Whitefly (Bemisia tabaci) Management (WFM) Strategies for Sustainable Agriculture: A Review
Abstract
1. Introduction
2. Taxonomy of Bemisia tabaci Gennadius
3. Life Cycle of Bemicia tabaci
4. Host Plants
5. Whitefly Management (WFM) Strategies
5.1. Traditional Strategies
5.1.1. Cultural Strategies
5.1.2. Miscellaneous
Fermented Curd Water
Cow Dung/Urine and Botanical Extracts
Ash
Kerosene
5.1.3. Botanical Extracts
5.1.4. Mechanical Strategies
5.1.5. Drawbacks of Traditional Strategies
5.2. Biological Strategies
5.2.1. Predators
5.2.2. Parasitoids
5.2.3. Entomopathogenic Organisms
5.2.4. Drawbacks of Biological Strategies
5.3. Biotechnological Strategies for Whitefly Control
5.3.1. Transgenesis and Whitefly Control
5.3.2. Exogenous Application of dsRNA to Control Whiteflies
5.3.3. Control of B. tabaci through Nanotechnology
5.3.4. Disadvantages of Biotechnological Strategies
5.4. Synthetic Chemicals
Drawbacks of Synthetic Chemicals
5.5. Pesticide Resistance and B. tabaci Control
5.6. IPM Strategies
Drawbacks of IPM Strategies
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oliveira, M.R.V.; Henneberry, T.J.; Anderson, P. History current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef]
- Cruz-Estrada, A.; Gamboa-Angulo, M.; Borges-Argáez, R.; Ruiz-Sánchez, E. Insecticidal effects of plant extracts on immature whitefly Bemisia tabaci Genn.(Hemiptera: Aleyroideae). Electron. J. Biotechnol. 2013, 16, 6. [Google Scholar]
- Brown, J.K.; Bird, J. Whitefly transmitted geminiviruses and associated disorders in the Americas and the Caribbean basin. Plant Dis. 1992, 76, 220–226. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Kanakala, S.; Ghanim, M. Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PLoS ONE 2019, 14, e0213946. [Google Scholar]
- Lee, M.H.; Lee, H.K.; Lee, H.G.; Lee, S.G.; Kim, J.S.; Kim, S.E.; Kim, Y.S.; Suh, J.K.; Youn, Y.N. Effect of cyantraniliprole against of Bemisia tabaci and prevention of tomato yellow leaf curl virus (TYLCV). Korean J. Pestic. Sci. 2018, 18, 33–40. [Google Scholar] [CrossRef][Green Version]
- Burnett, T. The effect of temperature on an insect host-parasite population. Ecology 1949, 30, 113–134. [Google Scholar] [CrossRef]
- Rodríguez, E.; Téllez, M.; Janssen, D. Whitefly control strategies against tomato leaf curl New Delhi virus in greenhouse zucchini. Int. J. Environ. Res. Public health. 2019, 16, 2673. [Google Scholar] [CrossRef]
- Wintermantel, W.M. Emergence of Greenhouse Whitefly (Trialeurodes vaporariorum) Transmitted Criniviruses as Threats to Vegetable and Fruit Production in North America; APSnet Features: Saint Paul, MN, USA, 2004. [Google Scholar] [CrossRef]
- CABI. Trialeurodes Vaporariorum (Whitefly, Greenhouse). 2015. Available online: http://www.cabi.org/isc/datasheet/54660 (accessed on 21 November 2021).
- Sani, I.; Ismail, S.I.; Abdullah, S.; Jalinas, J.; Jamian, S.; Saad, N. A review of the biology and control of whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), with special reference to biological control using entomopathogenic fungi. Insects 2020, 11, 619. [Google Scholar] [CrossRef]
- Gangwar, R.K.; Charu, G. Lifecycle, distribution, nature of damage and economic importance of whitefly, Bemisia tabaci (Gennadius). Acta Sci. Agric. 2018, 2, 36–39. [Google Scholar]
- Perring, T.M.; Stansly, P.A.; Liu, T.X.; Smith, H.A.; Andreason, S.A. Whiteflies: Biology, ecology, and management. In Sustainable Management of Arthropod Pests of Tomato, 1st ed.; Wakil, W., Brust, G.E., Perring, T.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 73–110. [Google Scholar]
- Solanki, R.D.; Jha, S. Population dynamics and biology of whitefly (Bemisia tabaci Gennadius) on sunflower (Helianthus annuus L.). J. Pharmacogn. Phytochem. 2018, 7, 3055–3058. [Google Scholar]
- Khan, I.A.; Wan, F.H. Life history of Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) biotype B on tomato and cotton host plants. J Entomol. Zool. Stud. 2018, 3, 117–121. [Google Scholar]
- Smith, P.E. Crop and Food Research. In Whitefly: Identification and Biology in New Zealand Greenhouse Tomato Crops; Smith, P.E., Ed.; AsureQuality Ltd.: Auckland, New Zealand, 2009; pp. 1–8. [Google Scholar]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Götz, M.; Winter, S. Diversity of Bemisia tabaci in Thailand and Vietnam and indications of species replacement. J. Asia Pac. Entomol. 2016, 19, 537–543. [Google Scholar] [CrossRef]
- Lu, S.; Chen, M.; Li, J.; Shi, Y.; Gu, Q.; Yan, F. Changes in Bemisia tabaci feeding behaviors caused directly and indirectly by cucurbit chlorotic yellows virus. Virol. J. 2019, 16, 1–14. [Google Scholar] [CrossRef]
- Kedar, S.C.; Saini, R.K.; Kumaranag, K.M. Biology of cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) on cotton. J. Entomol. Res. 2014, 38, 135–139. [Google Scholar]
- Legg, J.P.; Shirima, R.; Tajebe, L.S.; Guastella, D.; Boniface, S.; Jeremiah, S.; Nsami, E.; Chikoti, P.; Rapisarda, C. Biology and management of whitefly vectors of cassava virus pandemics in Africa. Pest Manag. Sci. 2014, 70, 1446–1453. [Google Scholar] [CrossRef]
- Hasanuzzaman, A.T.M.; Islam, M.N.; Zhang, Y.; Zhang, C.Y.; Liu, T.X. Leaf morphological characters can be a factor for intra-varietal preference of whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) among eggplant varieties. PLoS ONE 2016, 11, e0153880. [Google Scholar] [CrossRef]
- Nwezeobi, J.; Onyegbule, O.; Nkere, C.; Onyeka, J.; van Brunschot, S.; Seal, S.; Colvin, J. Cassava whitefly species in eastern Nigeria and the threat of vector-borne pandemics from east and central africa. PLoS ONE 2020, 15, e0232616. [Google Scholar] [CrossRef]
- Zhang, X.; Ferrante, M.; Wan, F.; Yang, N.; Lövei, G.L. The parasitoid Eretmocerus hayati is compatible with barrier cropping to decrease whitefly (Bemisia tabaci MED) densities on cotton in China. Insects 2020, 11, 57. [Google Scholar] [CrossRef]
- Vafaie, E.K.; Pemberton, H.B.; Gu, M.; Kerns, D.; Eubanks, M.D.; Heinz, K.M. Using multiple natural enemies to manage sweetpotato whiteflies (Hemiptera: Aleyrodidae) in commercial poinsettia (malpighiales: Euphorbiaceae) production. J. Integr. Pest Manag. 2021, 12, 18. [Google Scholar] [CrossRef]
- Pereyra, J.G.; Martínez, G.N.; De los Santos Villalobos, S.; Graciano, R.R.; Montelongo, A.M.; Roldan, H.M. Formulation of a bioinsecticide based on neem and chamomile used for the greenhouse control of the glasshouse whitefly Trialeurodes Vaporariorum. Mod. Environ. Sci. Eng. 2021, 7, 119–125. [Google Scholar]
- Tegene, B.G.; Tenkegna, T.A. Mode of action, mechanism and role of microbes in bioremediation service for environmental pollution management. J. Biotechnol. Bioinform. Res. 2020, 116, 39–50. [Google Scholar]
- Taggar, G.K.; Singh, R. Evaluation of some nonconventional insecticides against whitefly Bemisia tabaci in black gram. Indian J. Entomol. 2020, 82, 294–297. [Google Scholar] [CrossRef]
- Chen, J.C.; Wang, Z.H.; Cao, L.J.; Gong, Y.J.; Hoffmann, A.A.; Wei, S.J. Toxicity of seven insecticides to different developmental stages of the whitefly Bemisia tabaci MED (Hemiptera: Aleyrodidae) in multiple field populations of China. Ecotoxicology 2018, 27, 742–751. [Google Scholar] [CrossRef]
- Natikar, P.K.; Balikai, R.A. Bio-efficacy of insecticides against major insect pests of potato during kharif season in India. Potato Res. 2022, 65, 379–393. [Google Scholar] [CrossRef]
- Rehman, H. Use of Chrysoperla carnea larvae to control whitefly (Aleyrodidea: Hemiptera) on tomato plant in greenhouse. Pure Appl. Biol. 2020, 9, 2128–2137. [Google Scholar] [CrossRef]
- Kumar, R.; Kranthi, S.; Nagrare, V.S.; Monga, D.; Kranthi, K.R.; Rao, N.; Singh, A. Insecticidal activity of botanical oils and other neem-based derivatives against whitefly, Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) on cotton. Int. J. Trop Insect Sci. 2019, 39, 203–210. [Google Scholar] [CrossRef]
- Tian, J.; Diao, H.; Liang, L.; Arthurs, S.; Ma, R. Pathogenicity of Isaria fumosorosea to Bemisia tabaci, with some observations on the fungal infection process and host immune response. J. Invertebr. Pathol. 2015, 130, 147–153. [Google Scholar] [CrossRef]
- Iqbal, M.; State, K.; Academy, M.; Naeem, M.; Aziz, U.; Khan, M. An overview of cotton leaf curl virus disease, persistent challenge for cotton production an overview of cotton leaf curl virus disease, persistent challenge for cotton production. Bulg. J. Agaric Sci. 2014, 20, 405–415. [Google Scholar]
- Shukla, A.K.; Upadhyay, S.K.; Mishra, M.; Saurabh, S.; Singh, R.; Singh, H.; Srivastava, S. Expression of an insecticidal fern protein in cotton protects against whitefly. Nat. Biotech. 2016, 34, 10461051. [Google Scholar] [CrossRef]
- Hunter, W.B.; Wintermantel, W.M. Optimizing Efficient RNAi-Mediated Control of Hemipteran Pests (Psyllids, Leafhoppers, Whitefly): Modified Pyrimidines in dsRNA Triggers. Plants 2021, 9, 1782. [Google Scholar] [CrossRef]
- Wawdhane, P.A.; Nandanwar, V.N.; Mahankuda, B.; Ingle, A.S.; Chaple, K.I. Bio-efficacy of insecticides and bio pesticides against major sucking pests of Bt cotton. J. Entomol. Zoo Stud. 2020, 8, 829–833. [Google Scholar]
- Kamlesh, M.; Raghavendra, K.V.; Kumar, M. Vector management strategies against Bemisia tabaci (Gennadius) transmitting potato apical leaf curl virus in seed potatoes. Potato Res. 2021, 64, 167–176. [Google Scholar] [CrossRef]
- Papnai, G.; Nautiyal, P.; Joshi, N.; Supyal, V. Traditional knowledge and indigenous practices still in vogue among rural populace of Garhwal Hills, Uttarakhand, India. J. Pharmacogn Phytochem. 2020, 9, 145–147. [Google Scholar]
- Deguine, J.P.; Aubertot, J.N.; Flor, R.J.; Lescourret, F.; Wyckhuys, K.A.; Ratnadass, A. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev. 2021, 41, 1–35. [Google Scholar] [CrossRef]
- Gullan, P.J.; Martin, J.H. Sternorrhyncha:(Jumping plant-lice, whiteflies, aphids, and scale insects). In Encyclopedia of Insects; Academic Press: Cambridge, MA, USA, 2009; pp. 957–967. [Google Scholar]
- Boykin, L.M.; Bell, C.D.; Evans, G.; Small, I.; De Barro, P.J. Is agriculture driving the diversification of the Bemisia tabaci species complex (Hemiptera: Sternorrhyncha: Aleyrodidae)? Dating, diversification and biogeographic evidence revealed. BMC Evol. Biol. 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Liu, T.X.; Stansly, P.A.; Gerling, D. Whitefly parasitoids: Distribution, life history, bionomics, and utilization. Annu. Rev. Entomol. 2015, 60, 273–292. [Google Scholar] [CrossRef]
- Njoroge, M.K.; Mutisya, D.L.; Miano, D.W.; Kilalo, D.C. Whitefly species efficiency in transmitting cassava mosaic and brown streak virus diseases. Cogent Biol. 2017, 3, 1311499. [Google Scholar] [CrossRef]
- Chandrashekar, K.; Rao, A.; Gorane, A.; Verma, R.; Tripath, S. Aleurothrixus trachoides (Back) can transmit begomovirus from Duranta to potato, tomato and bell pepper. J. Biosci. 2020, 45, 36. [Google Scholar] [CrossRef]
- Dinsdale, A.; Cook, L.G.; Riginos, C.; Buckley, Y.M.; De Barro, P. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- Frohlich, D.R.; Torres-Jerez, I.; Bedford, D.; Markham, P.G.; Brown, J.K. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol. Ecol. 1999, 8, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Boykin, L.M.; Shatters, R.G.; Rosell, R.C., Jr.; McKenzie, C.L.; De Barro, P.; Frohlich, D.R. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using bayesian analysis of mitochondrial COI DNA sequences. Mol. Phylogenet Evol. 2007, 44, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Van den Elsen, F.H. Resistance Mechanisms against Bemisia Tabaci in Wild Relatives of Tomato. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2013; pp. 9–15. [Google Scholar]
- Shatters, R.G., Jr.; Powell, C.A.; Boykin, L.M.; Liansheng, H.; McKenzie, C.L. Improved DNA barcoding method for Bemisia tabaci and related Aleyrodidae: Development of universal and Bemisa tabaci biotype specific mitochondrial cytochrome oxidase I polymerase chain reaction primers. J. Econ. Entomol. 2009, 102, 750–758. [Google Scholar] [CrossRef]
- Guo, Q.; Tao, Y.; Chu, D. Characterization and comparative profiling of miRNAs in invasive Bemisia tabaci (Gennadius) B and Q. PLoS ONE. 2013, 8, e59884. [Google Scholar] [CrossRef]
- De Marchi, B.R.; Kinene, T.; Mbora Wainaina, J.; Krause-Sakate, R.; Boykin, L. Comparative transcriptome analysis reveals genetic diversity in the endosymbiont hamiltonella between native and exotic populations of Bemisia tabaci from Brazil. PLoS ONE 2018, 13, e0201411. [Google Scholar]
- Shadmany, M.; Boykin, L.M.; Muhamad, R.; Omar, D. Genetic diversity of Bemisia tabaci (Hemiptera: Aleyrodidae) species complex across Malaysia. J. Econ. Entomol. 2019, 112, 75–84. [Google Scholar] [CrossRef]
- Bedford, I.D.; Pinner, M.; Liu, S.; Markham, P.G. Bemisia tabaci potential infestation, phytotoxicity and virus transmission within European agriculture. In Proceedings of the Brighton Crop Protection Conference: Pests and Diseases 3, Brighton, UK, 21–24 November 1994; The British Crop Protection Council: Farnham, UK, 1994; pp. 911–916. [Google Scholar]
- Pan, H.; Li, X.; Ge, D.; Wang, S.; Wu, Q.; Xie, W.; Jiao, X.; Chu, D.; Liu, B.; Xu, B.; et al. Factors affecting population dynamics of maternally transmitted endosymbionts in Bemisia tabaci. PLoS ONE 2012, 7, e30760. [Google Scholar]
- Capinera, J. Handbook of Vegetable Pests; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Fekrat, L.; Shishehbor, P. Some biological features of cotton whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) on various host plants. Pak. J. Biol. Sci. 2007, 10, 3180–3184. [Google Scholar]
- Lindquist, R.K.; Cloyd, R.A. Identification of Insects and Related Pests of Horticultural Plants; Cuthbert, C., Carver, S.C., Eds.; OFA Services, Inc.: Columbus, OH, USA, 2005; pp. 1–50. [Google Scholar]
- Qiu, J.; Song, F.; Mao, L.; Tu, J.; Guan, X. Time-dose-mortality data and modeling for the entomopathogenic fungus. Can. J. Microbiol. 2013, 101, 97–101. [Google Scholar] [CrossRef]
- Baldin, E.L.L.; Fanela, T.L.; Pannuti, L.E.; Kato, M.J.; Takeara, R.; Crotti, A.E. Botanical extracts: Alternative control for silverleaf whitefly management in tomato Extratos botânicos: Controle alternativo para o manejo de mosca-branca em tomateiro. Hortic. Bras. 2015, 33, 59–65. [Google Scholar] [CrossRef]
- Leite, G.L.; Picanço, M.; Guedes, R.N.; Moreira, M.D. Factors affecting attack rate of whitefly on the eggplant. Pesqui. Agropecuária Bras. 2003, 38, 545–549. [Google Scholar] [CrossRef]
- Tressia, W.N. Evaluation of living and synthetic mulches with and without imidacloprid for suppression of whiteflies and aphids and insects transmitted viral diseases in zucchini squash. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2007. [Google Scholar]
- Lot, H.; Delecolle, B.; Lecoq, H. A whitefly transmitted virus causing muskmelon yellows in France. Acta Hortic. 1982, 127, 175–182. [Google Scholar] [CrossRef]
- Gonzalez, M.S.; Lima, B.G.; Oliveira, A.F.; Nunes, D.D.; Fernandes, C.P.; Santos, M.G.; Tietbohl, L.A.; Mello, C.B.; Rocha, L.; Feder, D. Effects of essential oil from leaves of Eugenia sulcata on the development of agricultural pest insects. Rev. Bras Farmacogn. 2014, 24, 413–418. [Google Scholar] [CrossRef]
- Qiu, B.L.; De Barro, P.J.; He, Y.R.; Ren, S.X. Suitability of Bemisia tabaci (Hemiptera: Aleyrodidae) instars for the parasitization by Encarsia bimaculata and Eretmocerus sp nr. furuhashii (Hymenoptera: Aphelinidae) on glabrous and hirsute host plants. Biocontrol. Sci. Technol. 2007, 17, 823–839. [Google Scholar] [CrossRef]
- Javaid, S.; Amin, I.; Jander, G.; Mukhtar, Z.; Saeed, N.A.; Mansoor, S. A transgenic approach to control hemipteran insects by expressing insecticidal genes under phloem-specific promoters. Sci. Rep. 2016, 6, 34706. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Y.; Wang, Z.; Wu, M.; Fu, J.; Guo, J. Inaccessibility of doublestranded RNAs in plastids restrict RNA interference in Bemisia tabaci (whitefly). Pest Manag. Sci. 2020, 76, 3168–3176. [Google Scholar]
- Maranha, E.A.; Maranha, E. Host plant influences pathogenicity of Beauveria bassiana to Bemisia tabaci and its sporulation on cadavers. Biocontrol 2006, 51, 519–532. [Google Scholar]
- Prayogo, Y.; Bayu, M.S.Y.I. Biological control of Bemisia tabaci gennadius by using entomopathogenic fungi Aschersonia aleyrodis. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Malang, Indonesia, 2–3 October 2019; IOP Publishing Ltd.: Bristol, UK; Volume 456, pp. 1–8.
