Relationship of Textures from Tomato Fruit Images Acquired Using a Digital Camera and Lycopene Content Determined by High-Performance Liquid Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Image Analysis
2.3. Lycopene Extraction
2.4. HPLC Analysis of Lycopene
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gwóźdź, E.; Gębczyński, P. Owoce pomidora źródłem karotenoidów [Tomato fruit as a source of carotenoids]. Med. Rodz. 2017, 20, 211–214. [Google Scholar]
- Fanasca, S.; Colla, G.; Maiani, G.; Veneria, E.; Rouphael, Y.; Azzini, E.; Saccardo, F. Changes in antioxidant content of tomato fruits in response to cultivar and nutrient solution composition. J. Agric. Food Chem. 2006, 54, 4319–4325. [Google Scholar] [CrossRef] [PubMed]
- Zalewska-Korona, M.; Jabłońska-Ryś, E.; Michalak-Majewska, M. Wartości odżywcze i prozdrowotne owoców pomidora gruntowego [The nutritional and health benefits of ground tomato fruits]. Bromatol. I Chem. Toksykol. 2013, 2, 200–205. [Google Scholar]
- Gullett, N.P.; Amin, A.R.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.-J.; Kucuk, O. Cancer prevention with natural compounds. Semin. Oncol. 2010, 37, 258–281. [Google Scholar] [CrossRef] [PubMed]
- Kelkel, M.; Schumacher, M.; Dicato, M.; Diederich, M. Antioxidant and anti-proliferative properties of lycopene. Free. Radic. Res. 2011, 45, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Laukkanen, J.A.; Makikallio, T.H.; Ronkainen, K.; Kurl, S. Serum β-carotene and the risk of sudden cardiac death in men: A population-based follow-up study. Atherosclerosis 2013, 226, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Igielska-Kalwat, J.; Gościańska, J.; Nowa, I. Carotenoids as natural antioxidants. Postepy Hig. I Med. Doświadczalnej 2015, 69, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Bramley, P. Is lycopene beneficial to human health? Phytochemistry 2000, 54, 233–236. [Google Scholar] [CrossRef]
- Knoblich, M.; Anderson, B.; Latshaw, D. Analyses of tomato peel and seed byprod-ucts and their use as a source of carotenoids. J. Sci. Food Agric. 2005, 85, 1166–1170. [Google Scholar] [CrossRef]
- Hernandez, M.; Rodriguez, E.; Diaz, C. Free hydroxycinnamic acids, lycopene and color parameters in tomato cultivars. J. Agric. Food Chem. 2007, 55, 8604–8615. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Teleky, B.-E.; Ranga, F.; Roman, I.; Khaoula, H.; Boudaya, E.; Ltaief, A.B.; Aouani, W.; Thiamrat, M.; Vodnar, D.C. Carotenoid Recovery from Tomato Processing By-Products through Green Chemistry. Molecules 2022, 27, 3771. [Google Scholar] [CrossRef] [PubMed]
- Kyriakoudi, A.; Tsiouras, A.; Mourtzinos, I. Extraction of Lycopene from Tomato Using Hydrophobic Natural Deep Eutectic Solvents Based on Terpenes and Fatty Acids. Foods 2022, 11, 2645. [Google Scholar] [CrossRef] [PubMed]
- Mare, R.; Maurotti, S.; Ferro, Y.; Galluccio, A.; Arturi, F.; Romeo, S.; Procopio, A.; Musolino, V.; Mollace, V.; Montalcini, T.; et al. A Rapid and Cheap Method for Extracting and Quantifying Lycopene Content in Tomato Sauces: Effects of Lycopene Micellar Delivery on Human Osteoblast-Like Cells. Nutrients 2022, 14, 717. [Google Scholar] [CrossRef] [PubMed]
- Moraru, C.; Lee, T.C. Kinetic studies of lycopene isomerization in a tributyrin model system at gastric pH. J. Agric. Food Chem. 2005, 53, 8997–9004. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, A.J.; Perrone, D.; Martucci, R.B.; Borojevic, R. Lycopene isomerisation and storage in an in vitro model of murine hepatic stellate cells. Eur. J. Nutr. 2009, 48, 261–268. [Google Scholar] [CrossRef]
- Richelle, M.; Sanchez, B.; Tavazzi, I.; Lambelet, P.; Bortlik, K.; Williamson, G. Lycopene isomerisation takes place within enterocytes during absorption in human subjects. Br. J. Nutr. 2010, 103, 1800–1807. [Google Scholar] [CrossRef]
- FAOSTAT. Food Balance Sheets. 2014. Available online: http://faostat.fao.org (accessed on 14 July 2022).
