Abstract
Bovine red offal is one of the main sources of animal proteins. The monitoring of the sanitary status of these foods is mandatory to protect human and animal health against transmitted diseases. The aim of this study was to establish an epidemiological situation of these diseases in the El Oued region. To do so, the registries of red offal seizures from three successive years (2018–2020) of 14,478 slaughtered cattle were retrospectively analyzed. The results showed a continuous evolution of the number of red offal seizures cases mainly in the liver and the lungs. The annual average prevalence of lung lesions increased progressively from 3.45% in 2018 to 10.50% in 2020 while a decrease of about 1% in pulmonary and hepatic hydatid cysts prevalence was observed. For liver diseases, the frequency of seizures increased, also predominately fasciolosis which reached 2.8% in 2020. Concerning tuberculosis, the prevalence did not show significant variations. These findings confirm the value of hygienic control along the entire red offal production chain and the need to strengthen zoonotic diseases prevention to reduce economic losses and to safeguard public health in this arid developing region.
1. Introduction
According to recent official statistics, there are 1.79 million cattle in Algeria [1]. Reared often under the traditional system [2], these animals represent a large part of the Algerian animal wealth, a primordial source of meat and milk and income for the population, thus enhancing food country security [2,3]. However, these foods could represent a source of danger to the consumer [4]. Especially since meats and red offal could be exposed to micro-organisms multiplication or several parasitic diseases [5] and other human harmful substances such as antibiotics residues and hormones [6,7,8]. The liver, the main red offal concerned by fascioliasis [9], and lungs are the predilection sites of bovine hydatid cysts [10], tubercles [11] and other infectious lesions such as abscesses [12]. The condemnation of these organs represents one of the financial losses caused by these diseases [13,14,15].
Edible red offal (liver, lung and heart) has recently received special attention [16]. These meat by-products have a good nutritive quality and their economic value depends entirely on the culture and country in question [17]. In Algeria, as well as in North African countries, red offal is appreciated by Algerians to prepare ethnic meat products such as sausages, and is considered to be a nutrient-rich product that ensures health and well-being [18]. In slaughterhouses, all the conditions and measures necessary to ensure, at all stages of the chain, the safety and wholesomeness of meat, is a matter of concern to any veterinarian. This is done by setting up monitoring and control methods [19,20]. However, in some cases, the absence of veterinary services in rural rearing zones could raise the possibility of major disease outbreaks in cattle herds [21]. Furthermore, this could suggest that most of the animals brought for slaughter may carry subclinical pathologies which are often difficult to identify during antemortem procedures.
From this perspective, slaughterhouse meat and red offal inspection archives are an inexhaustible source of information that could play a significant role in the epidemiology and the struggle against some diseases of concern and prevention campaigns among the population [22,23,24].
In northern Algeria, under the Mediterranean climate, the results of slaughterhouse surveys on the effect of the season vary between a significant presence [25] and absence [26] of a seasonal variation in the condemnation of cattle red offal. In the arid region of southern Algeria, this effect has not been widely discussed. In this area, a single study on ruminant fascioliasis [27] conducted in a slaughterhouse (province of Ouargla) between March 2008 and February 2009 did not report a seasonal variation in liver infestation across the few cases recorded only in cattle.
The purpose of the present study was to investigate the occurrences and prevalence of the main seizure patterns affecting red offal of slaughtered cattle in an arid region of Algeria (El Oued) during a 3-year period (2018 to 2020). The outcomes of this study could serve as remedial actions to preserve consumer health and protect the population against pathological entities.
2. Materials and Methods
2.1. Study Area and Fieldwork
The study was conducted in the El-Oued region which is located in the south-east of Algeria. This region is characterized by a large surface and a long border with Tunisia at the east. The great Oriental Erg covers 2/3 of its territory. Geographically, it is bounded to the north, by the wilayas of Tebessa and Khenchela, to the north and northwest by the wilaya of Biskra and to the south and southeast by the wilaya of Ouargla (Figure 1).
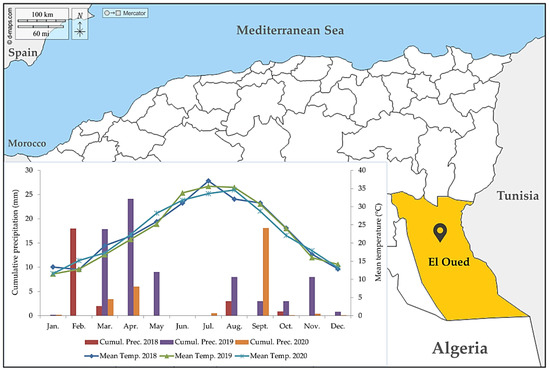
Figure 1.
Geographical location and climatic data (2018–2020) of the wilaya of El Oued.
The prevailing climate in El Oued is desert-like. There is practically no precipitation all year round and the summers are very long. According to the Köppen–Geiger classification, the climate is BWh [28]. The climatic data (temperature and precipitation) for the survey periods (2018, 2019, 2020) are summarized in Figure 1.
The fieldwork was carried out in the new “Royal” slaughterhouse. Located in Sidi Mestour (El Oued), this private establishment represents the main source of meat in the region. The production capacity of this slaughterhouse can reach 13,085 tonnes of red meat per day. In addition to its slaughtering activity via a modern slaughter chain, it ensures the transportation, refrigeration and storage of meat (a capacity of 6000 sheep carcasses and 600 bovine carcasses in five cold rooms).
The slaughterhouse is equipped with an incinerator that meets international technical standards, with an incineration capacity of 15 kg/hour of non-consumable meat and waste from the slaughtering operation. It also has a wastewater treatment unit. However, there is not a food safety assurance system implemented in this establishment.
A postmortem inspection was performed daily by two experienced veterinary inspectors, firstly by the veterinarian of the slaughterhouse and secondly by the veterinary inspector under the direction of the agricultural services of the wilaya of El-Oued.
2.2. Data Collection
Data concerning cattle red offal seizures were collected from the registry records of the slaughterhouse. The balance sheets for three years: 2018, 2019 and 2020 were retrospectively studied. However, missing data during these monitoring years were observed for the months of December 2018 and June and July 2019.