- Cabanillas, H.E.; Jones, W.A. Pathogenicity of Isaria sp. (Hypocreales: Clavicipitaceae) against the sweet potato whitefly B biotype, Bemisia tabaci (Hemiptera: Aleyrodidae). Crop Prot. 2009, 28, 333–337. [Google Scholar] [CrossRef]
- Elango, K.; Sobhana, E.; Sujithra, P.; Bharath, D.; Ahuja, A. Traditional agricultural practices as a tool for management of insects and nematode pests of crops: An overview. J Entomol Zool Stud. 2020, 8, 237–245. [Google Scholar]
- Soumia, P.S.; Pandi, G.G.; Krishna, R.; Ansari, W.A.; Jaiswal, D.K.; Verma, J.P.; Singh, M. Whitefly-transmitted plant viruses and their management. In Emerging Trends in Plant Pathology; Springer: Singapore, 2020; pp. 175–195. [Google Scholar]
- Razza, J.M.; Liburd, O.E.; Nuessly, G.S.; Samuel-Foo, M. Evaluation of bioinsecticides for management of Bemisia tabaci (Hemiptera: Aleyrodidae) and the effect on the whitefly predator Delphastus catalinae (Coleoptera: Coccinellidae) in organic squash. J. Econ. Entomol. 2016, 109, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.B.; Monteiro, T.R.; Cabral, G.B.; Aragão, F.J. RNAi-mediated resistance to whitefly (Bemisia tabaci) in genetically engineered lettuce (Lactuca sativa). Tran. Res. 2017, 26, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.J. Newsletter of work group on Bemisia tabaci. Newsletter 1992, 5, 1–3. [Google Scholar]
- Cohen, S.; Antignus, Y. Tomato yellow leaf curl virus, a whitefly-borne geminivirus of tomatoes. In Advances in Disease Vector Research; Springer: New York, NY, USA, 1994; pp. 259–288. [Google Scholar]
- Calvo, J.; Bolckmans, K.; Stansly, P.A.; Urbaneja, A. Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. Biocontrol 2009, 54, 237. [Google Scholar] [CrossRef]
- Calvo, F.J.; Torres-Ruiz, A.; Velázquez-González, J.C.; Rodríguez-Leyva, E.; Lomeli-Flores, J.R. Evaluation of Dicyphus hesperus for biological control of sweet potato whitefly and potato psyllid on greenhouse tomato. BioControl 2016, 61, 237–246. [Google Scholar] [CrossRef]
- Bughdady, A.; Mehna, A.E.; Amin, T. Effectiveness of synthetic insecticides against the whitefly, (Bemisia tabaci G.) on tomato, (Lycopersicon esculentum MILL.) and infestation impacts on certain photosynthetic pigments concentrations of tomato plant leaves. J. Product. Dev. 2020, 25, 307–321. [Google Scholar] [CrossRef]
- Islam, M.T.; Olleka, A.; Ren, S. Influence of neem on susceptibility of Beauveria bassiana and investigation of their combined efficacy against sweetpotato whitefly, Bemisia tabaci on eggplant. Pestic. Biochem Physiol. 2010, 98, 45–49. [Google Scholar] [CrossRef]
- Islam, T.; Shunxiang, R. Effects of sweetpotato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) infestation on eggplant (Solanum melongena L.) leaf. J. Pest Sci. 2009, 82, 211–215. [Google Scholar] [CrossRef]
- Li, Q.; Tan, W.; Xue, M.; Zhao, H.; Wang, C. Dynamic changes in photosynthesis and chlorophyll fluorescence in Nicotiana tabacum infested by Bemisia tabaci (Middle East–Asia Minor 1) nymphs. Arthropod-Plant Interact. 2013, 7, 431–443. [Google Scholar] [CrossRef]
- Li, Q.; Tan, W.; Xue, M.; Zhao, H. Dynamic changes in energy metabolism and electron transport of photosystem II in Nicotiana tabacum infested by nymphs of Bemisia tabaci (Middle East-Asia Minor 1). Arthropod Plant Interact. 2018, 12, 505–515. [Google Scholar] [CrossRef]
- Saeedi, Z.; Ziaee, M. Biochemical responses of two sugarcane varieties to whitefly Neomaskellia andropogonis infestation and its control by a new butenolide insecticide, flupyradifurone. Agric. For. 2020, 66, 69–81. [Google Scholar] [CrossRef]
- Al-Shareef, L.A. Impact of whitefly, Bemisia tabaci (Gennadius) infestation on chlorophyl and carotene concentrations, as well as moisture content in some vegetable plants in a greenhouse. Egypt J. Exp. Biol. 2011, 7, 11–15. [Google Scholar]
- McAuslane, H.J.; Chen, J.; Carle, R.B.; Schmalstig, J. Influence of Bemisia argentifolii (Homoptera: Aleyrodidae) infestation and squash silverleaf disorder on zucchini seedling growth. J Econo Entomol. 2004, 97, 1096–10105. [Google Scholar] [CrossRef]
- Shen, B.B.; Ren, S.X.; Musa, P.H.; Chen, C. A study on economic threshold of Bemisia tabaci. Acta Univ. Agric. Silvic. 2004, 27, 234–237. [Google Scholar]
- Chand, R.; Jokhan, A.; Prakash, R. Egg deposition by spiralling whiteflies (Aleurodicus dispersus) reduces the stomatal conductance of cassava (Manihot esculenta). Wētā 2018, 52, 55–60. [Google Scholar]
- Schutze, I.X.; Yamamoto, P.T.; Malaquias, J.B.; Naranjo, S.E. Network correlation to evidence the influence of Bemisia tabaci feeding in the photosynthesis and foliar sugar and starch composition in soybean. Ph.D. Thesis, University Sao Paulo, São Paulo, Brazil, 2021. [Google Scholar]
- Martinez, A. Georgia Plant Disease Loss Estimates; Annual Publication 102-10; University of Georgia Cooperative Extension: Griffin, GA, USA, 2007. [Google Scholar]
- Little, E.L. Georgia Plant Disease Loss Estimates; Annual Publication; University of Georgia Cooperative Extension: Athens, GA, USA, 2016; pp. 102–109. [Google Scholar]
- Norman, J.W.J.R.; Riley, D.G.; Stansly, P.A.; Ellsworth, P.C.; Toscano, N.C. Management of Silverleaf Whitefly: A Comprehensive Manual on the Biology, Economic Impact and Control Tactics. 1991. Available online: https://ucanr.edu/sites/CottonIPM/files/181441.pdf (accessed on 9 October 2021).
- Attaway, D. Cucurbit Leaf Crumple Virus Found in South Carolina Cucurbit Crops. 2019. Available online: https://news.clemson.edu/cucurbit-leaf-crumple-virus-found-in-south-carolina-cucurbit-crops/ (accessed on 9 October 2021).
- Chandel, R.S.; Banyal, D.K.; Singh, B.P.; Malik, K.; Lakra, B.S. Integrated management of whitefly, Bemisia tabaci (Gennadius) and potato apical leaf curl virus in India. Potato Res. 2010, 53, 129–139. [Google Scholar] [CrossRef]
- Selvaraj, K.; Sumalatha, B.V.; Poornesha, B.; Ramanujam, B.; Shylesha, A.N. Biological control of invasive rugo siralling whitefly in coconut. In Biological and Utilizatin of Insect in North East; Hebbal: Bangalore, India, 2019; pp. 1–14. [Google Scholar]
- Prasannath, K.; Dharmadasa, N.; Menike, N.; De Costa, D.M. Evaluation of the effects of an eco-friendly crop protection system on management of whitefly-vectored chilli leaf curl virus disease in Sri Lanka. Phytoparasitica 2020, 48, 117–129. [Google Scholar] [CrossRef]
- Dent, D. Insect Pest Management, 1st ed.; CABI: Iver, UK, 1991. [Google Scholar]
- Padhi, N.N.; Misra, R.P. Control of Rotylenchulus reniformis on French bean (Phaseolus vulgaris L.). Indian J. Nematol. 1987, 17, 130–131. [Google Scholar]
- Isman, M.B. Bridging the gap: Moving botanical insecticides from the laboratory to the farm. Ind. Crops Prod. 2017, 110, 10–14. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Galle, C.L.; Keith, S.R.; Kalscheur, N.A.; Kemp, K.E. Effect of commercially available plantderived essential oil products on arthropod pests. J. Econ. Entomol. 2009, 102, 1567–1579. [Google Scholar] [CrossRef]
- Mullins, J.W. Imidacloprid: A new nitroguanidine insecticide. Am. Chem. Soc. Symp. 1993, 524, 183–198. [Google Scholar]
- Riley, D.G. Insecticide control of sweetpotato whitefly in south Texas. Subtrop Plant Sci. 1994, 46, 45–49. [Google Scholar]
- Liu, T.X.; Meister, C.W. Managing Bemisia argentifolii on spring melons with insect growth regulators, entomopathogens and imidacloprid in south Texas. Subtrop Plant Sci. 2001, 53, 44–48. [Google Scholar]
- Shejulpatil, S.J.; Kakad, M.N.; Lande, G.K. Effect of insecticides against whitefly on brinjal under field condition. Int. J. Chem. Stud. 2019, 7, 1100–1103. [Google Scholar]
- Quesada-Moraga, E.E.; Maranhao, E.A.; Valverde-García, P.; Santiago-Álvarez, C. Selection of Beauveria bassiana isolates for control of the whiteflies Bemisia tabaci and Trialeurodes vaporariorum on the basis of their virulence, thermal requirements, and toxicogenic activity. Biol. Control. 2006, 36, 274–287. [Google Scholar] [CrossRef]
- Simmons, A.M.; Kousik, C.S.; Levi, A. Combining reflective mulch and host plant resistance for sweetpotato whitefly (Hemiptera: Aleyrodidae) management in watermelon. Crop Prot. 2010, 29, 898–902. [Google Scholar] [CrossRef]
- Athar, H.U.R.; Bhatti, A.R.; Bashir, N.; Zafar, Z.U.; Farooq, A. Modulating infestation rate of white fly (Bemicia tabaci) on okra (Hibiscus esculentus L.) by nitrogen application. Acta Physiol. Plant. 2010, 33, 843–850. [Google Scholar] [CrossRef]
- Lapidot, M.; Legg, J.P.; Wintermantel, W.M.; Polston, J.E. Chapter Three—Management of Whitefy-Transmitted Viruses in Open-Field Production Systems. In Advances in Virus Research; Loebenstein, G., Katis, N., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 147–206. Volume 90. [Google Scholar]
- Abd-Rabou, S.; Simmons, A.M. Effect of three irrigation methods on incidences of Bemisia tabaci (Hemiptera: Aleyrodidae) and some whitefly-transmitted viruses in four vegetable crops. Trends Entomol. 2012, 8, 21–26. [Google Scholar]
- Togni, P.H.; Marouelli, W.A.; Inoue-Nagata, A.K.; Pires, C.S.; Sujii, E.R. Integrated cultural practices for whitefly management in organic tomato. J. Appl. Entomol. 2018, 142, 998–1007. [Google Scholar] [CrossRef]
- Simmons, A.M.; Abd-Rabou, S. Population of the sweet potato whitefly in response to different rates of three sulfur-containing fertilizers on ten vegetable crops. Int. J. Veg. Sci. 2008, 5, 7–70. [Google Scholar]
- Ellsworth, P.C.; Martinez-Carrillo, J.L. IPM for Bemisia tabaci: A case study from North America. Crop Prot. 2001, 20, 853–869. [Google Scholar] [CrossRef]
- Mohamed, M. Impact of planting dates, spaces and varieties on infestation of cucumber plants with whitefly, Bemisia tabaci (Genn.). J. Basic Appl. Zool. 2012, 65, 17–20. [Google Scholar] [CrossRef]
- Hilje, L.; Costa, H.S.; Stansly, P.A. Cultural practices for managing Bemisia tabaci and associated viral diseases. Crop Prot. 2001, 20, 801–812. [Google Scholar] [CrossRef]
- Ahsan, M.I.; Hossain, M.S.; Parvin, S.; Karim, Z. Effect of varieties and planting dates on the incidence of aphid and white fly attack on tomato. Int. J. Sustain. Agric Technol. 2005, 1, 26–30. [Google Scholar]
- Nyoike, T.W.; Liburd, O.E. Effect of living (buckwheat) and UV reflective mulches with and without imidacloprid on whiteflies, aphids and marketable yields of zucchini squash. Int. J. Pest Manag. 2009, 56, 31–39. [Google Scholar] [CrossRef]
- Hilje, L.; Stansly, P.A. Living ground covers for management of Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) and Tomato yellow mottle virus (ToYMoV) in Costa Rica. Crop Prot. 2008, 7, 10–16. [Google Scholar] [CrossRef]
- Manandhar, R.; Cerruti, R.; Hooks, R.; Wright, M.G. Influence of cover crop and intercrop systems on Bemisia argentifolli (Hemiptera: Aleyrodidae) infestation and associated Squash silverleaf disorder in zucchini. Environ. Entomol. 2009, 38, 442–449. [Google Scholar] [CrossRef]
- Smith, H.A.; Koenig, R.L.; McAuslane, H.J.; McSorley, R. Effect of silver reflective mulch and a summer squash trap crop on densities of immature Bemisia argentifolii (Homoptera: Aleyrodidae) on organic bean. J. Econ. Entomol. 2000, 93, 726–731. [Google Scholar] [CrossRef]
- Summers, C.G.; Mitchell, J.P.; Stapleton, J.J. Management of aphid-borne viruses and Bemisia argentifolii (Homoptera: Aleyrodidae) in zucchini squash by using UV reflective plastic and wheat straw mulches. Environ. Entomol. 2005, 33, 1447–1457. [Google Scholar] [CrossRef]
- Nasruddin, A.; Agus, N.; Saubil, A.; Jumardi, J.; Rasyid, B.; Siriniang, A.; Nasruddin, A.D.; Firdaus, F.; Said, A.E. Effects of mulch type, plant cultivar, and insecticide use on sweet potato whitefly population in chili pepper. Scientifica 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Schuster, D.J. Squash as a trap crop to protect tomato from whitefly-vectored tomato yellow leaf curl. Int. J. Pest Manag. 2004, 50, 281–284. [Google Scholar] [CrossRef]
- El-Serwiy, S.A.; Ali, A.A.; Razoki, I.A. Effect of intercropping of some host plants with tomato on population density of tobacco whitefly, Bemisia tabaci (Genn.), and the incidence of Tomato yellow leaf curl virus (TYLCV) in plastic houses. J. Agric Water Resour. Res. 1987, 6, 79–81. [Google Scholar]
- Musa. A.A. Incidence, economic importance, and control of tomato yellow leaf curl in Jordan. Plant Dis. 1982, 66, 561–563. [Google Scholar] [CrossRef]
- Verma, A.K.; Mitra, P.; Saha, A.K.; Ghatak, S.S.; Bajpai, A.K. Effect of trap crops on the population of the whitefly Bemisia tabaci (Genn.) and the diseases transmitted by it. Bull Indian Aca Seri. 2011, 15, 99–106. [Google Scholar]
- Afifi, F.M.L.; Haydar, M.F.; Omar, H.I.H. Effect of different intercropping systems on tomato infestation with major insect pests; Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae), Myzus persicae Sulzer (Homoptera: Aphididae) and Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae). Bull Fac. Agric. 1990, 41, 885–900. [Google Scholar]
- Schuster, D.J. Preference of Bemisia argentifolii (Homoptera: Aleyrodidae) for selected vegetable hosts. J. Agric Urban Entomol. 2003, 20, 59–67. [Google Scholar]
- Rajasri, M.; Lakshmi, K.V.; Reddy, K.L. Management of whitefly transmitted Tomato leaf curl virus using guard crops in tomato. Indian J. Plant Prot. 2009, 37, 101–103. [Google Scholar]
- Yang, Z.; Ma, C.; Wang, X.; Long, H.; Liu, X.; Yang, X. Preference of Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) to four vegetable hosts. Acta Entomol. Sin. 2004, 47, 612–627. [Google Scholar]
- Asawalam, E.F.; Chukwu, E.U. The effect of intercropping okra with ginger on the population of flea beetle (Podagrica sjostedti Jacoby Coleoptera: Chrysomelidae) and whitefly (Bemisia tabaci Genn Homoptera: Aleyrodidae) and the yield of okra in Umudike Abia State, Nigeria. J. Agric Biol. Sci. 2012, 3, 300–304. [Google Scholar]
- Sharma, A.; Neupane, K.R.; Regmi, R.; Neupane, R.C. Effect of intercropping on the incidence of jassid (Amrasca biguttula biguttula Ish.) and whitefly (Bemesia tabaci Guen.) in okra (Abelmoschus esculentus L. Moench). J. Agric Nat. Resour. 2018, 1, 179–188. [Google Scholar] [CrossRef]
