 2023, 13, 146. https://doi.org/10.3390/agriculture13010146
Staniak M, Szpunar-Krok E, Kocira A. Responses of Soybean to Selected Abiotic Stresses—Photoperiod, Temperature and Water. Agriculture. 2023; 13(1):146. https://doi.org/10.3390/agriculture13010146
Chicago/Turabian StyleStaniak, Mariola, Ewa Szpunar-Krok, and Anna Kocira. 2023. "Responses of Soybean to Selected Abiotic Stresses—Photoperiod, Temperature and Water" Agriculture 13, no. 1: 146. https://doi.org/10.3390/agriculture13010146
APA StyleStaniak, M., Szpunar-Krok, E., & Kocira, A. (2023). Responses of Soybean to Selected Abiotic Stresses—Photoperiod, Temperature and Water. Agriculture, 13(1), 146. https://doi.org/10.3390/agriculture13010146