Records of the monthly monitoring of red offal seizures were mainly focused on the liver and the lungs. These organs were examined by visual inspection based on morphological identification of collected flukes (Fasciola hepatica) and lesions of cholangitis for fasciolosis, hydatid cysts and the presence of granulomatous lesions or specific tubercles, miliary and caseous tubercles for tuberculosis. This is in combination with palpation and incision (including lymph nodes) according to FAO and WHO directives [29]. Otherwise, the other types of lesions were mentioned as “other” on the registry records. This denomination was used to describe lesions (e.g., abscesses) that are non-pathognomonic or not specific to a disease with significant economic and public health impact.
2.3. Statistical Analyses
Statistical analyses of the collected data were performed using SPSS software v26.0 (IBM SPSS Statistics for Windows, Version 26.0, Armonk, NY: IBM Corp, 2019). The distribution of monthly frequency of variables according to the year and season was analyzed using the Chi-square test (χ2) and a multiples comparison of proportion was performed via the “z” test. In addition, multiple comparisons by the Tukey HSD test (ANOVA) were used to compare the mean prevalence of the different lesions encountered.
Categorical Principal Components Analysis (CATPCA) was conducted to account for patterns of variation of lesions in a single set of variables of mixed optimal scaling levels and to reduce the dimensionality of the dataset to two dimensions and components. This type of multiple correspondence analysis was performed because some of the variables were ordinal or numerical. It is used to compute the correlations between non-numerical scales and analyze them using standard principal components or a factor-analysis approach. Moreover, the correlation of Pearson “r” was applied to quantify the possible relationship between lesions prevalence and continuous variables. A critical significance level of 0.05 was considered for all statistical tests.
3. Results
3.1. Slaughtered Cattle
The total number of slaughtered cattle shown in Figure 2 decreased from 2018 to 2020 with a seasonal variation during these three years (Table 1). A remarkable increase during the Ramadan holy month in the number of slaughtered bovines was observed during May and June 2018 and May 2020. However, it considerably reduced during the Eid El Adha periods around September 2018, August and September 2019 and August 2020.
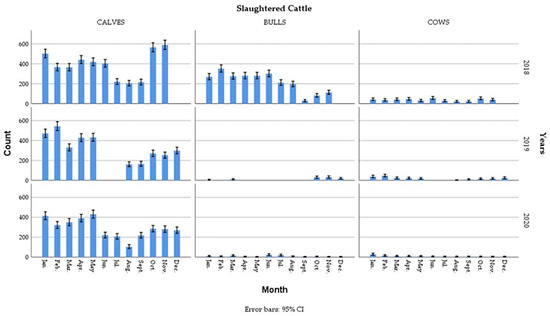
Figure 2.
Monthly number of slaughtered cattle during 2018, 2019 and 2020.

Table 1.
Frequency of cattle slaughtering according to the year and season.
The categorization used for the age of slaughtered animals is that adopted by the Algerian Ministry of Agriculture and Rural Development [1], which classifies animals into categories of young (calves aged 12 to 18 months) and adults (bulls and cows). During the three years of study, young bulls represent the dominant category of slaughtered animals. This could be explained by the meat quality of these fattened animals which is highly prized by customers. A remarkable decrease in the number of bulls slaughtered was observed during 2019 and 2020. This decrease is logically related to the decrease in total slaughtered animals. Cow slaughter was consistently low in all three years (Table 1).
The distribution of the number of animals slaughtered according to the seasons showed that for calves and cows, the frequency of slaughter was lower in summer than in other seasons, while for bulls it decreased to its lowest value, but not significantly, in autumn (Table 1).
3.2. Liver Prevalent Lesions
The monitoring of monthly variation of the prevalence of liver lesions recorded in all three years (Figure 3) changed from year to year, with a high number of liver seizure cases during the fall season (October, November). Compared to the prevalences recorded in 2019 and 2020, those of 2018 were the lowest with two peaks, the first in January (6%) and the second in November (7%).
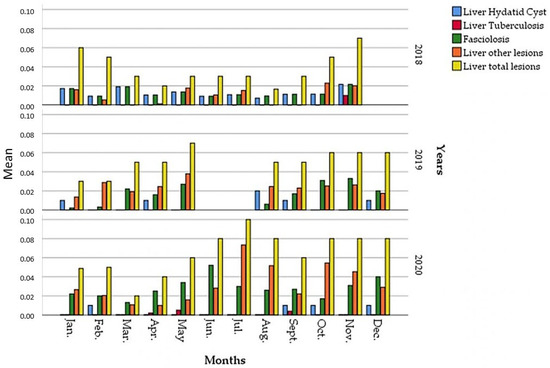
Figure 3.
Monthly prevalences of the different liver seizures reasons during 2018, 2019 and 2020.
In 2019, a high prevalence was recorded in May (7%) and November (6%). The highest prevalence of liver lesions (10%) was recorded in July 2020. In the same year, a second prevalence peak was observed in November (8%), which is not significantly higher than those recorded in previous years during the same period.
For the examined livers at the slaughterhouse, there was not a dominant reason for the seizure. The hydatid cyst (Figure 4) was the most frequent type of lesion encountered in the 2018 winter, with values that exceeded 3% (3.1% in the month of January and 3.6% in the month of February). The dominance of this type of liver lesion was also reported in September and October without exceeding 2%.
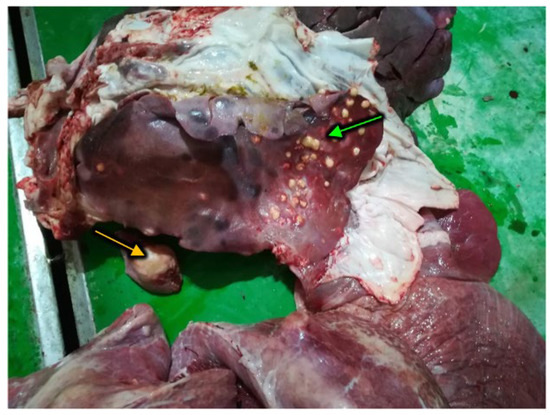
Figure 4.