- Kumar, A.; Raj Bhansali, R.; Mali, P.C. Response of biocontrol agents in relation to acquired resistance against leaf curl virus in chilli. In Proceedings of Asian Congress of Mycology Plant Pathology, Mysore, India, 1–4 October 2002; University of Mysore: Mysore, India; Indian Society of Mycology and Plant Pathology: Udaipur, India, 2002; p. 167. [Google Scholar]
- Karthikeyan, C.; Veeraragavathatham, D.; Karpagam, Firdouse, A.A. Cow Based Indigenous technologies in dry farming. Indian J. Tradit Knowl. 2006, 5, 47–50. [Google Scholar]
- Singh, R.S.; Sitaramaiah, K. Effect of decomposing green leaves, sawdust and urea on the incidence of root-knot of okra and tomato. Indian Phytopath. 1967, 20, 349–355. [Google Scholar]
- Bhattacharya, D.; Goswami, B.K. Comparative efficacy of neem and groundnut oil-cakes with aldicarb against Mezuidugyne incognita in tomato. Revue Nématol. 1987, 10, 467–470. [Google Scholar]
- Patel, C.C.; Singh, D.; Sridhar, V.; Choudhary, A.; Dindod, A.; Padaliya, S.R. Bioefficacy of cow urine and different types of bio-pesticide against major sucking insect pests of cowpea. Int. J. Chem. Stud. 2019, 7, 4664–4667. [Google Scholar]
- Shailaja, B.; Patnaik, H.P.; Mukherjee, S.K. Assessment of botanicals fermented in cow urine alone and along with panchagavya against brinjal shoot and fruit borer. J. Eco-Friendly Agric. 2012, 7, 24–28. [Google Scholar]
- Radhakrishnan, T.; Anandaraja, M.; Ramasubramanian, M.; Nirmala, L.; Israel Thomas, M. Traditional Agricultural Practices-Applications and Technical Implements; New India Publishing Agency: New Delhi, India, 2009. [Google Scholar]
- Patel, N.B.; Korat, D.M.; Acharya, R.R. Impact evaluation of cow-urine and vermiwash on insect pests of brinjal. Int. J. Trop Agric. 2017, 35, 591–595. [Google Scholar]
- Haroon, S.A.; Hassan, B.A.; Hamad, F.M. The efficiency of some natural alternatives in root-knot nematode control. Adv. Plants Agric Res. 2018, 8, 355–362. [Google Scholar]
- Karkar, D.B. Evaluation of cow urine and vermi-wash against insect pests of brinjal. Karnataka J. Agric Sci. 2014, 27, 528–530. [Google Scholar]
- Mandal, S.; Padamshali, S.; Rana, N.; Kolhekar, S. ITK based pest management module for sucking pest on brinjal (Solanum melongena L.) under terai agro-ecological system of West Bengal. J. Pharmacogn. Phytochem. 2018, 7, 2065–2070. [Google Scholar]
- Singh, S.; Yadav, G.S.; Das, A.; Das, B.; Devi, H.L.; Raghuraman, M.; Kumar, A. Bioefficacy, environmental safety and synergistic impacts of biorational formulations against whitefly, leafhopper and blister beetle in organic okra ecosystem. J. Agric Sci. 2021, 159, 373–384. [Google Scholar] [CrossRef]
- Celsia, S.; Janarthanan, P. Indigenous technology knowledge of rice. Int. J. Curr. Res. 2019, 11, 1810–1811. [Google Scholar]
- Van der Werf, E. Pest Management in Ecological Agriculture. AME Foundation, Groenekan/Holland. In Plant in Pest Control—Garlic and Onion; Vijayalakshmi, K., Subhashini, B., Shivani, V.K., Eds.; Centre for Indian Knowledge System: Chennai, India, 1985; pp. 1–20. [Google Scholar]
- Oparaeke, A.M.; Dike, M.C.; Amatobi, C.I. Fermented cow dung: A home-produced insecticide against post flowering insect pests of cowpea, Vigna unguiculata (L.) Walp. Sam. J. Agric. 2003, 19, 121–125. [Google Scholar]
- Yano, E. Control of the greenhouse whitefly, Trialeurodes vaporariorum westwood (Homoptera: Aleyrodidae) by the integrated use of yellow sticky traps and the parasite Encarsia formosa Gahan (Hymenoptra: Aphelinidae). Appl. Entomol. Zool. 1986, 22, 159–165. [Google Scholar] [CrossRef]
- Gu, X.S.; Bu, W.J.; Xu, W.H.; Bai, Y.C.; Liu, B.M.; Liu, T.X. Population suppression of Bemisia tabaci (Hemiptera: Aleyrodidae) using yellow sticky traps and Eretmocerus rajasthanicus (Hymenoptera: Aphelinidae) on tomato plants in greenhouses. Insect Sci. 2008, 15, 263–270. [Google Scholar] [CrossRef]
- Nair, I.J.; Sharma, S.; Shera, P.S. Impact of sticky traps of different colours and shapes against sucking pests of tomato under protected conditions: A randomized controlled trial. Int. J. Trop Insect Sci. 2021, 41, 2739–2746. [Google Scholar] [CrossRef]
- Lu, Y.; Bei, Y.; Zhang, J. Are yellow sticky traps an effective method for control of sweetpotato whitefly, Bemisia tabaci, in the greenhouse or field? J. Insec. Sci. 2012, 12, 113. [Google Scholar] [CrossRef]
- Hoelmer, K.A.; Roltsch, W.J.; Chu, E.C.; Hekneberry, T.J. Selectivity of whitefly traps in cotton for Eretmocerus eremicus (Hymenoptera: Aphelinidae), a native parasitoid of Bemisia argentifolii (Homoptera: Aleyrodidae). Environ. Entomol. 1998, 27, 1039–1044. [Google Scholar] [CrossRef][Green Version]
- Moreau, T.L.; Isman, M.B. Trapping whiteflies? A comparison of greenhouse whitefly (Trialeurodes vaporariorum) responses to trap crops and yellow sticky traps. Pest Manag. Sci. 2011, 67, 408–413. [Google Scholar] [CrossRef]
- Bhutto, N.N.; Shar, Z.U.; Kalroo, M.A.; Rind, A.B.; Solangi, U.A. Management of sucking insect pests of cotton crop through yellow sticky traps under field conditions. Int. J. Farm Alli Sci. 2021, 10, 36–39. [Google Scholar]
- Chabra, H.K.; Grewal, P.S.; Singh, A. Efficacy of some plant extracts on root knot nematode (Meloidogyne incognita). J. Tree Sci. 1988, 7, 24–25. [Google Scholar]
- Wagan, T.A.; Dhaunroo, A.A.; Jiskani, W.M.; Sahito, M.H.; Soomro, A.A.; Lakho, A.B.; Wagan, S.A.; Memon, Q.U.; Tunio, S.K. Evaluation of four-color sticky traps for monitoring whitefly and thrips on Okra crops at Tando Jam, Pakistan. J. Biol. Agric Health. 2017, 7, 12–15. [Google Scholar]
- Charavan, R.; Yeotikar, S.; Gaikwad, B.; Dongarjal, R. Management of major pests of tomato with biopesticides. J. Entomol. Res. 2015, 39, 213. [Google Scholar] [CrossRef]
- Hussein, H.S.; Salem, M.Z.M.; Soliman, A.M. Repellent, attractive, and insecticidal effects of essential oils from Schinus terebinthifolius fruits and Corymbia citriodora leaves on two whitefly species, Bemisia tabaci, and Trialeurodes ricini. Sci. Hortic. 2017, 216, 111–119. [Google Scholar] [CrossRef]
- Vite-Vallejo, O.; Barajas-Fernández, M.G.; Saavedra-Aguilar, M.; Cardoso-Taketa, A. Insecticidal effects of ethanolic extracts of Chenopodium ambrosioides, Piper nigrum, Thymus vulgaris, and Origanum vulgare against Bemisia tabaci. Southwest Entomol. 2018, 43, 383–393. [Google Scholar] [CrossRef]
- Bissdorf, J.K. How to Grow Crops without Endosulfan—Field Guide to Non-Chemical Pest Management; Webber, C., Ed.; Pesticide Action Network (PAN): Hamburg, Germany, 2008; p. 71. [Google Scholar]
- Mkenda, P.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.; Mtei, K.; Belmain, S.R. Extracts from field margin weeds provide economically viable and environmentally benign pest control compared to synthetic pesticides. PLoS ONE 2015, 10, e0143530. [Google Scholar]
- Tembo, Y.; Mkindi, A.G.; Mkenda, P.A.; Mpumi, N.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.A.; Belmain, S.R. Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Front Plant Sci. 2018, 9, 1425. [Google Scholar] [CrossRef]
- Ravindran, P.N.; Babu, K.N.; Sivaraman, K. (Eds.) Turmeric: The Genus Curcuma; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kumar, P.; Poehling, H.M. Persistence of soil and foliar azadirachtin treatments to control sweetpotato whitefly Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) on tomatoes under controlled (laboratory) and field (netted greenhouse) conditions in the humid tropics. J Pest Sci. 2006, 79, 189–199. [Google Scholar] [CrossRef]
- El Shafie, H.A.F.; Abdelraheem, B.A. Field evaluation of three biopesticides for integrated management of major pests of tomato, Solanum lycopersicum L. Agric. Biol. J. N. Am. 2012, 3, 340–344. [Google Scholar] [CrossRef]
- Castillo-Sánchez, L.E.; Jiménez-Osornio, J.J.; Delgado-Herrera, M.A.; Candelaria-Martínez, B.; Sandoval-Gío, J.J. Effects of the hexanic extract of neem Azadirachta indica against adult whitefly Bemisia tabaci. J. Entomol-Ogy Zool. Stud. 2015, 5, 95–99. [Google Scholar]
- Barati, R.; Golmohammadi, G.; Ghajarie, H.; Zarabi, M.; Mansouri, R. Efficiency of some herbal pesticides on reproductive parameters of silverleaf whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Arch. Phytopathol. Plant Prot. 2013, 47, 212–221. [Google Scholar] [CrossRef]
- Diabate, D.; Gnago, J.A.; Koffi, K.; Tano, Y. The effect of pesticides and aqueous extracts of Azadirachta indica (A. Juss) and Jatropha carcus L. on Bemisia tabaci (Gennadius) (Homoptera: Aleyrididae) and Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) found on tomato plants in Côte d’Ivoire. J. Appl. Biosci. 2014, 80, 7132–7143. [Google Scholar] [CrossRef]
- Nzanza, B.; Mashela, P.W. Control of whiteflies and aphids in tomato (Solanum lycopersicum L.) by fermented plant extracts of neem leaf and wild garlic. Afr. J. Biotechnol. 2012, 11, 16077–16082. [Google Scholar]
- Fanela, T.L.; Baldin, E.L.; Pannuti, L.E.; Cruz, P.L.; Crotti, A.E.; Takeara, R.; Kato, M.J. Lethal and inhibitory activities of plant-derived essential oils against Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) biotype B in tomato. Neotrop. Entomol. 2016, 45, 201–210. [Google Scholar] [CrossRef]
- Nottingham, S.F.; Chalfant, R.B. Whiteflies (Bemicia tabaci) on vegetable crops. Proc. Ha. State Hort. Soc. 1994, 107, 163–167. [Google Scholar]
- Hammad, E.A.; Nemer, N.M.; Hawi, Z.K.; Hanna, L.T. Responses of the sweetpotato whitefly, Bemisia tabaci, to the chinaberry tree (Melia azedarach L.) and its extracts. Ann. Appl. Biol. 2000, 137, 79–88. [Google Scholar] [CrossRef]
- Azam, K.M.; Bowers, W.S.; Srikandakumar, A.; Al-Mahmuli, I.H.; Al-Raeesi, A.A. Insecticidal action of plant extracts against nymphs of whitefly, Bemisia tabaci Gennadius. Crop Res. 2002, 24, 390–393. [Google Scholar]
- Zhang, W.; McAuslane, H.J.; Schuster, D.J. Repellency of ginger oil to Bemisia argentifolii (Homoptera: Aleyrodidae) on tomato. J. Econo. Entomol. 2004, 97, 1310–1318. [Google Scholar] [CrossRef]
- Aroiee, H.; Mosapoor, S.; Karimzadeh, H. Control of greenhouse whitefly (Trialeurodes vaporariorum) by thyme and peppermint. Curr. Appl. Sci. Technol. 2005, 5, 511–514. [Google Scholar]
- Aldana Lllanos, A.; Valdés Estrada, M.E.; Figueroa Brito, R.; Pérez Ramírez, A. Control of whitefly Bemisia tabaci with extracts of Trichillia havanensis and Passiflora edulis in the laboratory. In Proceedings of the Interamerican Society for Tropical Horticulture; Inter-American Society for Tropical Horticulture: Homestead, FL, USA, 2006; Volume 50, pp. 717–774. [Google Scholar]
- Porras, M.F.; López-Ávila, A. Effect of extracts from Sapindus saponaria on the glasshouse whitefly Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Rev. Colomb. Entomol. 2009, 35, 7–11. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wu, D.C.; Yu, J.Z.; Chen, B.H.; Wang, C.L.; Ko, W.H. Control of silverleaf whitefly, cotton aphid and kanzawa spider mite with oil and extracts from seeds of sugar apple Neotrop. Entomol. 2009, 38, 531–536. [Google Scholar]
- Ateyyat, M.A.; Al-Mazra’awi, M.; Abu-Rjai, T.; Shatnawi, M.A. Aqueous extracts of some medicinal plants are as toxic as Imidacloprid to the sweet potato whitefly, Bemisia tabaci. J. Insect Sci. 2009, 9, 15. [Google Scholar] [CrossRef]
- Sayeda, F.F.; Torkey, H.M.; Hala, M.A. Natural extracts and their chemical constituents in relation to toxicity against whitefly (Bemisia tabaci) and aphid (Aphis craccivora). Aust. J. Basic Appl. Sci. 2009, 3, 3217–3223. [Google Scholar]
- Pinheiro, P.V.; Quintela, E.D.; Oliveira, J.P.; Seraphin, J.C. Toxicity of neem oil to Bemisia tabaci biotype B nymphs reared on dry bean. Pesq. Agropec. Bras. 2009, 44, 354–360. [Google Scholar] [CrossRef]
- Yang, N.W.; Li, A.L.; Wan, F.H.; Liu, W.X.; Johnson, D. Effects of plant essential oils on immature and adult sweetpotato whitefly, Bemisia tabaci biotype B. Crop Prot. 2010, 29, 1200–1207. [Google Scholar] [CrossRef]
- Lynn, O.M.; Song, W.G.; Shim, J.K.; Kim, J.E.; Lee, K.Y. Effects of azadirachtin and neem-based formulations for the control of sweetpotato whitefly and root-knot nematode. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 598–604. [Google Scholar] [CrossRef]
- Zandi-Sohani, N. Efficiency of Labiateae plants essential oils against adults of cotton whitefly (Bemisia tabaci). Indian J. Agric Sci. 2011, 81, 1164. [Google Scholar]
- Regnault-Roger, C.; Charles, V.; John, T.A. Essential oils in insect control: Low-risk products in a highstakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Baloc, H.A.; Marissa, P.; Bulong, M.P. Efficacy of fermented botanical plant extracts in the management of white flies and 28-Spotted beetles in tomato. Int. J. Sci. Res. 2015, 4, 2566–2569. [Google Scholar]
- Barkman, B. Repellent, irritant and toxic effects of essential oil constituents on Bemisia tabaci (Gennadius). Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lee, D.H.; Nyrop, J.P.; Sanderson, J.P. Non-consumptive effects of the predatory beetle Delphastus catalinae (Coleoptera: Coccinellidae) on habitat use patterns of adult whitefly Bemisia argentifolii (Hemiptera: Aleyrodidae). Appl. Entomol. Zool. 2014, 49, 599–606. [Google Scholar] [CrossRef]
- Rehmana, H.; Nadeema, M.; Ayyazb, M.; Beguma, H.A. Comparative efficacy of neem oil and lambdacyhalothrin against whitefly (Bemesia tabaci) and Jassid (Amrasca Devastans Dist.) in okra field. Russ. Agric. Sci. 2015, 41, 138–145. [Google Scholar] [CrossRef]
- Sawsan, S.M.; Sharaby, A.; Ebadah, I.M.; El-Behery, H. Efficiency of zinc sulfate and some volatile oils on some insect pests of the tomato crop. Glob. Adv. Res. J. Agric Sci. 2015, 4, 182–187. [Google Scholar]
- Ezzat, A.S.; El-Awady, A.A.; Tawfik, A.A. Using some plant extracts to control of mechanical injured, pest management, increasing productivity and storability of potato (Solanum tuberosum L.). J. Plant Prod. 2015, 7, 801–811. [Google Scholar] [CrossRef]