- Apel, W.; Bock, R. Enhancement of carotenoid biosynthesis in transplastomic tomatoes by induced lycopene-to-provitamin A conversion. Plant Physiol. 2009, 151, 59–66. [Google Scholar] [CrossRef]
- Fantini, E.; Falcone, G.; Frusciante, S.; Giliberto, L.; Giuliano, G. Dissection of Tomato Lycopene Biosynthesis through Virus-Induced Gene Silencing. Plant Physiol. 2013, 163, 986–998. [Google Scholar] [CrossRef]
- Lihong, L.; Zhiyong, S.; Min, Z.; Qiaomei, W. Regulation of Carotenoid Metabolism in Tomato. Mol. Plant 2015, 8, 28–39. [Google Scholar]
- Ropelewska, E. The application of image processing for cultivar discrimination of apples based on texture features of the skin, longitudinal section and cross-section. Eur. Food Res. Technol. 2021, 247, 1319–1331. [Google Scholar] [CrossRef]
- Ropelewska, E.; Sabanci, K.; Aslan, M.F. Authentication of tomato (Solanum lycopersicum L.) cultivars using discriminative models based on texture parameters of flesh and skin images. Eur. Food Res. Technol. 2022, 248, 1959–1976. [Google Scholar] [CrossRef]
- Ropelewska, E.; Popińska, W.; Sabanci, K.; Aslan, M.F. Cultivar identification of sweet cherries based on texture parameters determined using image analysis. J. Food Process Eng. 2021, 44, e13724. [Google Scholar] [CrossRef]
- Tanksley, S. The Genetic, Developmental, and Molecular Bases of Fruit Size and Shape Variation in Tomato. Plant Cell 2004, 16, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ropelewska, E.; Mieszczakowska-Frąc, M.; Kruczyńska, D. The evaluation of the usefulness of textures from cross-section images obtained using a digital camera and a flatbed scanner for cultivar discrimination of quince (Cydonia oblonga Mill.). Food Control 2022, 131, 108447. [Google Scholar] [CrossRef]