Liver lesions, consistent with the presence of an Echinococcus granulosus cyst (yellow arrow) complicated by a bacterial infection (presence of caseating abscesses, green arrow).
Fasciolosis due to Fasciola hepatica was the major lesion in March (1.9%), with a peak in November (2.2%). The hepatic tuberculosis cases were not often observed, the only records were reported in November, with a low prevalence (0.8%).
Although their prevalences were the highest (mainly in May, June, July and October) compared to the previous hepatic lesions (hydatid, fasciolosis and tuberculosis), the other hepatic lesions such as abscesses were infrequently noticed.
An overview of the data (Figure 5) shows us that during the three year study, the seizures consecutive to liver injuries showed a gradual and steady change from 3.75% in 2018 to 6.67% in 2020.
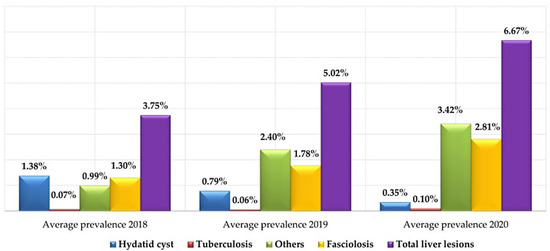
Figure 5.
The annual average prevalence of different types of liver lesions (2018, 2019 and 2020).
Regarding the seasonal mean prevalence (Table 2), although the variation was not significant (p < 0.05), there was a greater occurrence in autumn of the different liver lesions (6%), especially of the major lesions, namely fasciolosis (2.22%), tuberculosis (0.16%) and hydatidosis (0.82%).

Table 2.
Comparative distribution of liver lesions prevalence over seasons.
3.3. Lungs Prevalent Lesions
During the study period, the total number of lung lesions varied monthly without following significant monthly kinetics (Figure 6), except for the common fall period (September and October) which was characterized by an acute peak.
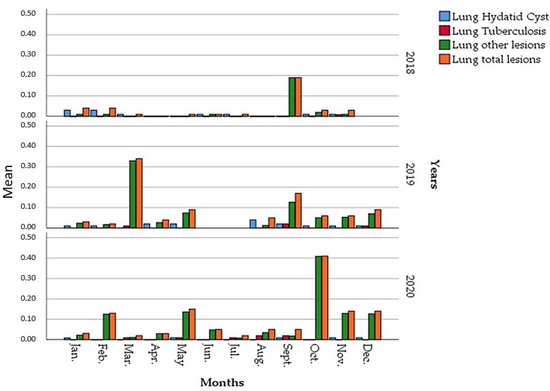
Figure 6.
Monthly prevalences of the different lungs seizures reasons during 2018, 2019 and 2020.
In 2018, low values were found in all months of the year except for September (19%). Subsequently, two peaks were recorded in 2019; the first was notable in the first month of spring (34%) while the second was lower in September (17%). However, it was close to the prevalence recorded in the same period of 2018.
The important increase in cases of lung lesions in 2020 was distributed over several months, especially in the months of February, May, November and December, with a very acute development of 41% in October.
During 2018, seizures related to pulmonary hydatid cysts (Figure 7 and Figure 8) were recorded with low prevalence except for two months in the winter: January and February with 2.9% and 3.3%, respectively. Other reasons for seizures (abscesses, pneumonia, etc.) were present throughout 2018 at very low prevalences, with a peak of 18.6% in September.
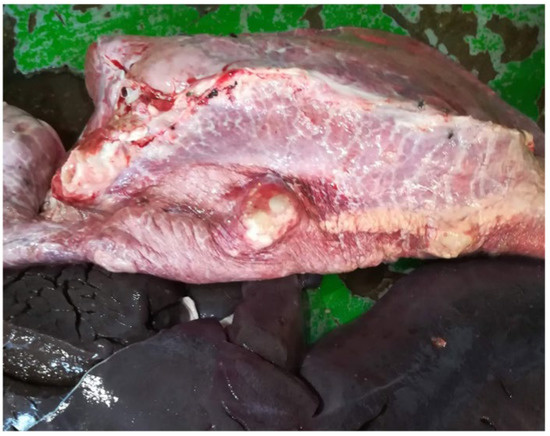
Figure 7.
Superinfected pulmonary hydatid cyst (immune response in progress).
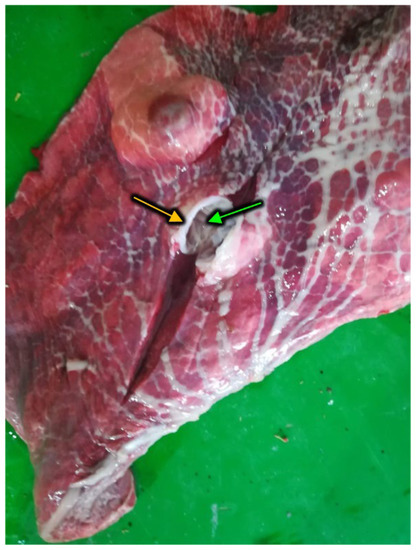
Figure 8.
Well-isolated pulmonary hydatid cysts, host derived collagenous layer (connective tissue, yellow arrow) and laminar layer (green arrow) visible on section.
In 2019, hydatid cysts lesions were present throughout the year with low monthly prevalence (<1%) except for two periods in August (4%) and September (2%). The other pulmonary lesions (abscesses, pneumonia, etc.) were mostly present with variable values. However, no prevalences were mentioned in Figure 6 for June and July due to the lack of data on the control sheets of the slaughterhouse.
For the year 2020, a remarkable decrease in the number of hydatid cyst cases was observed with low prevalences that did not exceed 1%. On the other hand, the cases of tuberculosis were spread over several months, especially in autumn, compared to the other years. The other lung lesions were the most prevalent and have been observed with a peak in October (41%) and with values that are more or less than 10% in February, May, November and December.