- Deletre, E.; Chandre, F.; Barkman, B.; Menut, C.; Martin, T. Naturally occurring bioactive compounds from four repellent essential oils against Bemisia tabaci whiteflies. Pest Man. Sci. 2016, 72, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Sarker, S. Efficacy of some botanical extracts on plant growth, yield and pest management in eggplant field. J. Environ. Sci. Nat. Resour. 2017, 10, 137–140. [Google Scholar] [CrossRef][Green Version]
- Moghadam, A.; Saidi, M.; Abdossi, V.; Mirab-Balou, M.; Tahmasebi, Z. Insecticidal effect of extracts from six native plants on Bemisia tabaci and some physiological effects on cucumber as host plant. Pak J. Agric. Sci. 2018, 55, 563–568. [Google Scholar]
- Ghosal, A.; Chatterjee, M.L.; Bhattacharyya, A. Field bio-efficacy of some new insecticides and tank mixtures against whitefly on cotton in New Alluvial Zone of West Bengal. Pestic Res. J. 2018, 30, 31–36. [Google Scholar] [CrossRef]
- Sayed, W.A.A.; El-Bendary, H.; El-Helaly, A. Increasing the efficacy of the cotton leaf worm Spodoptera littoralis nucleopolyhedrosis virus using certain essential oils. Egypt. J. Biol. Pest Control 2020, 30, 1–7. [Google Scholar] [CrossRef]
- Okolo, E.T.; Iledun, O.C. Insecticidal effect of neem (Azadirachta indica) extracts obtained from leaves and seeds on pests of cowpea (Vigna Unguiculata). Sumerianz J. Agric Vet. 2019, 2, 20–28. [Google Scholar]
- Fabrick, J.A.; Yool, A.J.; Spurgeon, D.W. Insecticidal activity of marigold Tagetes patula plants and foliar extracts against the hemipteran pests, Lygus hesperus and Bemisia tabaci. PLoS ONE 2020, 15, e0233511. [Google Scholar]
- Peres, M.C.; de Souza Costa, G.C.; dos Reis, L.E.; da Silva, L.D.; Peixoto, M.F.; Alves, C.C.; Forim, M.R.; Quintela, E.D.; Araújo, W.L.; de Melo Cazal, C. In natural and nanoencapsulated essential oils from Xylopia aromatica reduce oviposition of Bemisia tabaci in Phaseolus vulgaris. J. Pest Sci. 2020, 93, 807–821. [Google Scholar] [CrossRef]
- Sweetha, G. Is lemon peel responsible for controlling whitefly? A review article. Int. J. Sci. Dev. Res 2021, 6, 1–3. [Google Scholar]
- Kobenan, K.C.; Bini, K.K.; Kouakou, M.; Kouadio, I.S.; Zengin, G.; Ochou, G.E.; Boka, N.R.; Menozzi, P.; Ochou, O.G.; Dick, A.E. Chemical composition and spectrum of insecticidal activity of the essential oils of Ocimum gratissimum L. and Cymbopogon citratus Stapf on the main insects of the cotton entomofauna in Côte d’Ivoire. Chem. Biodivers. 2021, 27, e2100497. [Google Scholar] [CrossRef]
- De Carvalho, S.S.; do Prado Ribeiro, L.; Forim, M.R.; Bicalho, K.U.; Fernandes, J.B.; Vendramim, J.D. Avocado kernels, an industrial residue: A source of compounds with insecticidal activity against silverleaf whitefly. Environ. Sci. Poll Res. 2021, 28, 2260–2268. [Google Scholar] [CrossRef]
- Soares, M.C.E.; Baldin, E.L.L.; do Prado Ribeiro, L. Lethal and sublethal effects of Annona spp. derivatives on Bemisia tabaci MEAM 1 (Hemiptera: Aleyrodidae) in Tomato. Neotrop. Entomol. 2021, 50, 966–975. [Google Scholar] [CrossRef]
- Cohen, S.; Berlinger, M.J. Transmission and cultural control of whitefly-borne viruses. Agric Ecosyst Environ. 1986, 17, 89–97. [Google Scholar] [CrossRef]
- Antignus, Y.; Lachman, O.; Pearlsman, M.; Koren, A.; Matan, E.; Tregerman, M.; Ucko, O.; Messika, Y.; Omer, S.; Unis, H. Development of an IPM system to reduce the damage of squash leaf curl begomovirus in zucchini squash crops. In Proceedings of the 2nd European Whitefly Symposium, Cavtat, Croatia, 5–9 October 2004. [Google Scholar]
- Berlinger, M.J.; Dahan, R.; Mordechi, S.; Liper, A.; Katz, J.; Levav, N. The use of nets to prevent the penetration of Bemisia tabaci into greenhouse. Hassadeh 1991, 71, 1579–1583. [Google Scholar]
- Antignus, Y.; Lapidot, M.; Hadar, D.; Messika, Y.; Cohen, S. UV absorbing screens serve as optical barriers to protect vegetable crops from virus diseases and insect pests. J. Econ. Entomol. 1998, 91, 1401–1405. [Google Scholar] [CrossRef]
- Diaz, B.M.; Fereres, A. Ultraviolet-blocking materials as a physical barrier to control insect pests and pathogens in protected crops. Pest Tech. 2007, 1, 85–95. [Google Scholar]
- Ben-Yakir, D.; Hadar, M.D.; Offir, Y.; Chen, M.; Tregerman, M. Protecting crops from pests using OptiNet® and ChromatiNet® shading nets. Acta Hortic. 2008, 770, 205–212. [Google Scholar] [CrossRef]
- Legarrea, S.; Karnieli, A.; Fereras, A.; Weintraub, P.G. Comparison of UV-absorbing nets in pepper crops, spectral properties, effects on plants and pest control. Photochem. Photobiol. 2010, 86, 324–330. [Google Scholar] [CrossRef]
- Saady, R.H. Combined effect of mechanical and biological control strategies for managing Bemisia tabaci (hemiptera: Aleyrodidae). Asian J. Biol. 2022, 5, 14–18. [Google Scholar]
- Dougoud, J.; Toepfer, S.; Bateman, M. Efficacy of homemade botanical insecticides based on traditional knowledge. A review. Agron. Sustain Dev. 2019, 39, 1–22. [Google Scholar] [CrossRef]
- Angioni, A.; Dedola, F.; Minelli, E.V.; Barra, A.; Cabras, P.; Caboni, P. Residues and half-life times of pyrethrins on peaches after field treatments. J. Agric Food Chem. 2005, 53, 4059–4063. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Sarais, G.; Angioni, A.; Garcia, A.J.; Lai, F.; Dedola, F. Residues and persistence of neem formulations on strawberry after field treatment. J. Agric Food Chem. 2006, 54, 10026–10032. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides: For richer, for poorer. Pest Manag. Sci. 2008, 64, 8–11. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Pavela, R.; Žabka, M.; Bednář, J.; Tříska, J.; Vrchotová, N. New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.). Ind. Crop Prod. 2016, 83, 275–282. [Google Scholar] [CrossRef]
- Daniel, C.; Wyss, E. Field applications of Beauveria bassiana to control the European cherry fruit fly Rhogoletis cerasi. J. Appl. Entomol. 2010, 134, 9–10. [Google Scholar] [CrossRef]
- Soloneski, S.; Kujawski, M.; Scuto, A.; Larramendy, M.L. Carbamates: A study on genotoxic, cytotoxic, and apoptotic effects induced in Chinese hamster ovary (CHO-K1) cells. Toxicol Vitr. 2015, 29, 834–844. [Google Scholar] [CrossRef]
- Singh, H.; Kaur, T. Pathogenicity of entomopathogenic fungi against the aphid and the whitefly species on crops grown under greenhouse conditions in India. Egypt J. Biol. Pest Control. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Gerling, D.; Alomar, Ò.; Arnò, J. Biological control of Bemisia tabaci using predators and parasitoids. Crop Prot. 2001, 20, 779–799. [Google Scholar] [CrossRef]
- Arnó, J.; Gabarra, R.; Liu, T.X.; Simmons, A.M.; Gerling, D. Natural enemies of Bemisia tabaci: Predators and parasitoids. In Bemisia: Bionomics and Management of a Global Pest; Stansly, P.A., Naranjo, S.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 385–421. [Google Scholar]
- Khan, M.M.; Fan, Z.Y.; O’Neill Rothenberg, D.; Peng, J.; Hafeez, M.; Chen, X.Y.; Pan, H.P.; Wu, J.H.; Qiu, B.L. Phototoxicity of Ultraviolet-A against the Whitefly Bemisia tabaci and Its Compatibility with an Entomopathogenic Fungus and Whitefly Parasitoid. Oxid. Med. Cell. Longev. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Tan, X.; Hu, N.; Zhang, F.; Ramirez-Romero, R.; Desneux, N.; Wang, S.; Ge, F. Mixed release of two parasitoids and a polyphagous ladybird as a potential strategy to control the tobacco whitefly Bemisia tabaci. Sci. Rep. 2016, 6, 28245. [Google Scholar] [CrossRef][Green Version]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Kheirodin, A.; Simmons, A.M.; Legaspi, J.C.; Grabarczyk, E.E.; Toews, M.D.; Roberts, P.M.; Chong, J.H.; Snyder, W.E.; Schmidt, J.M. Can generalist predators control Bemisia tabaci? Insects 2020, 11, 823. [Google Scholar] [CrossRef]
- Alomar, O.; Riudavets, J.; Castañe, C. Macrolophus caliginosus in the biological control of Bemisia tabaci on greenhouse melons. Biol. Control. 2006, 36, 154–162. [Google Scholar]
- Adly, D. Use of predators for controlling the whitefly, Bemisia tabaci Genn. and the two spotted spider mite, Tetranychus urticae koch, in cucumber greenhouses in Egypt. Egypt J. Biol. Pest Control. 2016, 26, 701–706. [Google Scholar]
- Chung, B.K.; Xia, C.; Song, Y.H.; Lee, J.M.; Li, Y.; Kim, H.; Chon, T.S. Sampling of Bemisia tabaci adults using a pre-programmed autonomous pest control robot. J. Asia Pac. Entomol. 2014, 17, 737–743. [Google Scholar] [CrossRef]
- Karut, K.; Kazak, C.; Döker, I. Potential of single and combined releases of Eretmocerus mundus and Macrolophus melanotoma to suppress Bemisia tabaci in protected eggplant. Biol. Control. 2018, 126, 1–6. [Google Scholar] [CrossRef]
- Hoddle, M.S. Biological control of whiteflies on ornamental crops. In Biocontrol in Protected Culture; Heinz, K., Van Driesche, R.G., Parrella, M.P., Eds.; Ball Publishing: Batavia, NY, USA, 2004. [Google Scholar]
- Stansly, P.A.; Natwick, E.T. Integrated systems for managing Bemisia tabaci in protected and open field agriculture. In Bemisia: Bionomics and Management of a Global Pest; Stansly, P.A., Naranjo, S.E., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 467–497. [Google Scholar]
- Kumar, V.; Houben, K.; McKenzie, C.L.; Osborne, L.S. Efficacy of Eretmocerus eremicus and cyantraniliprole on Bemisia tabaci (MED whitefly). Arthropod Manag. Tests 2017, 42, 1–2. [Google Scholar]
- Hanan, A.; He, X.Z.; Wang, Q. Insight into the success of whitefly biological control using parasitoids: Evidence from the Eretmocerus warrae-Trialeurodes vaporariorum system. Pest Manag. Sci. 2017, 3, 2294–2301. [Google Scholar] [CrossRef]
- Shandhu, S.S.; Sharma, A.K.; Beniwal, V.; Goel, G.; Batra, P.; Kumar, A.; Jaglan, S.; Malhotra, S. Myco-biocontrol of insect pests: Factors involved, mechanism, and regulation. J. Pathog. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Eslamizadeh, R.; Sajap, A.S.B.; Omar, D.B.; Azura, N.; Adam, B. Evaluation of different isolates of the entomopathogenic fungus, Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) against Bemisia tabaci (Hemiptera: Aleyrodidae). Biol. Control Plant Prot. 2015, 2, 82–90. [Google Scholar]
- Lenteren, J.C.; Martin, N.A. Biological control of whiteflies. In Integrated Pest and Disease Management in Green-House Crops; Springer: Dordrecht, The Netherlands, 1999; pp. 202–216. [Google Scholar]
- Head, J.; Lawrence, A.J.; Walters, K.F.A. Efficacy of the entomopathogenic nematode, Steinernema feltiae, against Bemisia tabaci in relation to plant species. J. Appl. Entomol. 2004, 128, 543–547. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.; Mathers, J.J.; Northing, P.; Prickett, A.J.; Walters, K.F. The integrated use of chemical insecticides and the entomopathogenic nematode, Steinernema carpocapsae (Nematoda: Steinernematidae), for the control of sweetpotato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). J. Insect Sci. 2008, 15, 447–453. [Google Scholar] [CrossRef]
- Harris-Shultz, K.; Knoll, J.; Punnuri, S.; Niland, E.; Ni, X. Evaluation of strains of Beauveria bassiana and Isaria fumosorosea to control sugarcane aphids on grain sorghum. Agrosystems Geosci. Environ. 2020, 3, e20047. [Google Scholar] [CrossRef]
- Lacey, L.A.; Fransen, J.J.; Carruthers, R.I. Global distribution of naturally occurring fungi of Bemisia, their biologies and use as biological control agents. In Bemisia: 1995 Taxonomy, Biology, Damage, Control and Management; Gerling, D., Mayer, R.T., Eds.; Intercept Ltd.: Andover UK, 1996; pp. 401–433. [Google Scholar]
- Olleka, A.; Mandour, N.; Ren, S. Effect of host plant on susceptibility of whitefly Bemisia tabaci (Homoptera: Aleyrodidae) to the entomopathogenic fungus Beauveria bassiana (Ascomycota: Hypocreales). Biocontrol Sci. Technol. 2009, 19, 717–727. [Google Scholar] [CrossRef]
- Wraight, S.; Carruthers, R.; Jaronski, S.; Bradley, C.; Garza, C. Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol. Control. 2000, 17, 203–217. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Kobori, N.N.; Quintela, E.D.; Delalibera, I., Jr. The virulence of entomopathogenic fungi against Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) and their conidial production using solid substrate fermentation. BioControl 2013, 66, 209–218. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.; Walters, K.F.; Deppe, C. Compatibility of the entomopathogenic fungus Lecanicillium muscarium and insecticides for eradication of sweetpotato whitefly, Bemisia tabaci. Mycopathologia 2005, 160, 35–41. [Google Scholar] [CrossRef]
- James, R.R.; Elzen, G.W. Antagonism between Beauveria bassiana and imidacloprid when combined for Bemisia argentifolii (Homoptera: Aleyrodidae) control. J. Econ. Èntomol. 2001, 94, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Pirzadfard, S.; Zandi-Sohani, N.; Sohrabi, F.; Rajabpour, A. Intraguild interactions of a generalist pred ator, Orius albidipennis, with two Bemisia tabaci parasitoids. Int. J. Trop. Insect Sci. 2020, 40, 259–265. [Google Scholar] [CrossRef]
- Shahpouri, A.; Yarahmadi, F.; Zandi Sohani, N. Functional response of the predatory species Orius albidipennis Reuter (Hemiptera: Anthocoridae) to two life stages of Bemisia tabaci (Genn.)(Hemiptera: Aleyrodidae). Egyp J. Biol. Pest Control. 2019, 29, 1–6. [Google Scholar] [CrossRef]
- Faria, M.; Wraight, S.P. Biological control of Bemisia tabaci with fungi. Crop Prot. 2001, 20, 767–778. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C. The contribution of conservation biological control to integrated control of Bemisia tabaci in cotton. Biol. Control. 2009, 51, 458–470. [Google Scholar] [CrossRef]
- Gould, J.; Hoelmer, K.; Goolsby, J. Classical Biological Control of Bemisia Tabaci in the United States: A Review of Interagency Research and Implementation; Goolsby, J., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 191–204. [Google Scholar]