- Szczypiński, P.M.; Strzelecki, M.; Materka, A.; Klepaczko, A. MaZda—A software package for image texture analysis. Comput. Methods Programs Biomed. 2009, 94, 66–76. [Google Scholar] [CrossRef]
- Ropelewska, E. The Application of Machine Learning for Cultivar Discrimination of Sweet Cherry Endocarp. Agriculture 2021, 11, 6. [Google Scholar] [CrossRef]
- Ropelewska, E. Diversity of Plum Stones Based on Image Texture Parameters and Machine Learning Algorithms. Agronomy 2022, 12, 762. [Google Scholar] [CrossRef]
- Bohoyo-Gil, D.; Dominguez-Valhondo, D.; García-Parra, J.J.; González-Gómez, D. UHPLC as a suitable methodology for the analysis of carotenoids in food matrix. Eur. Food Res. Technol. 2012, 235, 1055–1061. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Int. J. Sci. Rep. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Lenucci, M.S.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant composition in cherry and high-pigment tomato cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E.; Zalewska-Koron, A.M. Ocena barwy oraz zawartości barwników karotenoidowych w owocach pomidora nowych linii hodowlanych [The Colour Analysis and The Content of Carotenoids in Fruits of New Breeding Lines of Tomatoes]. Bromatol. I Chem. Toksykol. 2009, 42, 926–931. [Google Scholar]
- Choi, S.H.; Kim, D.-S.; Kozukue, N.; Kim, H.-J.; Nishitani, Y.; Mizuno, M.; Levin, C.; Friedman, M. Protein, free amino acid, phenolic, β-carotene, and lycopene content, and antioxidative and cancer cell inhibitory effects of 12 greenhouse-grown commercial cherry tomato varieties. J. Food Compos. Anal. 2014, 34, 115–127. [Google Scholar] [CrossRef]