Recapitulating, the number of lung seizure cases considerably increased. The lowest prevalence (3.45%) was recorded in 2018, 9.57% in 2019 and 10.50% in 2020 (Figure 9). The overall balance of lung lesions established during the three years of the study summarized in Figure 9 showed a decrease in the annual average prevalence of hydatid cysts in 2020. On the other hand, a sensible and continuous increase in tuberculosis cases was recorded between 2018 (0.1%) and 2020 (0.6%). Nevertheless, the increase in other lung lesions proportionally explains the increase in the number of lung seizures between 2018 and 2020.
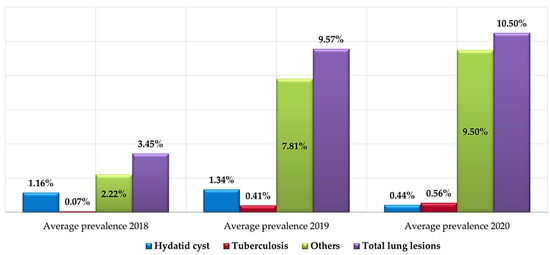
Figure 9.
The annual average prevalence of different types of lungs lesions (2018, 2019 and 2020).
For the seasonal dynamics of the occurrence of pulmonary lesions, it seems that it did not vary significantly (p < 0.05) despite a higher average prevalence in autumn (12.67%) including that of tuberculosis (0.53%). However, an increase in their mean in winter for hydatidosis (1.83%) was noted (Table 3).

Table 3.
Comparative distribution of lungs lesions prevalence over seasons.
3.4. Factorial Analysis of Liver and Lungs Lesions Variation
To display the relationship between quantitative variables and categorical factors, a two-dimensional diagram (Figure 10) was created using Categorical Principal Components Analysis. Responses in the same category are plotted close to each other but those in different categories are plotted as far apart as possible.
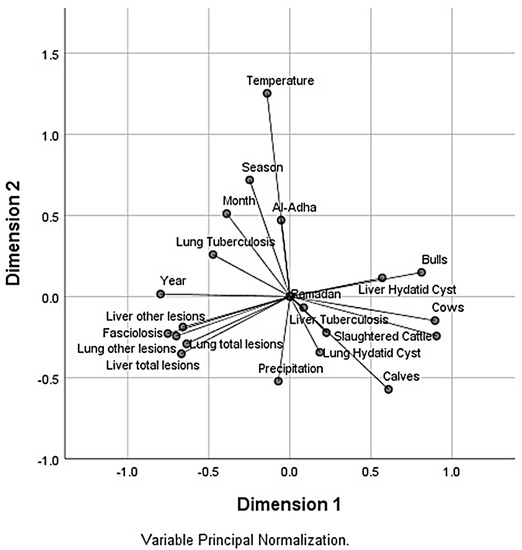
Figure 10.
Component loadings plot for the first two components from Principal Components Analysis for Categorical Data (CATPCA).
The Cronbach’s alpha, 0.84 for dimension 1 (Table 4) exceed the lower limit (0.70) of the acceptable values of alpha Cronbach’s alpha, ranging from 0.70 to 0.95 [30]. That of dimension 2 is equal to 0.699 and is at the limit of this acceptance range.

Table 4.
Dimensions pattern of normalized variables affecting dynamics of liver and lung lesions using CATPCA.
Dimension 1 was well correlated with year, number of cattle slaughtered, number of bulls, number of cows, fasciolosis, other lesions of liver, liver total lesions, liver hydatid cyst, lung other lesions and lung total lesions. Liver lesions except hydatid cyst seems related to the time (year) but they were opposed to the number and sexes of animals slaughtered, which could indicate that axis 1 characterized the dynamic of liver lesions, mainly due to an increase in fasciolosis prevalence dependant on the COVID-19 pandemic (the year 2020) (r = 0.58, p < 0.01) that negatively affected the number of cattle slaughtered (r = 0−0.44, p< 0.05) since the registration of a close number of cases (95 cases in 2018 and 101 cases in 2020) lead to a lower number of slaughtered animals (7112 head in 2018 vs. 3695 head in 2020). Furthermore, this pandemic situation negatively (Table 5) influenced the number of adult animals slaughtered compared to the number of young animals (3753 calves, 1122 bulls, 251 cows in 2018 vs. 3419 calves, 481 bulls, 182 cows in 2020). This pattern of head number could explain the negative correlation between reduction in bulls and mainly the cow number with the increase of fasciolosis prevalence (r = −0.38, p < 0.05 and r = −0.58, p < 0.01, respectively).

Table 5.
Pearson correlation of liver and lung lesions with continuous variables.
Dimension 2 was associated with season, precipitation, temperature and to a limited extent with Eid al-Adha. It could give a good explanation of the climatic changes according to the seasons without, however, showing the seasonal variation of the recorded lesions since the Cronbach’s alpha value of this dimension is at the minimum threshold value of acceptance.
4. Discussion
Algeria is characterized by a specialization of the agro-ecological zones in terms of cattle breeding, which remains confined to the north of the country with a low frequency in the south. In this latter desert environment, it is the extensive system that dominates, and the hostile climatic conditions have an advantage to goat, sheep and camel breeding compared to cattle livestock [31]. Looking to the national herd size [1], cattle breeding in the region of El Oued is limited (1.15%) compared to those of camel (13.18%), goat (9.94%) and sheep (2.51%). In southern Algeria, cattle livestock has experienced a progression in numbers with the state efforts to intensify its production, but it is still hampered by the lack of management culture of intensive breeding among farmers [31]. In the study area, the farmers seem to be essentially interested in fattening cattle for meat given the dominance of calves (12 to 18 months old) number (56.64% of the bovine population of El Oued). During the three years of our study, a total of 14,478 cattle were slaughtered at the Royal abattoir. This number of slaughtered animals is considered significant when compared to the official statistics for the El Oued’s cattle population estimated at 20,585 head in 2019 [1]. This unequal number of slaughtered animals could be explained in part by an animal supply from the north of the country where the traditional semi-extensive system (grazing practice) and climatic conditions are favorable for the development and proliferation of internal parasites. This partly explains the relationship between variations in the number and category of animals slaughtered with prevalence of fasciolosis established by the Principal Components Analysis for Categorical Data (CATPCA).