- Nomikou, M.; Janssen, A.; Schraag, R.; Sabelis, M.W. Phytoseiid predators as potential biological control agents for Bemisia tabaci. Experim. Appl. Acarol. 2001, 25, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Zandi-Sohani, N.; Shishehbor, P. Temperature effects on the development and fecundity of Encarsia acaudaleyrodis (Hymenoptera: Aphelinidae), a parasitoid of Bemisia tabaci (Homoptera: Aleyrodidae) on cucumber. BioControl 2011, 56, 257–263. [Google Scholar] [CrossRef]
- Hagler, J.R.; Blackmer, F. Identifying inter- and intra-guild feeding activity of an arthropod predator assemblage. Ecol. Entomol. 2013, 38, 258–271. [Google Scholar] [CrossRef]
- Vandervoet, T.F.; Ellsworth, P.C.; Carrière, Y.; Naranjo, S.E. Quantifying conservation biological control for management of Bemisia tabaci (Hemiptera: Aleyrodidae) in cotton. J Econ Entomol. 2018, 111, 1056–1068. [Google Scholar] [CrossRef]
- Legaspi, J.C.; Simmons, A.M.; Legaspi, B.C. Prey preference by Delphastus catalinae (Coleoptera: Coccinellidae) on Bemisia argentifolii (Homoptera: Aleyrodidae): Effects of plant species and prey stages. Fla. Entomol. 2006, 89, 218–222. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Hernandez, Y.V.; Kumar, V.; Francis, A.; Skelley, P.; Rohrig, E.; McKenzie, C.; Osborne, L.; Mannion, C. Pallidus beetle, Delphastus pallidus LeConte (Insecta: Coleoptera: Coccinellidae), a native predatory beetle of whitefly species in Florida; FDACS-P-01782, Issue No. 435; Florida Department of Agriculture and Consumer Services, Division of Plant Industry: Tallahassee, FL, USA, 2017; p. 10. [Google Scholar]
- Kumar, S.; Sachan, S.K.; Singh, R.; Singh, D.V. Bio-efficacy of some newer insecticides and bio-pesticides against whitefly (Bemisia tabaci Gennadius) in brinjal ecosystem. IJCS 2020, 8, 1883–1888. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C. Mortality dynamics and population regulation in Bemisia tabaci Entomol. Exp. Appl. 2005, 116, 93–108. [Google Scholar] [CrossRef]
- Hagler, J.R.; Naranjo, S.E. Use of a gut content ELISA to detect whitefly predator feeding activity after field exposure to different insecticide treatments. Biocontrol Sci. Technnol. 2005, 15, 321. [Google Scholar] [CrossRef]
- Montserrat, M.; Albajes, R.; Castañé, C. Functional response of four heteropteran predators preying on greenhouse whitefly (Homoptera: Aleyrodidae) and western flower thrips (Thysanoptera: Thripidae). Environ. Entom. 2000, 29, 1075–1082. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, Z.F.; Han, Y.Y.; Wang, X.M.; Wang, Z.Q.; Musa, P.D.; Qiu, B.L.; Ali, S. Effects of Aschersonia aleyrodis on the life table and demographic parameters of Bemisia Tabaci. J. Integr. Agric. 2018, 17, 389–396. [Google Scholar] [CrossRef]
- Koike, M.; Higashio, T.; Komori, A.; Akiyama, K.; Kishimoto, N.; Masuda, E.; Sasaki, M.; Yoshida, S.; Tani, M.; Kuramoti, K.; et al. Verticillium lecanii (Lecanicillium spp.) as epiphyte and its application to biological control of arthropod pests and diseases. IOBC/Wprs Bull 2004, 27, 41–44. [Google Scholar]
- Kim, J.J.; Lee, M.H.; Yoon, C.S.; Kim, H.S.; Yoo, J.K.; Kim, K.C. Control of cotton aphid and greenhouse whitefly with a fungal pathogen. In Biological Control of Greenhouse Pests; Food & Fertilizer Technology Center Extension Bulletin 502; Fertilizer Technology Center: Taipei, Taiwan; pp. 8–15.
- Rahim, E.; Ahmad, S.S.; Dzolkhifli, O.; Nur, A.A. First record of Isaria fumosorosea Wize (Deuteromycotina: Hyphomycetes) infecting Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) in Malaysia. J. Entomol. 2013, 10, 182–190. [Google Scholar]
- Zafar, J.; Freed, S.; Khan, B.A.; Farooq, M. Effectiveness of Beauveria bassiana against cotton whitefly, Bemisia tabaci (Gennadius) (Aleyrodidae: Homoptera) on different host plants. Pak J Zool. 2016, 48, 91–99. [Google Scholar]
- Imam, I.I. Role of certain Beauveria bassiana isolate as biological control agent against whitefly, Bemisia tabaci (Genn.) and its effect on the predator Chrysopela carnea (stephens). Egypt J. Desert Res. 2017, 67, 351–359. [Google Scholar] [CrossRef][Green Version]
- Iqbal, M.; Arif, M.J.; Saeed, S.; Javed, N. Biorational approach for management of whitefly, Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae), on cotton crop. Inter. J. Trop Insect Sci. 2021, 42, 1461–1469. [Google Scholar] [CrossRef]
- Nada, M.S.; Gaffar, S.A.; Taman, A. Comparative effect of three entomopathogenic fungi against whitefly Bemisia tabaci (Gennadius) infesting eggplant under field conditions at kafr el-Sheik gov Egypt. J. Plant Prot. Path. 2021, 12, 239–244. [Google Scholar] [CrossRef]
- Schoeller, E.N.; Redak, R.A. Climate and seasonal effects on phenology and biological control of giant whitefly Aleurodicus dugesii (Hemiptera: Aleyrodidae) with parasitoids in southern California, USA. BioControl 2020, 65, 559–570. [Google Scholar] [CrossRef]
- Xu, X.R.; Li, N.N.; Bao, X.Y.; Douglas, A.E.; Luan, J.B. Patterns of host cell inheritance in the bacterial symbiosis of whitefies. Insect Sci. 2018, 27, 938–946. [Google Scholar] [CrossRef]
- Ou, D.; Ren, L.M.; Liu, Y.; Ali, S.; Wang, X.M.; Ahmed, M.Z.; Qiu, B.L. Compatibility and efficacy of the parasitoid Eretmocerus hayati and the entomopathogenic fungus Cordyceps javanica for biological control of whitefly Bemisia tabaci. Insects 2019, 10, 425. [Google Scholar] [CrossRef]
- Reichelderfer, K.H. Economic feasibility of biological control of crop pests. BioControl Crop Prod. 1981, 5, 403–417. [Google Scholar]
- Van emdem, H.F.; Service, M.W. Pest and Vector Control; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Kidane, D.; Yang, N.W.; Wan, F.H. Effect of cold storage on the biological fitness of Encarsia sophia (Hymenoptera: Aphelinidae), a parasitoid of Bemisia tabaci (Hemiptera: Aleyrodidae). Eur. J. Entomol. 2015, 112, 460–469. [Google Scholar] [CrossRef]
- Bar, L.; Czosnek, H.; Sobol, I.; Ghanim, M.; Hariton Shalev, A. Down regulation of dystrophin expression in pupae of the whitefly Bemisia tabaci inhibits the emergence of adults. Insect Mol. Biol. 2019, 28, 662–675. [Google Scholar] [CrossRef]
- Koul, B.; Srivastava, S.; Sanyal, I.; Tripathi, B.; Sharma, V.; Amla, D.V. Transgenic tomato line expressing modified Bacillus thuringiensis cry1Ab gene showing complete resistance to two lepidopteran pests. SpringerPlus 2014, 3, 1–13. [Google Scholar] [CrossRef][Green Version]
- Koul, B.; Yadav, R.; Sanyal, I.; Amla, D.V. Comparative performance of modified full-length and truncated Bacillus thuringiensis-cry1Ac genes in transgenic tomato. SpringerPlus 2015, 4, 1–14. [Google Scholar] [CrossRef][Green Version]
- Grover, S.; Jindal, V.; Banta, G.; Taning, C.N.T.; Smagghe, G.; Christiaens, O. Potential of RNA interference in the study and management of the whitefly, Bemisia tabaci. Arch. Insect Biochem Physiol. 2018, 100, e21522. [Google Scholar] [CrossRef]
- Thakur, N.; Upadhyay, S.K.; Verma, P.C.; Chandrashekar, K.; Tuli, R.; Singh, P.K. Enhanced whitefly resistance in transgenic tobacco plants expressing double stranded RNA of v-ATPase A gene. PLoS ONE 2014, 9, e87235. [Google Scholar]
- Wamiq, G.; Khan, J.A. Over-expression of ghrmiR166b generates resistance against Bemisia tabaci infestation in Gossypium hirsutum plants. Planta 2018, 247, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Eakteiman, G.; Moses-Koch, R.; Moshitzky, P.; Mestre-Rincon, N.; Vassão, D.G.; Luck, K. Targeting detoxification genes by phloem-mediated RNAi: A new approach for controlling phloem-feeding insect pests. Insect Biochem. Mol. Biol. 2018, 100, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Suhag, A.; Yadav, H.; Chaudhary, D.; Subramanian, S.; Jaiwal, R.; Jaiwal, P.K. Biotechnological interventions for the sustainable management of a global pest, whitefly (Bemisia tabaci). Insect Sci. 2021, 28, 1228–1252. [Google Scholar] [CrossRef]
- Zotti, M.; Smagghe, G. RNAi technology for insect management and protection of beneficial insects from diseases: Lessons, challenges and risk assessments. Neotrop. Entomol. 2018, 44, 197–213. [Google Scholar] [CrossRef]
- Cagliari, D.; Dias, N.P.; Galdeano, D.M.; dos Santos, E.Á.; Smagghe, G.; Zotti, M.J. Management of pest insects and plant diseases by non-transformative RNAi. Front Plant Sci. 2019, 10, 1319. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Aleynova, O.A.; Kalachev, A.V.; Suprun, A.R.; Ogneva, Z.V.; Kiselev, K.V. Induction of transgene suppression in plants via external application of synthetic dsRNA. Int. J. Mol. Sci. 2019, 20, 1585. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.; Papadopoulou, K.K. GMO-free RNAi: Exogenous application of RNA molecules in plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef]
- Gogoi, A.; Sarmah, N.; Kaldis, A.; Perdikis, D.; Voloudakis, A. Plant insects and mites uptake double-stranded RNA upon its exogenous application on tomato leaves. Planta 2017, 246, 1233–1241. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Zheng, Y.; Weng, Q.; Biondi, A.; Desneux, N.; Wu, K. Assessment of potential sublethal effects of various insecticides on key biological traits of the tobacco whitefly, Bemisia tabaci. Int. J. Biol. Sci. 2013, 9, 246–255. [Google Scholar] [CrossRef]
- Christofoli, M.; Costa, E.C.; Bicalho, K.U.; de Cássia Domingues, V.; Peixoto, M.F.; Alves, C.C.; Araújo, W.L.; de Melo Cazal, C. Insecticidal effect of nanoencapsulated essential oils from Zanthoxylum rhoifolium (Rutaceae) in Bemisia tabaci populations. Ind. Crops Prod. 2015, 70, 301–308. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Wang, X.; Qiu, B.; Cuthbertson, A.G.S.; Du, C. Isaria fumosorosea-based zero- valent iron nanoparticles affect the growth and survival of sweet potato whitefly, Bemisia tabaci (Gennadius). Pes. Manag. Sci. 2019, 75, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.J.; Raza, A.; Amin, I.; Scheffler, J.A.; Scheffler, B.E.; Brown, J.K.; Mansoor, S. RNAi-mediated mortality of the whitefly through transgenic expression of double-stranded RNA homologous to acetylcholinesterase and ecdysone receptor in tobacco plants. Sci. Rep. 2016, 6, 1–11. [Google Scholar]
- Zubair, M.; Khan, M.Z.; Rauf, I.; Raza, A.; Shah, A.H.; Hassan, I.; Amin, I.; Mansoor, S. Artificial micro-RNA (amiRNA)-mediated resistance against whitefly (Bemisia tabaci) targeting three genes. Crop Prot. 2020, 137, 105308. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Mirabella, R.; Diergaarde, P.J.; Van Doorn, A.; Tissier, A.; Kant, M.R.; Prins, M.; De Vos, M.; Haring, M.A.; Schuurink, R.C. Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl. Acad. Sci. USA 2012, 109, 20124–20129. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Q.; Luan, J.; Chung, S.H.; Van Eck, J.; Turgeon, R.; Douglas, A.E. Towards an understanding of the molecular basis of effective RNAi against a global insect pest, the whitefly Bemisia tabaci. Insect Biochem. Mol. Biol. 2017, 88, 21–29. [Google Scholar] [CrossRef]
- Gul, A.; Hussain, G.; Iqbal, A.; Rao, A.Q.; Yasmeen, A.; Shahid, N.; Ahad, A.; Latif, A.; Azam, S.; Samiullah, T.R.; et al. Constitutive expression of asparaginase in Gossypium hirsutum triggers insecticidal activity against Bemisia tabaci. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Ghanim, M.; Kontsedalov, S.; Czosnek, H. Tissue-specific gene silencing by RNA interference in the whitefly Bemisia tabaci (Gennadius). Insect Biochem. Mol. Biol. 2007, 37, 732–738. [Google Scholar] [CrossRef]
- Luan, J.B.; Ghanim, M.; Liu, S.S.; Czosnek, H. Silencing the ecdysone synthesis and signaling pathway genes disrupts nymphal development in the whitefly. Insect Biochem. Mol. Biol. 2013, 43, 740–746. [Google Scholar] [CrossRef]
- Ludba, K. Evaluating Plant Root Uptake of dsRNA for Application in Pest Management. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2018. [Google Scholar]
- Jin, S.; Zhang, X.; Daniell, H. Pinellia ternata agglutinin expression in chloroplasts confers broad spectrum resistance against aphid, whitefly, lepidopteran insects, bacterial and viral pathogens. Plant Biotechnol. J. 2012, 10, 313–327. [Google Scholar] [CrossRef]
- Anwar, W.; Ali, S.; Nawaz, K.; Iftikhar, S.; Javed, M.A.; Hashem, A.; Alqarawi, A.A.; Abd Allah, E.F.; Akhter, A. Entomopathogenic fungus Clonostachys rosea as a biocontrol agent against whitefly (Bemisia tabaci). BiocontrolSci. Technol. 2018, 28, 750–760. [Google Scholar] [CrossRef]
- Puri, H.; Jindal, V. Target of rapamycin (TOR) gene is vital for whitefly survival and reproduction. J. Biosci. 2021, 46, 1–2. [Google Scholar] [CrossRef]
- Brookes, G.; Barfoot, P. Environmental impacts of genetically modified (GM) crop use 1996–2015: Impacts on pesticide use and carbon emissions. GM Crops Food. 2016, 8, 117–147. [Google Scholar] [CrossRef]
- Sheldon, C.C.; Finnegan, E.J.; Dennis, E.S.; Peacock, W.J. Quantitative effects of vernalization on FLC and SOC1 expression. Plant J. 2006, 45, 871–883. [Google Scholar] [CrossRef]
- Stansly, P.A. Seasonal abundance of silverleaf whitefly in southwest Florida vegetable fields. Proc Fla State Hort Soc. 1996, 108, 234–242. [Google Scholar]
- Stansly, P.A.; Liu, T.X.; Vavrina, C.V. Response of Bemisia argentifolii (Homoptera: Aleyrodidae) in bioassay, greenhouse tomato transplants and field plants of tomato and eggplant. J. Econ. Entomol. 1998, 91, 686–692. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 14, 148. [Google Scholar] [CrossRef]
- World Health Organization. Public Health Impact of Pesticides used in Agriculture; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Alewu, B.; Nosiri, C. Pesticides and human health. In Pesticides in the Modern World Effects of Pesticides Exposure; Stoytcheva, M., Ed.; InTech Open: London, UK, 2011; pp. 231–250. [Google Scholar]
- Zheng, S.; Chen, B.; Qiu, X.; Chen, M.; Ma, Z.; Yu, X. Distribution and risk assessment of 82 pesticides in Jiulong river and estuary. Chemosphere 2016, 144, 1177–1192. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.; Walters, K.F.; Northing, P. The susceptibility of immature stages of Bemisia tabaci to the en-tomopathogenic fungus Lecanicillium muscarium on tomato and verbena foliage. Mycopathologia 2005, 159, 23–29. [Google Scholar] [CrossRef]