- Del Giudice, R.; Raiola, A.; Tenore, G.C.; Frusciante, L.; Baron, A.; Monti, D.M.; Rigano, M.M. Antioxidant bioactive compounds in tomato fruits at different ripening stages and their effects on normal and cancer cells. J. Funct. Foods 2015, 18, 83–94. [Google Scholar] [CrossRef]
- Yuan, L.; Wenquan, N.; Miles, D.; Jingwei, W.; Xiaoyang, Z. Yields and Nutritional of Greenhouse Tomato in Response to Different Soil Aeration Volume at two depths of Subsurface drip irrigation. Sci. Rep. 2016, 6, 39307. [Google Scholar]
- Erba, D.; Casiraghi, M.C.; Ribas-Agustý, A.; Ca’Ceres, R.; Marfa, O.; Castellari, M. Nutritional value of tomatoes (Solanum lycopersicum L.) grown in greenhouse by different agronomic techniques. J. Food Compos. Anal. 2013, 31, 245–251. [Google Scholar] [CrossRef]
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chem. 2013, 140, 794–802. [Google Scholar] [CrossRef]
- Pugliese, A.; Loizzo, M.R.; Tundis, R.; O’Callaghan, Y.; Galvin, K.; Menichini, F.; O’Brien, N. The effect of domestic processing on the content and bioaccessibility of carotenoids from chili peppers (Capsicum species). Food Chem. 2013, 141, 2606–2613. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef]
- Ropelewska, E.; Jurczak, S.; Bilska, K.; Kulik, T.; Zapotoczny, P. Correlations between the textural features of wheat kernels and the quantity of DNA of Fusarium fungi. Eur. Food Res. Technol. 2019, 245, 1161–1167. [Google Scholar] [CrossRef]