Compared to other categories of cattle, a much higher frequency of slaughtered calves (76.88%) was observed while a consistently low number of cows were slaughtered during the three years of the survey. This finding is in line with the Algerian regulations which only authorize the slaughter of old or sick females [32].
The effect of the number of young animals on the variation of the different liver and lung lesions was not significant for parasitic diseases and was moderately significant for tuberculosis. All lesion frequencies were related to the change in the number of older animals slaughtered. These findings may be due, on the one hand, to the short-term exposure of young animals to infectious agents compared to the chronic evolution in older animals [33], and on the other hand, to the intensive fattening system of young animals that is characterized by the use of deworming.
The analysis of the data concerning the frequency of slaughtering according to the seasons showed a greater frequency of slaughtering during the relatively wet seasons than during the summer which could be linked in part to the livestock market movement active during the winter tourism period, the period of dates (fruit) in autumn, and in part to the holy month of Ramadan. However, this movement became limited from July–August 2020 onwards due to movement restrictions and the closure of the livestock market related to the COVID-19 pandemic.
Slaughterhouse health inspection plays a key role in controlling the dangers to the consumer from the consumption of unsafe meat or offal. The results of our survey undertaken in the Royal slaughterhouse of El Oued concerning the inspection of slaughtered animals and the main reasons for seizure of red offal allowed us to note a greater frequency of lesions due to hydatidosis, fasciolosis and tuberculosis compared to other hepatic lesions, whereas for the lungs, a greater frequency of other reasons was noted compared to hydatidosis and tuberculosis.
Compared to previous studies carried out in slaughterhouses located in other bioclimatic zones of Algeria (humid and semi-arid), our results are close to those obtained recently in the humid zone with a Mediterranean climate by Ayad et al. [25] in Bejaia during a retrospective study from 2009 to 2016 with a prevalence of 2.83% for fascioliasis and 2.49% for hydatidosis and by Hamiroun et al. [34] in Jijel (prevalence of 6.9% for fascioliasis, 4.8% for hydatidosis and 1.0% for tuberculosis). In addition, Gherroucha et al. [26], in their work in the slaughterhouses of Constantine, a semiarid region, were able to record a frequency of 2% for lesions due to fascioliasis infestation, whereas for the lungs and liver, hydatid lesions dominate with respective rates of around 24% and 8%.
Nevertheless, the prevalences recorded during our work are very low compared to the infestation rates of 52.4% for fascioliasis and 30.9% for hydatidosis recorded in 2011 by Boucheikhchoukh et al. [35] in the humid region of El Tarf (Algeria).
On the other hand, Mimoune et al. [5] also reported a rate of 11% for fasciolosis and rates ranging from 19.05% to 20.69% for hydatidosis of the liver and lungs in the Taher region in Jijel under the Algerian Mediterranean climate, while Hamed et al. [36] reported a prevalence of around 12.6% in their work in the abattoir of Sejnane (Tunisia).
Other works in Africa have also reported higher rates, with 24.4% in Ethiopia [37], 65.7% in Uganda [13] and 27.67% in Nigeria [38].
The analysis of these values shows a variability of prevalence that could be linked to many factors, such as the type of animal husbandry (intensive or extensive), biotopes, climate, age or the absence of a control programmes.
The monthly frequencies of fasciolosis during the three years of monitoring corroborate those found by Ouchene-Khelifi et al. [27] in another province characterized by a dry climate (Ouargla) and Gherroucha et al. [26] in the semi-arid bioclimatic stage of the Constantine region, but are lower than the rate of 6.5 recorded in the same region by Mekroud et al. [39] and 12.3% reported by Meguini et al. [40] in the Souk Ahras region.
Regarding the influence of season, no significant difference was found for the data of the three years of the study. Similar findings were also reported by Gherroucha et al. [26] and Ayad et al. [25].
However, it should be noted that fasciolosis occurs where climatic conditions are favorable and that these conditions are related to two factors, temperature and humidity. Indeed, these two factors are conditioning the forage production and the occurrence or not of fasciolosis seasons.
Compared to the humid regions of the country, the wilaya of El Oued is an arid region characterized by a dry desert climate, which is unfavorable to the development of the intermediate host (molluscs of fasciolosis) and therefore to the completion of the biological cycle of the parasite.
Concerning the reported results for monthly hydatidosis frequencies, these remain very low compared to those noted in other studies, 30% in the region of El Tarf [35], 24% in the region of Constantine [26], 22% in the region of El Djelfa [41] and 42.9% in the region of Sidi Kacem Province in Morocco [42]. The low rates recorded in our study for both liver and lung lesions can be attributed to several factors, such as the number of dogs in the area, deworming, prohibition of dog access to slaughterhouses and proper management of infested offal.
The study of the impact of season on Echinococcus granulosis infestation showed no significant difference. Hamiroun et al. [34] in northern Algeria (Jijel), under the Mediterranean climate, reported a higher frequency of infestation during the spring, explaining that during this season, the majority of animals are grazed outdoors, which favors the transmission of pathogens and the contamination of animals by various diseases. The difference in prevalence between these two bioclimatic stages (Mediterranean and arid) can be explained by the fact that heat negatively influences this parasite since it remains the most reliable method to destroy Echinococcus eggs [34].
Similar findings were also reported by Haffaf et al. [43] in the region of M’Sila and Ayad et al. [25] in the region of Bejaia, with a higher frequency of hydatidosis cases observed during the wet seasons compared to the dry seasons.
Hydatidosis, which represents a major zoonosis, is still present in Algeria and it requires particular attention, especially since the control remains difficult in the presence of numerous canid species carrying the adult worm, and numerous affected species carrying the larva. Finally, it is important because of its wide distribution around the world, resulting in various subspecies with different hosts and geographical distribution [44].