- Bacci, L.; Crespo, A.L.; Galvan, T.L.; Pereira, E.J.; Picanço, M.C.; Silva, G.A.; Chediak, M. Toxicity of insecticides to the sweetpotato whitefly (Hemiptera: Aleyrodidae) and its natural enemies. Pest Manag. Sci. 2007, 63, 699–706. [Google Scholar] [CrossRef]
- Bi, J.L.; Toscano, N.C. Current status of the greenhouse whitefly, Trialeurodes vaporariorum, susceptibility to neonicotinoid and conventional insecticides on strawberries in southern California. Pest Manag. Sci. 2007, 63, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M. Varietal performance and chemical control used as tactics against sucking insect pests of cotton. Sarhad J. Agric. 2011, 27, 255–261. [Google Scholar]
- Golmohammadi., G.; Hosseini-Gharalari, A.; Fassihi, M.; Arbabtafti, R. Efficacy of one botanical and three synthetic insecticides against silverleaf whitefly, Bemisia tabaci (Hem.: Aleyrodidae) on cucumber plants in the field. J. Crop Prot. 2014, 3, 435–441. [Google Scholar]
- Jamieson, L.E.; Page-Weir, N.E.M.; Chhagan, A.; Curtis, C. The efficacy of insecticides against australian citrus whitefly. NZ Plant Prot. 2010, 63, 254–261. [Google Scholar]
- Sathyan, T.; Murugesan, N.; Elanchezhyan, K.; Raj, A.S.; Ravi, G. Efficacy of synthetic insecticides against sucking insect pests in cotton, Gossypium hirsutum L. Int. J. Entomol. Res. 2016, 1, 16–21. [Google Scholar]
- Pachundkar, N.N.; Borad, P.K.; Patil, P.A. Evaluation of various synthetic insecticides against sucking insect pests of cluster bean. Int. J. Sci. Res. Publi. 2013, 3, 1–6. [Google Scholar]
- Oladimeji, A.; Kannike, M.A. Comparative studies on the efficacy of neem, basil leaf extracts and synthetic insecticide, lambda-cyhalothrin, against Podagrica spp. on okra. Afr. J. Microbiol. Res. 2010, 4, 33–37. [Google Scholar]
- Magsi, F.H.; Hussain, L.K.; Ahmed, C.M.; Bhutto, Z.; Channa, N.; Ahmed, J.A. Effectiveness of different synthetic insecticides against Bemisia tabaci (genn) on tomato crop. Int. J. Fauna Biol. Stud. 2017, 4, 6–9. [Google Scholar]
- Jha, S.K.; Kumar, M. Relative efficacy of different insecticides against whitefly, Bemisia tabaci on tomato under field condition. J. Entomol. Zool. Stud. 2017, 5, 728–732. [Google Scholar]
- Mohammadali, M.T.; Alyousuf, A.A.; Baqir, H.A.; Kadhim, A.A. Evaluation of the efficacy of different Neocontinoid insecticides against cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) on eggplant under greenhouse condition. Earth Environ. Sci. 2019, 388, 1–7. [Google Scholar] [CrossRef]
- Parhyar, R.A.; Mari, J.M.; Bukero, A.; Lanjar, A.G.; Hyder, M.; Khan, N.; Bukero, A.A.; Soomro, H.U. Relative efficacy of synthetic insecticides against sucking insect pests of chilli crop. Pure Appl. Biol 2019, 8, 2248–2256. [Google Scholar] [CrossRef]
- El, A.E.; Khaleid, M.S.; AbdAllah, S.A.; Ali, O.S. Effect of some insecticides alone and in combination with salicylic acid against aphid, Aphis gossypii, and whitefly Bemisia tabaci on the cotton field. Bull Natl. Res. Cent 2019, 43, 1–7. [Google Scholar]
- Thorat, S.S.; Kumar, S.; Patel, J.D. Bio efficacy of different pesticides against whitefly (Bemisia tabaci Gennadius) in tomato. J. Entomol. Zool. Stud. 2020, 8, 1428–1431. [Google Scholar]
- Zawrah, M.F.; El Masry, A.T.; Noha, L.; Saleh, A.A. Efficacy of certain insecticides against whitefly Bemicia tabaci (Genn.) infesting tomato plants and their associated predators. Plant Arch. 2020, 20, 2221–2228. [Google Scholar]
- Jain, D.; Kumar, H.; Chouhan, B.S.; Singh, B.; Sumeriya, H. Comparative efficacy of different bio and synthetic insecticides against sucking pests of okra (Abelmoschus esculentus L. Moench). Pharma Innov. J. SP. 2021, 10, 719–727. [Google Scholar]
- Sana, K.; Iqbal, T.; Usman, A. Comparative efficacy of botanicals and a synthetic insecticide against sucking insect pests of brinjal. Ann. Rom. Soc. Cell Biol. 2021, 25, 19381–19389. [Google Scholar]
- Dittrich, V.; Ernst, G.H.; Ruesch, O.; Uk, S. Resistance mechanisms in sweetpotato whitefly (Homoptera: Aleyrodidae) populations from Sudan, Turkey, Guatemala, and Nicaragua. J. Econ. Entomol. 1990, 83, 1665–1670. [Google Scholar] [CrossRef]
- Clark, J.M.; Yamaguchi, I. Scope and status of pesticide resistance. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2001; Volume 808, pp. 1–22. [Google Scholar]
- Koul, B.; Taak, P. Soil Pollution: Causes and Consequences. In Biotechnological Strategies for Effective Remediation of Polluted Soils; Springer: Singapore, 2018; pp. 1–37. [Google Scholar]
- Horowitz, A.R.; Antignus, Y.; Gerling, D. Management of Bemisia tabaci whiteflies. In the Whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) Interaction with Geminivirus-Infected Host Plants: Bemisia tabaci, Host Plants and Geminiviruses; Thompson, W.M.O., Ed.; Springer: Amsterdam, The Netherlands, 2011; pp. 293–322. [Google Scholar]
- Horowitz, A.R.; Denholm, I.; Morin, S. Resistance to insecticides in the TYLCV vector, Bemisia tabaci. In Tomato Yellow Leaf Curl Virus Disease: Management, Molecular Biology, Breeding for Resistance; Czosnek, H., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 305–325. [Google Scholar]
- Shah, R.; Al-Sadi, A.M.; Scott, I.M.; AlRaeesi, A.; AlJahdhami, A.A. Insecticide resistance monitoring in whitefly (Bemisia tabaci)(Hemiptera: Aleyrodidae) in Oman. J. Asia-Pacific Entomol 2020, 23, 1248–1254. [Google Scholar] [CrossRef]
- Naveen, N.C.; Chaubey, R.; Kumar, D.; Rebijith, K.B.; Rajagopal, R.; Subrahmanyam, B.; Subramanian, S. Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Sci. Rep. 2017, 7, 40634. [Google Scholar] [CrossRef]
- Khalid, M.Z.; Ahmed, S.l.; Ashkar, I.; Sabagh, A.E.L.; Liu, L.; Zhong, G. Evaluation of Resistance Development in Bemisia tabaci Genn. (Homoptera: Aleyrodidae) in Cotton against Different Insecticides Insects 2021, 12, 996. [Google Scholar]
- Pappas, M.L.; Migkou, F.; Broufas, G.D. Incidence of resistance to neonicotinoid insecticides in greenhouse populations of the whitefly, Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) from Greece. Appl. Entomol. Zool 2013, 48, 373–378. [Google Scholar] [CrossRef]
- Toscano, N.C.; Prabhaker, N.; Castle, S.J.; Henneberry, T.J. Inter-regional differences in baseline toxicity of Bemisia argentifolii (Homoptera: Aleyrodidae) to the two insect growth regulators, buprofezin and pyriproxyfen. J. Econ. Entomol. 2001, 94, 1538–1546. [Google Scholar] [CrossRef]
- Nauen, R.; Denholm, I. Resistance of insect pests to neonicotinoid insecticides: Current status and future prospects. Arch. Ins. Biochem. Physiol. 2005, 58, 200–215. [Google Scholar] [CrossRef]
- Shah, R.; Scott, I.M. Susceptibility of Bemisia tabaci (MEAM1) Gennadius (Hemiptera: Aleyrodidae) to Deltamethrin, Thiamethoxam and Pyriproxyfen in Oman . Int. J. Agric. Biol. 2020, 24, 279–284. [Google Scholar]
- Carrière, Y.; Ellers-Kirk, C.; Hartfield, K.; Larocque, G.; Degain, B.; Dutilleul, P.; Dennehy, T.J.; Marsh, S.E.; Crowder, D.W.P.; Li, X.; et al. Large-scale, spatially-explicit test of the refuge strategy for delaying insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 775–780. [Google Scholar] [CrossRef]
- Shelby, E.A.; Moss, J.B.; Andreason, S.A.; Simmons, A.M.; Moore, A.J.; Moore, P.J. Debugging: Strategies and considerations for efficient RNAi-mediated control of the whitefly Bemisia Tabaci. Insects 2020, 11, 723. [Google Scholar] [CrossRef]
- Xia, J.; Guo, Z.; Yang, Z.; Han, H.; Wang, S.; Xu, H.; Yang, X.; Yang, F.; Wu, Q.; Xie, W.; et al. Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 2021, 184, 1693–1705. [Google Scholar] [CrossRef]
- Zhang, P.J.; Broekgaarden, C.; Zheng, S.J.; Snoeren, T.A.; van Loon, J.J.; Gols, R.; Dicke, M. Jasmonate and ethylene signaling mediate whitefly-induced interference with indirect plant defense in Arabidopsis thaliana. N. Phytol. 2013, 197, 1291–1299. [Google Scholar] [CrossRef]
- Zhang, P.J.; Wei, J.N.; Zhao, C.; Zhang, Y.F.; Li, C.Y.; Liu, S.S.; Dicke, M.; Yu, X.P.; Turlings, T.C.J. Airborne host-plant manipulation by whiteflies via an inducible blend of plant volatiles. Proc. Natl. Acad. Sci. USA 2019, 116, 7387–7396. [Google Scholar] [CrossRef]
- Heidel-Fischer, H.M.; Vogel, H. Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci. 2015, 8, 8–14. [Google Scholar] [CrossRef]
- Malka, O.; Easson, M.L.A.E.; Paetz, C.; Go tz, M.; Reichelt, M.; Stein, B.; Luck, K.; Stanisic, A.; Juravel, K.; Santos-Garcia, D. Glucosylation prevents plant defense activation in phloem-feeding insects. Nat. Chem Biol. 2020, 16, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Nonomura, T.; Kakutani, K.; Kimbara, J.; Osamura, K.; Kusakari, S. Avoidance of an electric field by insects: Fundamental biological phenomenon for an electrostatic pest-exclusion strategy. J. Phys. Conf Ser. 2015, 646, 012003. [Google Scholar] [CrossRef]
- Javed, M.A.; Matthews, G.A. Bioresidual and integrated pest management status of a biorational agent and a novel insecticide against whitefly and its key parasitoids. Int. J. Pest Manag. 2002, 48, 13–17. [Google Scholar] [CrossRef]
- Jazzar, C.; Hammad, E.A. The efficacy of enhanced aqueous extracts of Melia azedarach leaves and fruits integrated with the Camptotylus reuteri releases against the sweetpotato whitefly nymphs. Bullet Insectol. 2003, 56, 269–276. [Google Scholar]
- Reddy, P.P. Organic Farming for Sustainable Horticulture; Scientific Publishers: Jodhpur, India, 2012; Volume 91. [Google Scholar]
- Tamilnayagan, T.; Suganthy, M.; Ganapathy, N.; Renukadevi, P.; Malathi, V.G. Integrated pest management strategies against Bemicia tabaci and tomato leaf curl New Delhi virus (TOLCNDV) affecting ash gourd (Benincasa hispida) in tamil nadu. J. Exp. Zool India. 2019, 22, 1133–1138. [Google Scholar]
- Arnemann, J.A.; Bevilaqua, J.G.; Bernardi, L.; da Rosa, D.O.; da Encarnação, F.A.; Pozebon, H.; Marques, R.P.; Moro, D.; Ribas, D.; Patias, L.S.; et al. Integrated management of tomato whitefly under greenhouse conditions. J. Agri Sci. 2019, 11, 443–453. [Google Scholar] [CrossRef]
- Riley, D.G.; Srinivasan, R. Integrated management of tomato yellow leaf curl virus and its whitefly vector in tomato. J. Econ. Entomol. 2019, 112, 1526–1540. [Google Scholar] [CrossRef]
- Abd-Allah, S.M.; Hendawy, M.A.; Heba, A.I. Efficiency of some pesticides on cotton whitefly, Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae), infesting soybeans plants, Glycine hispida (Max). J. Product Dev. 2015, 20, 47–60. [Google Scholar] [CrossRef]
- Baiomy, F. Efficacy of kaolin foliar application against tomato whitefly; Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Egypt Acad J. Biol. Sci. 2017, 10, 71–80. [Google Scholar]
- Jaber, L.R.; Araj, S.E.; Qasem, J.R. Compatibility of endophytic fungal entomopathogens with plant extracts for the management of sweetpotato whitefly Bemesia tabaci Gennadius (Homoptera: Aleyrodidae). Biol. Cont. 2018, 117, 164–1671. [Google Scholar] [CrossRef]
- Conboy, N.J.; McDaniel, T.; George, D.; Ormerod, A.; Edwards, M.; Donohoe, P.; Gatehouse, A.M.; Tosh, C.R. Volatile organic compounds as insect repellents and plant elicitors: An integrated pest management (IPM) strategy for glasshouse whitefly (Trialeurodes vaporariorum). J. Chem. Ecol. 2020, 46, 1090–10104. [Google Scholar] [CrossRef]
- Mokrane, S.; Cavallo, G.; Tortorici, F.; Romero, E.; Fereres, A.; Djelouah, K.; Verrastro, V.; Cornara, D. Behavioral effects induced by organic insecticides can be exploited for a sustainable control of the orange spiny whitefly Aleurocanthus spiniferus. Sci. Rep. 2020, 10, 15746. [Google Scholar] [CrossRef]
- Malinga, L.N.; Laing, M.D. Efficacy of three biopesticides against cotton pests under field conditions in South Africa. Crop Prot. 2021, 145, 105578. [Google Scholar] [CrossRef]
- Gill, G.S.; Chong, J.H. Efficacy of selected insecticides as replacement for neonicotinoids in managing sweetpotato whitefly on poinsettia. Hort Technol. 2021, 31, 745–752. [Google Scholar] [CrossRef]
- Parsa, S.; Medina, C.; Rodríguez, V. Sources of pest resistance in cassava. Crop Prot. 2015, 68, 79–84. [Google Scholar] [CrossRef]
- Dutcher, J.D. A review of resurgence and replacement causing pest outbreaks in IPM. In General Concepts in Integrated Pest and Disease Management; Ciancio, A., Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 27–43. [Google Scholar]
- Li, S.; Li, H.; Zhou, Q. Essential oils from two aromatic plants repel the tobacco whitefly Bemisia tabaci. J. Pest Sci. 2022, 95, 971–982. [Google Scholar] [CrossRef]
- Xia, C.; Chon, T.S.; Ren, Z.; Lee, J.M. Automatic identification and counting of small size pests in greenhouse conditions with low computational cost. Ecol. Inform. 2015, 29, 139–146. [Google Scholar] [CrossRef]

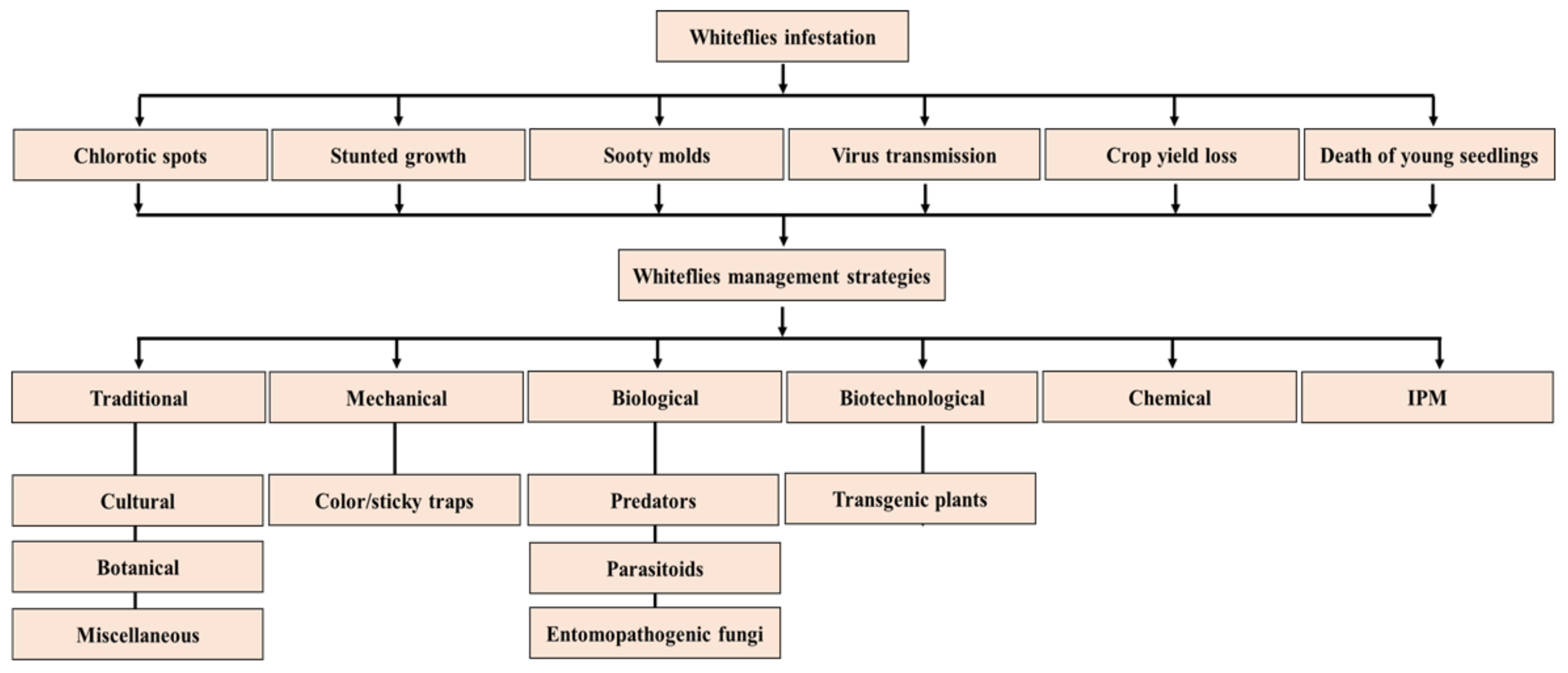
| Crop Name | Study Location | Damages Caused | Reference |
|---|---|---|---|
| Tomato | Florida | Economic loss of >125 million US dollars. | [75] |
| Tomato | Israel | Leaf curl, flower drop, short internodes, dwarfing, and leathery leaves. | [76] |
| Tomato | Spain | Multiple necrotic rings on the leaves. | [77] |
| Tomato | Spain | The average number of holes per leaf were 0.23 ± 0.10 and 0.3 ± 0.12 on the fruits during winter and summer experiments. | [78] |
| Tomato | Egypt | Reduction in chlorophyll A and B in infected tomato leaves by 8 and 12.8%, respectively. | [79] |
| Eggplant | China | Reduction in plant height: 12.6%, leaf area: 12.7%, dry matter: 8.2%, absolute growth rate: 26.0%, relative growth rate: 25.0%, and net assimilation rate: 22.2%. | [80] |
| Eggplant | China | Reduction in leaf area, fresh, and dry weight by 26.6, 21.8, and 19.27%, respectively. Reduction in chlorophyll content and photosynthetic by 9.7 and 65.9%, respectively. | [81] |
| Tobacco | China | Reduction in plant height: 32.7%, internode length: 4%, and photosynthetic rate: 81.5%. | [82] |
| Tobacco | China | At 11, 14, and 20 days, infected leaves had 42.36, 56.96, and 81.43% less chlorophyll A than the control plants. | [83] |