- Nazari, L.; Shaker, M.; Karimi, A.; Ropelewska, E. Correlations between the textural features and chemical properties of sorghum grain using the image processing method. Eur. Food Res. Technol. 2021, 247, 333–342. [Google Scholar] [CrossRef]



| Tomato Cultivar | Fruit Color | Lycopene Content (mg 100 g−1) |
|---|---|---|
| ‘Ożarowski’ | yellow | 0.37 d |
| ‘Marvel Striped’ | yellow-orange-pink | 1.19 c |
| ‘Green Zebra’ | green | 0.31 d |
| Sandoline F1 | red | 9.89 b |
| Cupidissimo F1 | red | 11.74 a |
| Sacher F1 | brown | 11.83 a |
| Texture Parameter | Correlation Coefficient |
|---|---|
| GHPerc90 | −0.99 |
| GHDomn01 | −0.99 |
| GHDomn10 | −0.99 |
| GS5SV1SumAverg | −0.99 |
| GS5SV3SumAverg | −0.99 |
| BHMaxm10 | 0.94 |
| BSGPerc50 | 0.94 |
| BSGPerc90 | 0.94 |
| BS4RZRLNonUni | −0.96 |
| BS4RNGLevNonU | −0.97 |
| Texture Parameter | Correlation Coefficient |
|---|---|
| LHSkewness | 0.92 |
| LHPerc90 | −0.94 |
| LHPerc99 | −0.97 |
| LHDomn01 | −0.91 |
| LHDomn10 | −0.91 |
| aS5SV1InvDfMom | 0.83 |
| aS4RHShrtREmp | −0.95 |
| aS4RVShrtREmp | −0.94 |
| aS4RVFraction | −0.83 |
| aS4RNShrtREmp | −0.83 |
| bHPerc50 | −0.92 |
| bHPerc90 | −0.98 |
| bHPerc99 | −0.99 |
| bHDomn01 | −0.93 |
| bHDomn10 | −0.97 |
| Texture Parameter | Correlation Coefficient |
|---|---|
| XHVariance | −0.98 |
| XHMaxm10 | 0.87 |
| YHVariance | −0.99 |
| YHPerc90 | −0.97 |
| YHPerc99 | −0.97 |
| YHMaxm10 | 0.98 |
| YS5SN5SumOfSqs | −0.94 |
| ZHPerc50 | −0.90 |
| ZHDomn01 | −0.93 |
| Regression Equation | Coefficient of Determination (R2) |
|---|---|
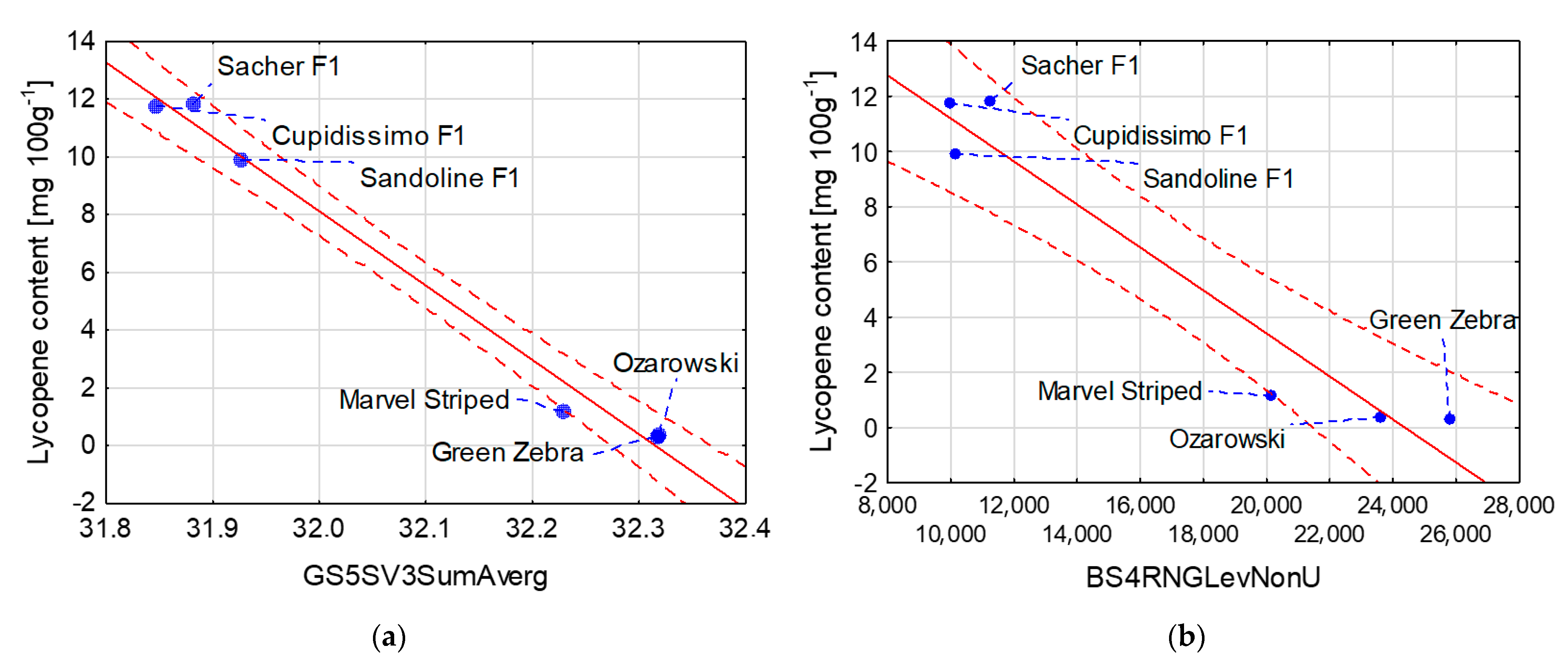
| Lycopene content [mg 100 g−1] = 831.95 – 25.74 × GS5SV3SumAverg | 0.99 |
| Lycopene content [mg 100 g−1] = 18.994 – 0.0008 × BS4RNGLevNonU | 0.94 |
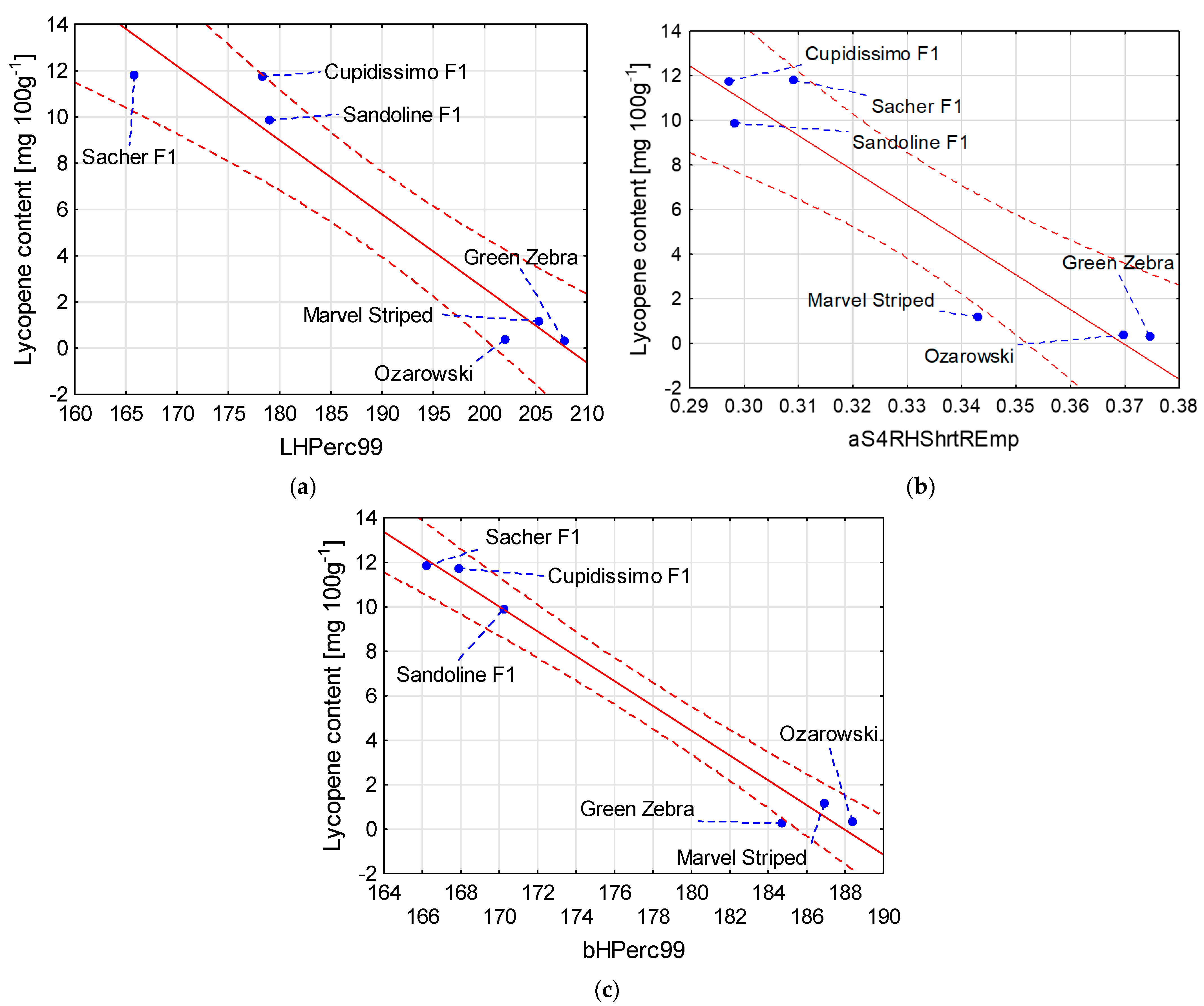