For tuberculosis, the study of monthly trends showed prevalences very close to those reported by Hamiroune et al. [34] where they noted rates ranging from 0.3% to 1.8%, those of Gherroucha et al. [26] from 0.72%–2.18% and slightly lower than those of Belakehal et al. (2021) with 4.78%. Considering the effect of the season, no significant difference was found between dry and wet seasons during the period of our study.
Belakehal et al. [45] reported a higher frequency of TB lesions during dry seasons.
Hamiroune et al. [34], on the other hand, noted a greater frequency of tuberculosis during the spring.
In Nigeria, Sa’idu et al. [46] and Boukary et al. [47] were able to find much higher rates in the early rainy season (July and August).
Awah-Ndukum et al. [48] explained, in their work, in slaughterhouses in the Littoral and Western Highlands regions of Cameroon, that the detection of TB lesions is not influenced by the season, but was high during stressful periods such as the off-season and the high season.
The low rates recorded in our study in particular and those in other regions in Algeria generally show a relatively controlled situation of this pathology compared to other developing countries and especially those in Africa where this zoonosis continues to affect livestock heavily [49].
According to Djafar et al. [50], in their work on bovine tuberculosis in the eastern region of Algeria, the intermediate epidemiological position of bovine tuberculosis in Algeria is influenced by certain risk factors that fit into the three mechanisms commonly known by the scientific community: “contamination by the introduction of an animal”, “neighborhood contamination” and “recurrence”.
Purulent lesions can have several causes, infectious or parasitic, whether for the liver or the lungs, or even as a result of lesions caused by foreign bodies.
5. Conclusions
Our retrospective analysis of monthly and annual red offal lesion reports for three consecutive years (2018, 2019 and 2020) recorded by the inspection at the Royal slaughterhouse in the El Oued region showed that lung and liver lesions are the main causes of seizure of bovine red offal. These lesions are dominated by non-specific injuries, with a lesser impact on public health, but they are still the cause of considerable economic losses. The kinetics of all lesions showed an increasing trend of seizure cases in relation to the decrease in the number of cattle slaughtered. Hepatic and pulmonary hydatid cysts showed a common decrease in prevalence, especially in 2020. This zoonosis did not show a characteristic seasonal pattern. Hepatic fasciolosis, which has a considerable economic impact, has shown a continuous increase without taking on the known seasonal aspect of a pathology considered to be specific to the wetland. The epidemiological situation seems to be stable for tuberculosis.
From an epidemiological point of view, the autumn season represents the period at risk in the region of El Oued in relation to the intense agricultural and commercial activity during the date fruit season. On the other hand, the Eid El Adha period represents the low-risk period due to the decrease in demand for these commodities.
The results obtained from our study confirm that the quality assurance of meat and red offal marketed in the region of El Oued and the food safety of its population requires the implementation of a more structured epidemiological approach based on the intensification of surveillance during risk periods such as the date palm season and the reinforcement of the control of the movement of animals coming from different climatic stages. The use of complementary laboratory diagnosis is strongly recommended to confirm cases of suspicion upon inspection and to identify and characterize the infectious agents involved. This could lead to the integration of the concept of molecular epidemiology in the control of these diseases. Finally, the implementation throughout the meat production chain (farms, livestock production and markets, slaughterhouse and points of sale) of a food safety system seems to be a necessary approach for an effective hygienic control program of zoonoses limiting economic losses and preserving public health.
Author Contributions
Conceptualization and methodology, A.H.; software, A.H. and M.B.; validation, A.H.; formal analysis, A.H.; investigation, A.H. and C.E.B.; resources and data curation, A.H. and C.E.B.; writing—original draft preparation, A.H., S.R. and M.B; writing—review and editing, A.H., S.R. and M.B.; visualization and supervision, A.H., S.R. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank the direction of the agricultural services of the wilaya of El Oued and the veterinary inspector of the slaughterhouse “Royal” for their cooperation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ministry of Agriculture and Rural Development. Statistique Agricole, Superficies et Productions, SERIE “B” 2019; M.A.R.D.: Algiers, Algeria, 2021; p. 87.
- Kardjadj, M. The Epidemiology of Cattle Abortion in Algeria. Trop. Anim. Health Prod. 2018, 50, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Kardjadj, M. The Epidemiology of Human and Animal Brucellosis in Algeria. J. Bacteriol. Mycol. 2016, 3, 1025. [Google Scholar]