| Sugarcane | Iran | Chlorophyll content reduced to 0.583 mg/g compared to 1.48 mg/g in the control group. | [84] |
| Cantaloupe, cucumber, and zucchini | Saudi Arabia | Average reduction in cantaloupe pigments: 0.87, cucumber: 1.12, zucchini: 0.54 compared to 1.13, 2.09, and 1.05 in the control. | [85] |
| Zucchini | Florida | Reduced chlorophyll content by 66% in petioles compared to leaf blades at lower infestation stage. | [86] |
| Zucchini | Florida | There was a reduction in fruits yield using varied number of whiteflies compared to control (control: 5.1 ± 0.5, 30 pairs: 3.9 ± 0.5, 60 pairs: 0.4 ± 0.1 and 120 pairs: 0). | [87] |
| Cassava | Fiji Island | Reduced average conductivity rates (M = 11.90 mmol m2s−1), compared to non-infested foliage (M = 17.80 mmol m2s−1). | [88] |
| Soybeans | Brazil | Reduction in grain weight (33 g/1000 grains) and loss in protein contents (440 kg/ha) were recorded. | [89] |
| Snap bean | Georgia | Up to 45% of snap bean was lost due to whiteflies infestation. | [90] |
| Squash | Georgia | Up to 35% of the squash was lost due to whiteflies infestation. | [91] |
| Vegetables | Texas | Economic loss of 29 million US dollars was recorded. | [92] |
| Vegetables | South Carolina | The infestations resulted in thickened and distorted leaves, which become curled and crumpled. | [93] |
| Potato | India | The percent incidence (40–75%) of whitefly transmitted viruses was reported. | [94] |
| Coconut palm | India | In severe cases, the nymphs covered almost 60% of the leaf, which led to yellowing, necrosis, and dehydration. | [95] |
| Chili | Sri Lanka | Chili leaf curl virus, carried by whitefly, has led to leaf distortion and stunted growth in chili plants. | [96] |
| Crop | Materials Used | Mode of Preparation | Effects | Reference |
|---|---|---|---|---|
| Tomato | Yellow sticky traps | The traps were placed at a height of 1.4 m in the middle of the greenhouse. | Up to 67 whiteflies were caught per trap. | [147] |
| Tomato | Yellow sticky traps | The traps were hung either vertically or parallel to tomato lines. | Vertically hung yellow sticky traps caught more whiteflies (66.57) per row in the fields. | [148] |
| Tomato | Several colored and shaped adhesive traps | The traps were placed at different rates: 2, 4, and 6 traps per 250 m2. | The yellow rectangular traps proved more effective with a mean of 5.7 whiteflies/trap. | [149] |
| Eggplant | Yellow traps | The traps were put at 30 cm above the plants at a rate of 1 trap per 5 m2 in the field. | Yellow sticky traps caught up to 27 whiteflies in 6 days. | [150] |
| Eggplant | Sludge/slurry, ashes, cattle urine, and dung | Wood ash sprinkled at 50 g/plants, cow urine, cow dung, slurry and water sprayed at 1:10 ratio for five days. | Lower pest densities, reduced production costs, and less harm to the non-target arthropods were recorded. | [142] |
| Eggplant | Cow urine and vermin-wash | They were prepared at 20, 30, 40, and 50% concentrations. | The whiteflies densities were reduced with 50% concentration being the most effective. | [141] |
| Eggplant | Cow urine, different plant extracts, and vermiwash | Cow urine (CU) alone formulated at 20, 30, 40, and 50%, then mixed with plant extracts and vermiwash. | Lowest whitefly mean number (2.22) was reported in CU 20% + neem leaf extract 10%. | [139] |
| Cotton | Non-sticky, yellow sticky, and colorless sticky card | The traps with 7.5 × 12.5-cm, 72 cm2 and 93.75 cm2 were used. | After 24 h, non-sticky cards trapped 264, sticky cards caught 523, while colorless sticky cards caught 37 whiteflies per card. | [151] |
| Pepper | Combination of trap crops with yellow traps | Yellow sticky traps and trap crops were evaluated separately and in combination. | Yellow sticky traps were more effective (42 whiteflies/traps). | [152] |
| Cotton | Yellow sticky traps | The traps were hung vertically at 45 cm above the plant using a wooden pole. | Average densities were 34.07 whiteflies/trap. The whitefly number decreased to 0.83/leaf. | [153] |
| Okra | Buttermilk | 10 L of buttermilk was fermented, 1 L of the fermented material was added to 9 L of water and sprinkled on the crops. | The formulation significantly reduced the whiteflies population by 60%. | [133] |
| Crop plants | Plants extracts and soap | Mixture of marigold and hot chili pods, filtrates diluted with water at 1:2, 1 teaspoon of soap was added per 1 liter of extracts and sprayed on the crops. | Most agricultural pests are curtailed/managed effectively. | [71] |
| Cowpea | Cow urine with botanical extracts | The cow urine was prepared at 25, 50, 75, and 100% with 1% extract of neem seed kernel. | Cow urine 100% + neem 1% proved most effective with 13.26/leaf. | [136] |
| Okra | Cow urine with plant extracts | Pepper, garlic, neem leaf, and cow urine combination at quaternary level were prepared and applied at 10% w/v. | Reduction in whitefly numbers (95.2%) was reported. | [143] |
| Crop plants | Cow urine, soap, and plant extract | 20 g crushed root of turmeric was steeped in 200 mL cattle urine. The mixture was diluted using 2–3 L of tap water (8–12 mL). | Sap-sucking insects including whiteflies, aphids, caterpillars, and red mites were significantly reduced. | [154] |
| Agricultural crops | Cow urine | Urine diluted in water (1: 20). | The treatment was effective against insects and pathogens and serves as fertilizer to the crops. | [98] |
| Crop plants | Buttermilk | ITK using fermented curd water (buttermilk). | White fly, jassids, aphids, etc. were managed/suppressed efficiently. | [132] |
| Agricultural crops | Cow dung and urine with fermented plant extracts | Fermented plant extracts, cow dung/urine in a ratio of 1:20 water. | The insect pests were well managed. | [138,140] |
| Okra | Colored sticky traps | 1500 mL empty Pepsi containers coated with yellow, green, purple, and black were kept in the field, 2 m apart and 0.6 m above the crops. | Yellow traps were found most promising with a mean of 61.13 whiteflies per trap. | [155] |
| Crop plants | Kerosene–soap–water emulsion | Indigenous technical knowledge (ITK) using kerosene–soap emulsion. | It had a detrimental effect on piercing-sucking insects. | [39,144] |
| Cotton | Traps/barrier crops and parasitoids | The intercropping and perimeter cropping strategies involving 3 intercrop schemes and 3 peripheral plantings were examined. | About 1.44 and 1.15/100 cm−2 of both nymph and adult whiteflies were recorded on the leaf surface. | [24] |
| Black gram | Soap, indoneem, neem, buttermilk, actara, and lisapol detergents. | The treatments were used separately and in combination | Lower whiteflies number (7.56) was found in treated plants compared to 37.11 whiteflies per leaf in untreated plants. The combined effect led to 26.50–27.35% reduction in whitefly number. | [28] |
| Crop Name | Plant Products Used | Results | Reference |
|---|---|---|---|
| Sweet potato | Use of plant extracts (petunia) | Whitefly controlled at 0.5 and 1 mg ml−1 concentrations (70% and 82% for adult and eggs mortality). | [170] |
| Tomato, cucumber, and bean | Aqueous, methanol and acetone fruits and leaf extracts of chinaberry | Methanol extract reduced the whitefly number to 1.44± 0.24 per plant. | [171] |
| Tomato | Seeds and leaf extracts from eight plant species | The highest lethality (41%) was caused by Jatropha dhofanica L. while 30.85% was caused by Azadirachta indica A. Juss as the lowest fatality rate. | [172] |
| Tomato | Ginger oils | The oils were effective in repelling the whitefly on tomatoes | [173] |
| Melon | Essential oils from thyme and peppermint | The extracts were effective with 62.78% (peppermint) and 100% (thyme) fatality rate. | [174] |
| Tomato | Seed extracts from Trichillia havanensis Jack. and Passiflora edulis Sims | Passiflora edulis Sims led to 60% lethality while Trichillia havanensis Jack. caused 70% whiteflies fatality. | [175] |
| Winged soapberry | Crude and semi-purified saponin extracts from Sapindus saponaria L.D. Benson fruits | Whitefly lethality increased as the quantity of unrefined and semi-purified saponin preparations increased (20 to 80%). | [176] |
| Soybean, cotton and melon | Oils of sugar apple | Whitefly nymphs shrunk and detached from the surface of the leaf after being exposed to the seed oil. | [177] |
| Coleus plant | Essential oils from various plant species | After one, two, and three weeks of treatment, none of the essential oil offered sufficient suppression of whitefly. | [100] |
| Sweet potato | Aqueous plant extracts | The extracts were as lethal as Imidacloprid to the sweet potato whitefly. | [178] |
| Laboratory | Mint and colothyn foliar extracts (crude or formulated) | At LC50, the extracts were effective (100% toxicity) against whiteflies and aphids. | [179] |
| Dry bean | Neem oils | On the 6th day after treatment, the fatality rate for first to third instars was above 80% at 1% concentration. | [180] |
| Laboratory | Essential oils from 4 different plants | Mortality rate of up to 79% was recorded from the report. | [181] |
| Sweet potato | Plant derived pesticides (neem) | The oviposition, egg hatching, and adult eclosion were reduced by 23.1, 53.2, and 26.6% compared to control. | [182] |
| Okra | Neem essential oils | Neem oil 5% caused 70.77% mortality in B. tabaci 72 h after application | [183] |
| Different crops | Essential oils from aromatic plants | The EOs acted as a repellant, insecticide, and growth inhibitors. | [184] |
| Tomato | Fermented botanicals from neem, kakawate, marigold, and makabuhay | Marigold was found to be most effective among the four extracts. | [185] |
| Laboratory | Essential oils and secondary metabolites from lants (cumin, cinnamon, lemongrass and citronella grass.) | Cinnamaldehyde (deterrent at 0.084 mg/L and deadly at 8.4 mg/L) and linalool (retardant at 0.006 mg/L with unknown lethality). | [186] |
| Y-tube olfactometer | Volatile compounds from six plants species. | There was more than 80% attraction response, more than 62% deterrent effect and more than 80% anti oviposition. | [82] |
| Tomato | Five different combinations of chemical treatment | 100% mortality on treatment 1–4 and 2 whiteflies on treatment number 5. | [187] |
| Eugenia Spring ex Mart. foliar extracts | 80–97% lethality rates on the insects. | [64] | |
| Okra | Plant extracts | Significant reduction on the whitefly population ranging from 5.19 to 63.17%. | [188] |
| Tomato | Clove and bitter orange essential oils | The mortality of whiteflies ranged from 70 to 90%. | [189] |
| Tomato | Essential oils from different plant species | Both adult and egg number decreased to 6.6 ± 0.93, 6.0 ± 2.39 compared to 22.6 ± 2.23 and 70.6 ± 19.29 in the control. | [169] |
| Potato | Extracts from five plant species viz: neem, licorice, turmeric, pomegranate, and thyme | The most efficient substance was neem oil, with 66.79 and 67.71% reduction of whiteflies density in the two seasons (2014 and 2015). | [190] |
| Tomato | Plant aqueous extracts | Up to 78% and 72.8% were recorded for ovicidal and mortality rate, respectively. | [60] |
| Laboratory | Essential oils from lemongrass, cumin, and cinnamon | After 24 h, cinnamaldehyde was the most poisonous (100%) to the whiteflies, followed by geraniol (32.1%) and citronellol (17.1%). | [191] |
| Laboratory | Essential oils from Gardenia jasminoides Ellis and its four primary chemical constituents | The extracts had fumigant activity against whitefly adults (81.48%) and acute toxicity against the larvae (77.28%). | [155] |
| Eggplants | Aqueous extracts of nine different plant species | Cotton seed extract demonstrated superior effects to pest infestation in eggplant fields. | [192] |
| Chilli | Aqueous plant extracts | Up to 96.67% mortality rate on the nymph of whiteflies | [64] |
| Cucumber | Plant extracts and commercial insecticides | Up to 80% whitefly mortality was reported. | [193] |
| Eggplant | Bio pesticides | Whitefly mortality was highest (83.94%) in n spiromesifen+ imidacloprid and lowest (64.04%) in d dinotefuran. | [194] |
| Cotton | Essential oils from four different plants | About 30.8% to 64.2% mortality rates were reported. | [195] |
| Cowpea | Plant extracts (Neem leaves) | Promising results on population reduction in whiteflies, aphids, and pod borer. | [196] |
| Diets bioassays | French marigold plant aqueous and methanolic extracts | Up to 80% rate and antioviopostion were recorded on whiteflies. | [197] |
| Common bean | Nanoencapsulated essential oils from the fruits and foliage of Xylopia aromatic Lam. Mart. | Up to 98% reduction in oviposition by the whiteflies was recorded on the snap bean leaves. | [198] |
| Different plants | Lemon peel essential oils | About 99 to 100% mortality rate in both whitefly and mealy bugs. | [199] |
| Tomato and Strawberry | Neem oils and chamomile extracts | Neem oil lethality (71.3%), chamomile and lechuguilla extracts (62%) while neem oil with cactus pectin led to 60% mortality. | [26] |
| Tomato and Strawberry | Essential oils of neem | 60 to 71.3% mortality was observed. | [26] |
| Cotton | Ocimum gratissimum Lam. and Cymbopogon citratus Stapf. volatile compounds | A lower dose of C. citratus reduced whitefly number to 3.77 ± 0.51/30 plants, while a high dose of 5% of O. gratissimum reduced whitefly numbers to 3.38 ± 0.53/30 plants. | [200] |
| Laboratory | Plant extract (Avacado Kernel) | The extracts caused a high mortality of 90% in adults and 98.3% in the nymphs of whiteflies. | [201] |
| Tomato | Ethanolic extracts of Anona species | At 13 days following treatment, fewer eggs (35.00%) had hatched in the LC90 treatment than in the other groups. | [202] |
| Crop Name | Biological Agents Involved | Effects | Reference(s) |
|---|---|---|---|
| Predators | |||
| Cotton | Geocoris pallens Geocoridae | A predator–prey ratio of 0.75 G. pallens per 100 sweeps to one B. tabaci nymph was recorded. | [253,254] |