| Lycopene content [mg 100 g−1] = 66.744 – 0.3208 × LHPerc99 | 0.94 |
| Lycopene content [mg 100 g−1] = 57.754 – 156.2 × aS4RHShrtREmp | 0.90 |
| Lycopene content [mg 100 g−1] = 104.82 – 0.5577 × bHPerc99 | 0.98 |
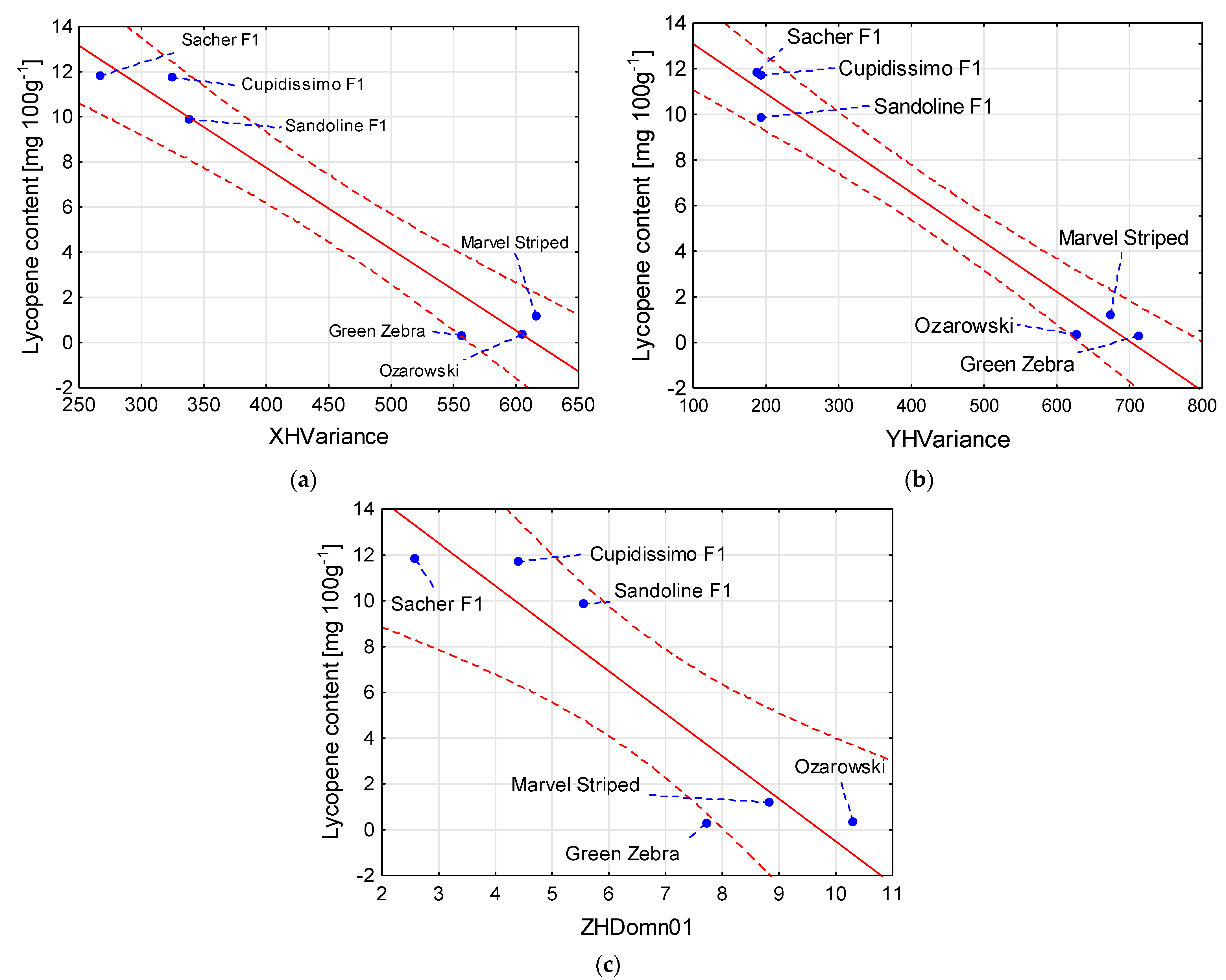
| Lycopene content [mg 100 g−1] = 22.166 – 0.0361 × XHVariance | 0.96 |
| Lycopene content [mg 100 g−1] = 15.251 – 0.0217 × YHVariance | 0.98 |
| Lycopene content [mg 100 g−1] = 18.090 – 1.860 × ZHDomn01 | 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ropelewska, E.; Szwejda-Grzybowska, J. Relationship of Textures from Tomato Fruit Images Acquired Using a Digital Camera and Lycopene Content Determined by High-Performance Liquid Chromatography. Agriculture 2022, 12, 1495. https://doi.org/10.3390/agriculture12091495
Ropelewska E, Szwejda-Grzybowska J. Relationship of Textures from Tomato Fruit Images Acquired Using a Digital Camera and Lycopene Content Determined by High-Performance Liquid Chromatography. Agriculture. 2022; 12(9):1495. https://doi.org/10.3390/agriculture12091495
Chicago/Turabian StyleRopelewska, Ewa, and Justyna Szwejda-Grzybowska. 2022. "Relationship of Textures from Tomato Fruit Images Acquired Using a Digital Camera and Lycopene Content Determined by High-Performance Liquid Chromatography" Agriculture 12, no. 9: 1495. https://doi.org/10.3390/agriculture12091495
APA StyleRopelewska, E., & Szwejda-Grzybowska, J. (2022). Relationship of Textures from Tomato Fruit Images Acquired Using a Digital Camera and Lycopene Content Determined by High-Performance Liquid Chromatography. Agriculture, 12(9), 1495. https://doi.org/10.3390/agriculture12091495