- Pluhar, E.B. Meat and Morality: Alternatives to Factory Farming. J. Agric. Environ. Ethics 2010, 23, 455–468. [Google Scholar] [CrossRef]
- Mimoune, N.; Hamiroune, M.; Boukhechem, S.; Mecherouk, C.; Harhoura, K.; Khelef, D.; Kaidi, R. Pathological Findings in Cattle Slaughtered in Northeastern Algeria and Associated Risk Factors. Vet. Sci. 2022, 9, 330. [Google Scholar] [CrossRef]
- Bailly, J.-D.; Brugère, H.; Chardon, H. Micro-Organismes et Parasites Des Viandes: Les Connaître Pour Les Maîtriser, de l’éleveur Au Consommateur; Cahiers sécurité des aliments; Centre D’information des Viandes: Paris, France, 2012. [Google Scholar]
- Mohammed, N.; Adare Mengistu, D.; Abdurehman, A.; Belina, D.; Mengistu, S. Determination of Tetracycline Residues in Kidney and Muscle of Beef Cattle Slaughtered in Dire Dawa and Harar Municipal Abattoirs, Eastern Ethiopia. Environ. Health Insights 2022, 16, 117863022211097. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-H.; Kang, D.-J.; Lim, M.-W.; Kang, C.-S.; Sung, H.-J. Risk Assessment of Growth Hormones and Antimicrobial Residues in Meat. Toxicol. Res. 2010, 26, 301–313. [Google Scholar] [CrossRef]
- Durr, P.A.; Tait, N.; Lawson, A.B. Bayesian Hierarchical Modelling to Enhance the Epidemiological Value of Abattoir Surveys for Bovine Fasciolosis. Prev. Vet. Med. 2005, 71, 157–172. [Google Scholar] [CrossRef]
- Mehmood, N.; Arshad, M.; Ahmed, H.; Simsek, S.; Muqaddas, H. Comprehensive Account on Prevalence and Characteristics of Hydatid Cysts in Livestock from Pakistan. Korean J. Parasitol. 2020, 58, 121–127. [Google Scholar] [CrossRef]
- Ayele, W.; Neill, S.; Zinsstag, J.; Weiss, M.; Pavlik, I. Bovine Tuberculosis: An Old Disease but a New Threat to Africa. Int. J. Tuberc. Lung Dis. 2004, 8, 924–937. [Google Scholar]
- Hathaway, S. Bonnes Pratiques Pour l’industrie de La Viande; Food & Agriculture Org.: Rome, Italy, 2006; Volume 2, ISBN 92-5-205146-5. [Google Scholar]
- Opio, L.G.; Abdelfattah, E.M.; Terry, J.; Odongo, S.; Okello, E. Prevalence of Fascioliasis and Associated Economic Losses in Cattle Slaughtered at Lira Municipality Abattoir in Northern Uganda. Animals 2021, 11, 681. [Google Scholar] [CrossRef]
- Wilson, C.S.; Jenkins, D.J.; Brookes, V.J.; Barnes, T.S.; Budke, C.M. Assessment of the Direct Economic Losses Associated with Hydatid Disease (Echinococcus Granulosus Sensu Stricto) in Beef Cattle Slaughtered at an Australian Abattoir. Prev. Vet. Med. 2020, 176, 104900. [Google Scholar] [CrossRef]
- Ejeh, E.F.; Raji, M.A.; Bello, M.; Lawan, F.A.; Francis, M.I.; Kudi, A.C.; Cadmus, S.I.B. Prevalence and Direct Economic Losses from Bovine Tuberculosis in Makurdi, Nigeria. Vet. Med. Int. 2014, 2014, 904861. [Google Scholar] [CrossRef] [Green Version]
- Im, M.C.; Seo, K.W.; Bae, D.H.; Lee, Y.J. Bacterial Quality and Prevalence of Foodborne Pathogens in Edible Offal from Slaughterhouses in Korea. J. Food Prot. 2016, 79, 163–168. [Google Scholar] [CrossRef]
- Toldrá, F.; Aristoy, M.-C.; Mora, L.; Reig, M. Innovations in Value-Addition of Edible Meat by-Products. Meat Sci. 2012, 92, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Boudechicha, H.-R. Ethnic Meat Products of the North African and Mediterranean Countries: An Overview. J. Ethn. Foods 2018, 5, 83–98. [Google Scholar] [CrossRef]
- Ren, Q.-S.; Fang, K.; Yang, X.-T.; Han, J.-W. Ensuring the Quality of Meat in Cold Chain Logistics: A Comprehensive Review. Trends Food Sci. Technol. 2022, 119, 133–151. [Google Scholar] [CrossRef]
- Borch, E.; Arinder, P. Bacteriological Safety Issues in Red Meat and Ready-to-Eat Meat Products, as Well as Control Measures. Meat Sci. 2002, 62, 381–390. [Google Scholar] [CrossRef]
- Mellau, L.; Nonga, H.E.; Karimuribo, E. A Slaughterhouse Survey of Liver Lesions in Slaughtered Cattle, Sheep and Goats at Arusha, Tanzania. Res. J. Vet. Sci. 2010, 3, 179–188. [Google Scholar] [CrossRef]
- Collins, D.S.; Huey, R.J. (Eds.) Gracey’s Meat Hygiene: Collins/Gracey’s Meat Hygiene; John Wiley & Sons, Ltd.: Chichester, UK, 2014; ISBN 978-1-118-64998-5. [Google Scholar]
- Törmä, K.; Kaukonen, E.; Lundén, J.; Fredriksson-Ahomaa, M.; Laukkanen-Ninios, R. A Comparative Analysis of Meat Inspection Data as an Information Source of the Health and Welfare of Broiler Chickens Based on Finnish Data. Food Control 2022, 138, 109017. [Google Scholar] [CrossRef]
- Horst, A.; Gertz, M.; Krieter, J. Challenges and Opportunities of Using Meat Inspection Data to Improve Pig Health Traits by Breeding: A Review. Livest. Sci. 2019, 221, 155–162. [Google Scholar] [CrossRef]
- Ayad, A.; Benhanifia, M.; Balla, E.-H.; Moussouni, L.; Ait-Yahia, F.; Benakhla, A. A Retrospective Survey of Fasciolosis and Hydatidosis in Domestic Ruminants Based on Abattoirs’ Data in Bejaia Province, Algeria. Veterinaria 2019, 68, 47–51. [Google Scholar]
- Gherroucha, D.; Benhamza, L.; Gharbi, M. Prevalence of Parasitic Lesions in Lungs and Livers of Cattle and Sheep at Constantine’s Slaughterhouse, Northeast Algeria. Rev. Elev. Med. Vet. Pays Trop. 2022, 75, 19–24. [Google Scholar] [CrossRef]
- Ouchene-Khelifi, N.; Ouchene, N.; Dahmani, H.; Dahmani, A.; Sadi, M.; Douifi, M. Fasciolosis Due to Fasciola Hepatica in Ruminants in Abattoirs and Its Economic Impact in Two Regions in Algeria. Trop. Biomed. 2018, 35, 181–187. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- FAO/WHO. Projet de Code d’usages En Matière d’hygiène Pour La Viande. In Rapport de La 10e Session Du Comité Du Codex Sur l’hygiène de La Viande; Alinorm 04/27/16; FAO/WHO: Rome, Italy, 2004. [Google Scholar]
- Tavakol, M.; Dennick, R. Making Sense of Cronbach’s Alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef]
- Senoussi, A.; Haïli, L.; Maïz, H. The Dairy Bovine Breeding Situation in the Region of Guerrara (Northern Algerian Sahara). Livest. Res. Rural Dev. 2010, 22, 220. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Décret Exécutif N° 91-514 Du 22 Décemebre 1991 Relatif Aux Animaux Interdits à l’abattage. J. Off. de la Repub. Algér. 1991, 68, 2180. [Google Scholar]
- Karim, M.R.; Mahmud, M.S.; Giasuddin, M. Epidemiological Study of Bovine Fasciolosis: Prevalence and Risk Factor Assessment at Shahjadpur Upazila of Bangladesh. Immun. Infect. Dis. 2015, 3, 25–29. [Google Scholar] [CrossRef]
- Hamiroune, M.; Dahmane, M.; Charef, A.; Cheniguel, H.; Foughalia, H.; Saidani, K.; Djemal, M. Evaluation of Fascioliasis, Hydatidosis, and Tuberculosis in Domestic Animals during Post-Mortem Inspection at Jijel Slaughterhouse (Algeria). J. Food Qual. Hazards Control 2020, 7, 149–156. [Google Scholar] [CrossRef]
- Boucheikhchoukh, M.; Righi, S.; Sedraoui, S.; Mekroud, A.; Benakhla, A. Principales Helminthoses Des Bovins: Enquête Épidémiologique Au Niveau de Deux Abattoirs de La Région d’El Tarf (Algérie). Tropicultura 2012, 30, 167–172. [Google Scholar]
- Hamed, N.; Ayadi, A.; Hammami, H. Epidemiological Studies on Fasciolosis in Northern Tunisia. Revue Méd. Vét 2014, 165, 49–56. [Google Scholar]
- Yusuf, M.; Nuraddis, I.; Tafese, W.; Deneke, Y. Prevalence of Bovine Fasciolosis in Municipal Abattoir of Haramaya, Ethiopia. Food Sci. Qual. Manag. 2016, 48, 38–43. [Google Scholar]
- Magaji, A.A.; Ibrahim, K.; Salihu, M.D.; Saulawa, M.A.; Mohammed, A.A.; Musawa, A.I. Prevalence of Fascioliasis in Cattle Slaughtered in Sokoto Metropolitan Abattoir, Sokoto, Nigeria. Adv. Epidemiol. 2014, 2014, 247258. [Google Scholar] [CrossRef]
- Mekroud, A.; Benakhla, A.; Vignoles, P.; Rondelaud, D.; Dreyfuss, G. Preliminary Studies on the Prevalences of Natural Fasciolosis in Cattle, Sheep, and the Host Snail (Galba Truncatula) in North-Eastern Algeria. Parasitol. Res. 2004, 92, 502–505. [Google Scholar] [CrossRef]
- Meguini, M.N.; Righi, S.; Bouchekhchoukh, M.; Sedraoui, S.; Benakhla, A. Investigation of Flukes (Fasciola hepatica and Paramphistomum sp.) Parasites of Cattle in North-Eastern Algeria. Ann. Parasitol. 2021, 67, 455–464. [Google Scholar] [CrossRef]
- Hamrat, K.; Achour, Y.; Ghadiri, Y.; Cozma, V. Epidemiologic Study of Hydatidosis in the Steppe Regions of Djelfa, Algeria. Sci. Parasitol. 2011, 12, 177–183. [Google Scholar]
- El Berbri, I.; Petavy, A.F.; Umhang, G.; Bouslikhane, M.; Fassi Fihri, O.; Boué, F.; Dakkak, A. Epidemiological Investigations on Cystic Echinococcosis in North-West (Sidi Kacem Province) Morocco: Infection in Ruminants. Adv. Epidemiol. 2015, 2015, 104025. [Google Scholar] [CrossRef]
- Haffaf, S.; Ragoub, F.Z.; Mammeri, A.; Nouari, Z.; Djaalab, I.; Zernine, A. Epidemiological Study of Hydatidosis in Sheep Slaughtered at M’Sila Abattoirs (Algeria). Bull. UASVM CN. Vet. Med. 2020, 77, 7–16. [Google Scholar] [CrossRef]
- Gessese, A.T. Review on Epidemiology and Public Health Significance of Hydatidosis. Vet. Med. Int. 2020, 2020, 8859116. [Google Scholar] [CrossRef]
- Belakehal, F.; Moser, I.; Naim, M.; Zenia, S.; Hamdi, T.-M. Tuberculosis Lesions of Bovine Carcasses in Algerian Municipal Abattoirs and Associated Risk Factors. JAHP 2021, 9, 379. [Google Scholar] [CrossRef]
- Sa’idu, A.S.; Mohammed, S.; Ashafa, M.; Gashua, M.M.; Mahre, M.B.; Maigado, A.I. Retrospective Study of Bovine Tuberculosis in Gombe Township Abattoir, Northeastern Nigeria. Int. J. Vet. Sci. Med. 2017, 5, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Boukary, A.R.; Thys, E.; Rigouts, L.; Matthys, F.; Berkvens, D.; Mahamadou, I.; Yenikoye, A.; Saegerman, C. Risk Factors Associated with Bovine Tuberculosis and Molecular Characterization of Mycobacterium Bovis Strains in Urban Settings in Niger: Risk Factors Associated with Bovine Tuberculosis and Molecular Characterization of Mycobacterium Bovis Strains. Transbound. Emerg. Dis. 2012, 59, 490–502. [Google Scholar] [CrossRef]
- Awah Ndukum, J.; Kudi, A.C.; Bradley, G.; Ane-Anyangwe, I.N.; Fon-Tebug, S.; Tchoumboue, J. Prevalence of Bovine Tuberculosis in Abattoirs of the Littoral and Western Highland Regions of Cameroon: A Cause for Public Health Concern. Vet. Med. Int. 2010, 2010, 495015. [Google Scholar] [CrossRef] [PubMed]
- Cosivi, O. Zoonotic Tuberculosis Due to Mycobacterium Bovis in Developing Countries. Emerg. Infect. Dis. 1998, 4, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Djafar, Z.R.; Benazi, N.; Bounab, S.; Sayhi, M.; Diouani, M.F.; Benia, F. Distribution of Seroprevalence and Risk Factors for Bovine Tuberculosis in East Algeria. Prev. Vet. Med. 2020, 183, 105127. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).