| Cotton, tomato, hibiscus, cowpea, collard | Delphastus catalinae (Horn) (Coleoptera: Coccinellidae | High rate of predation on whiteflies with highest effects on cotton and lowest on collard plants. | [255] |
| Cucumber | Chrysoperla carnea (Steph.), Orius albidipennis (Reuter) and Phytoseiulus persimilis Athias-Henrio | Individual predation suppressed whiteflies density on cucumber with highest effect recorded in the combination of the three predators. | [227] |
| Tomato | Dicyphus Hesperus Knight | About 88.8% decrease in whitefly density was recorded. | [78] |
| Cotton, cantaloupe | Hippodamia convergens Coccinellidae | Nymphal mortality per petri-dish reached 45.5%. | [253] |
| Cotton ficus hedge | Delphastus pallidus Coccinellidae | 68.0% and 55.1% eggs and nymph mortality on leaf disc, respectively. | [256,257] |
| Poinsettia | Serangium parcesetosum Coccinellidae | When four individuals/plant were used, B. tabaci fatality reached 60%. | [73] |
| Collards, soybean, and tomato | Nephaspis oculatus Coccinellidae | Within 24 h, up to 72.55% average predation on eggs was reported. | [255] |
| Cotton | Collops vittatus Melyridae | B. tabaci densities decreased by 86%. | [253,254] |
| Cotton | Geocoris punctipes Hemiptera | There was 36% nymphal predation petri dish. Predation on 4th instar nymphs led to major death of B. tabaci in the crops. | [73,258] |
| Cotton | Spanagonicus albofasciatus Miridae | 30–50% of the ova or mature females were reactive for B. tabaci antigen. | [259] |
| Cucumber | Macrolophus caliginosus Wagner, Dicyphus tamaninii Wagner, Orius majusculus Reuter, and O. laevigatus Feiber. | D. tamaninii consumed whitefly effectively at both lower and high densities while Orius majusculus and Macrolophus caliginosus were ineffective on whiteflies. | [260] |
| Entomopathogenic fungi | |||
| Melon, zucchini, squash, and cucumber | Beauveria bassiana (Balsamo-Crivelli) Vuillemin and Cordyceps fumosorosea (Wize) Kepler | More than 90% suppression of the whitefly recorded. | [242] |
| Cotton | Trachelas spp. Corinnidae | About 33.3% of individuals were reactive for B. tabaci DNA causing low species densities. | [253] |
| Eggplant | Aschersonia aleyrodis Aschal. | The rate of egg hatching in treated plants (85.3 ± 61.42) was less than the untreated groups (91.52 ± 2.10). The viability of the 1st (22.56 ± 1.20%), 2nd (39.30 ± 1.88%), and 3rd (39.30 ± 1.88%) instar nymphs were recorded. | [261] |
| Cucumber, melon, tomato | Verticillium lecanii (Zimm) strains | Reduction in whitefly population and symptoms of powdery mildew disease. | [262] |
| Cotton | Verticillium lecanii Zimm, Beauveria bassiana (Balsamo-Crivelli) Vuillemin, and Paecilomyces spp. | The mortality rate ranged from 57.1 to 100% depending on the strain deployed. | [263] |
| Eggplant | Isaria fumosoroseus Wize | It killed eggs, second, third, and fourth instars at a rate of 91, 90, 86, and 89%. | [264] |
| Soybean | Aschersonia aleyrodis Aschal. | Greatest mortality (99%) reported. | [69] |
| Cotton and tomato | Beauveria bassiana (Balsamo-Crivelli) Vuillemin | The fungi (Bb-01) reduced whitefly eggs by 65.30% and nymphs by 88.82%. | [265] |
| Cucumber, tomato, melon, and many other crops | Beauveria bassiana (Balsamo-Crivelli) Vuillemin | The mean fatality for larvae raised on cotton: 52.3 ± 7.3, cucumber: 91.8 ± 5.8. | [68] |
| Tomato | Aschersonia. Placenta Berk. | The fatality rate varied from 93% to 100%. | [59] |
| Sweet potato | Isaria spp. | LC50 and LT50 values when exposed to 1000 spores/mm2: LC50: second instar: 72–118 spores/mm2; third instar: 166–295 spores/mm2; fourth instar: 166–295 spores/mm2. | [70] |
| Cotton | Beauveria bassiana (Balsamo-Crivelli) Vuillemin | The fatality (56%) was observed at a higher dosage (1107 spores/mL) | [266] |
| Cucumbers | Isaria fumosoroseus (Wize) A.H.S | After 7 days of treatment, the 2nd instar was the most susceptible phase, with 83% fatalities. | [33] |
| Cotton | Beauveria bassiana (Balsamo-Crivelli) Vuillemin and Metarhizium anisopliae (Metschnikoff) Sorokin with synthetic insecticides | Mortality rate ranging from 62 to 84% was observed. | [267] |
| Eggplant | Metarhizium anisopliae (Metschnikoff) Sorokin, Verticillium lecanii Zimm, and Beauveria bassiana (Balsamo-Crivelli) Vuillemin | In plots of B. bassiana, V. lecanii, and M. anisopliae, the average density of whiteflies dropped from 126 ± 2.8 to 62.8 ± 3.3, 130 ± 3.8 to 61.4 ± 2, and 165.6 ± 2.2 to 62.4 ± 3.5, respectively. | [268] |
| Entomopathogenic nematodes | |||
| Cucumber and pepper | Steinernema feltiae Filipjev and Heterorhabditis bacteriophora Poinar | Both life stages of the whiteflies were susceptible to infection by the two nematode species. | [73] |
| Parasitoids | |||
| Eggplants | Metarhizium anisopliae (Metschnikoff) Sorokin | Mortality rate of up to 84.3% was recorded. | [215] |
| Hibiscus | Encarsia noyesi Hayat, Idioporous affinis LaSalle and Polaszek and Entedononecremnus krauteri Zolnerowich and Rose | Mean parasitism rates were 28 ± 2% for Idioporous affinis, 28.7 ± 1.9% for Encarsia noyesi, and 1 ± 0.0% by Entedononecremnus krauteri. | [269] |
| Tomato | Encarsia formosa Gahan and Encarsia sophia Girault and Dodd (Hymenoptera: Aphelinidae) | Up to 60% parasitism rate was observed on the whitefly population using individual predators. | [223] |
| Cotton | Encarsia sophia Girault and Dodd and Eretmocerus hayati Zolnerowich and Rose (Hymenoptera: Aphelinidae) | Encarsia sophia had a cumulative host consumption rate (C0) of 84.1 whiteflies per individual, while E. hayati had C0 of 17.6 whiteflies per individual. | [270] |
| Cotton | Eretmocerus hayati Zolnerowich and Ros | Eretmocerus hayati parasitized the entire nymphal phases of the whitefly with 2nd nymphs showing the greatest incidence (62.03%). | [271] |
| Poinsettias | Eretmocerus eremicus (Rose & Zolnerowich) and Amblyseius swirskii Athias-Henriot compared to synthetic insecticides | Average density (3.5 ± 1.09) of immature whiteflies per plant were recorded for the IPM. | [25] |
| Crop Name | Biotechnology Involved | Results | Reference |
|---|---|---|---|
| Cotton | RNA interference using v-ATPaseA | After consuming transgenic plants, the transcript level of v-ATPaseA in whiteflies was lowered by up to 62%. | [279] |
| Cotton | RNA interference using dsRNA | More than 90% mortality rate was recorded 24 h post treatment. | [291] |
| Lettuce | RNA interference using v-ATPaseA | After 5 days of feeding, whiteflies on modified plants die at a range of 83.8–98.1%. | [74] |
| Tobacco | RNA interference using | Transgenic plants showed tolerance to whitefly compared to untreated plants. | [292] |
| Cotton | RNA interference using expression of short interfering RNAs (siRNAs) | After 6 days of feeding on modified cotton, 70% mortality rate was recorded. | [73] |
| Tomato | Nuclear transgenics (transgenic plant) | Due to the toxic and repellency effects of 7-epizingiberene, developed tomato trichomes are resilient to whiteflies. | [293] |
| Tomato | Plant-mediated RNAi (A. tumefaciens) | Up to 50% whitefly mortality. | [294] |
| Tobacco | Nuclear transgenics (A. tumefaciens) | 100% mortality of Bemicia tabaci. | [66] |
| Cotton | Transgenic using ZmASN gene under constitutive promoter (A. tumefaciens) | There was a 95% death rate for whiteflies. | [295] |
| Arabidophsis | sRNA (307 bp) detoxifying gene BtGSTs5 is implicated in the neutralization of glucosides in B. tabaci | Knockdown of the BtBGSTs5 gene in the gut extends the developmental time of nymphs and reduces the number of B. tabaci. | [281] |
| Micro-injection (0.1–0.5 µg dsRNA) | dsRNA introduction into whiteflies (0.1–0.5 µg) | Up to 60% success was recorded from the study. | [296] |
| Micro-injection dsRNA | dsRNA introduction into whiteflies (0.1–0.5 µg) | Up to 70% decrease in whitefly population. | [296] |
| Micro-injection dsRNA | dsRNA introduction into whiteflies (0.1–0.5 µg) | There was 75% decline in the salivary gland as well 60% reduction in midgut expression. | [296] |
| Oral feeding | dsRNA introduction into whiteflies (Oral feeding) | Whitefly reproduction as well as survivability decreased significantly. | [278] |
| Tobacco | dsRNA applied exogenously to plants (0.5 mg/mL) | In 4th instar nymphs of whiteflies, Cyp315a1 was down-regulated by approximately 80%, while Cyp18a1 was down-regulated by 46%. | [297] |
| Citrus and cassava | Exogenous application of modified dsRNA via NRAi methods | Insects’ death rate rises from 12–35% in transformed species as related to non-modified ones. | [36] |
| Tomato | dsRNA applied exogenously to plants | The development of mature whiteflies was dramatically reduced (48.6%) in the pupae produced on Dys-dsRNA-treated plants. | [275] |
| Tomato | dsRNA applied exogenously to plants leaves. | The dsRNA, were molecularly detected in plants, aphids and mites but not in whiteflies. | [287] |
| Tomato | Application of dsRNA through the roots | The highest mortality (84%) was recorded at a concentration of 5 (µg/mL). | [298] |
| Tobacco | Chloroplast transgenics (Transplastomic plants) | B. tabaci density decreased by 91–93% in transplastomic plants compared to control plants. | [299] |
| Cotton | Nuclear transgenics | After six days, nymphs and adults of B. tabaci died at a rate of 18.37% and 9.65%, respectively. | [300] |
| Cotton | Nuclear transgenics | Genetically modified cotton harboring Tma12 gene at a concentration of 0.01% was effective against whitefly (>90% mortality). | [35] |
| Tomato | RNA interference induced by plants (via siRNA) transgenic | Decreased reproduction and increased lethality by 81.8% to 85.6% respectively. | [5] |
| Cotton | RNA interference induced by plants (via miRNA) transgenic | Up to 78% mortality rate was recorded. | [280] |
| Tobacco | Chloroplast-mediated RNAi | The transgenic plants harboring BtACTB had led to 80% mortality rates in B. tabaci. | [67] |
| Tobacco | Artificial miRNA mediated resistance | Abnormal egg hatching and poor nymphal development were observed on the modified plant compared to the control. | [292] |
| Citrus, cassava | Modified RNAi for dsRNA delivery | There was an increase in the mortality rate of the insects with 12–35% compared to non-modified plants. | [36] |
| Cotton | Gene silencing (RNAi) | Oral delivery of dsRNA led to 42.5% adult death, decreased fertility (36.57 eggs per female), with 62.50% larval death. | [301] |
| Crop Name | Pesticides Used | Result | Reference |
|---|---|---|---|
| Tomato and verbena | Buprofezin, teflubenzuron, imidacloprid, and nicotine | Highest mortality (79:8%) was recorded for buprofezin while imidacloprid caused 58:5% lower mortality. | [310] |
| Cabbage | Seventeen insecticides including abamectin, acephate, acetamiprid, cartap, imidacloprid, malathion, etc. | Cartap caused highest mortality (100%) while trichlorphon had less (4%) mortality. | [311] |
| Strawberry | Imidacloprid, thiamethoxam, and dinotefuran | Imidacloprid caused adult mortality of 63.58%, thiamethoxam had 41.95% mortality. | [312] |
| Cotton | Acetamiprid, imidacloprid, bifenthrin, cypermethrin, triazophos, cyhalothrin and rani. | The plots treated with bifenthrin had the highest number of whiteflies per leaf (2.773), followed by imidacloprid (1.83) compared to control plots (5107). | [313] |
| Cucumber | Imidacloprid, thiacloprid, deltamethrin, pyrethrum, thiamethoxam, and lambda-cyhalothrin | Pyrethrum was the most effective with 90.23% mortality rate followed by thiacloprid+ deltamethrin with 89.57%. | [314] |
| Eggplant | Four insecticides viz; fipronil, imidacloprid, buprofezin, and thiamethoxam along with emamectin benzoate | Confidor was the most effective with 69.0% whitefly mortality. | [215] |
| Citrus | Diazinon, endosulfan, imidacloprid | After 10 weeks of pesticide spraying, there was 100% fatality of whiteflies. | [315] |
| Cotton | Imidacloprid, bifenthrin, chlorpyrifos, and carbosulfan | Carbosulfan led to 40% adult whitefly mortality while chlorpyrifos had the least (25%). | [288] |
| Cotton | Diafenthiuron, quinalphos, flubendiamide, imidacloprid, thiamethoxam, triazophos, carbosulfan, phosalone, and chlorpyriphos | The whitefly found on treated plants range from 0.13 (fipronil 5 SC) to 2.1 (phosalone 35 EC) per leaf. | [316] |
| Cluster bean | Clothianidin, thiamethoxam spiromesifen, fipronil, acephate, imidacloprid, and carbosulfan | Spiromesifen was the best treatment with 1.61 whiteflies/3 leaves while imidacloprid had the least effect (3.46) whiteflies/3 leaves. | [317] |
| Okra | Lambdacyhalothrin | Up to 63.94% lethality at 7 days of treatment. However, a drop (18.99%) in its efficacy was recorded after 15 days of the treatment. | [318] |
| Eggplant | Thiamethoxam, imidacloprid, acephate, fipronil, thiacloprid, and dimethoate | Total control (100%) was reported using thiamethoxam 25 WG @ 100 g/ha 14 days post treatment. | [232] |
| Tomato | Transform (sulfoxaflor), polo (diafenthiuron), confidor (imidacloprid), and agrovista | Imidacloprid was the most effective having a mortality rate of up 93.24% 2 h post treatment. | [319] |
| Tomato | Profenophos, imidacloprid, cypermethrin, and indoxacarb. | Imidacloprid was the most effective treatment with 58.1% mortality while indoxacarb was the least effective (51.40%). | [320] |
| Cotton | Seven common insecticides: cyanantraniliprole, sulfoxaflor, spirotetramat, flonicamid, acetamiprid, etc. | Sulfoxaflor has the highest relative toxicity (13.86%). | [29] |
| Zucchini | Acetamiprid, pymetrozine with phosphoric soap, and spirotetramat along with azadirachtin | Up to 44% of whiteflies suppression was recorded from the study. | [8] |
| Eggplant | Actara a 25 WDG, calypso 480 SC, polo, confidor 5 G, and confidor 200 SL | After 14 days, the maximum effectiveness (89.06%) was achieved using actara foliar application. | [321] |
| Chilli | Spinetoram, novastar (bifenthrin + abamectin), and sulfoxaflor 50 WG | Bifenthrin + abamectin had proven to be the most effective for reducing whitefly populations (84.46%). | [322] |
| Cotton | Profenofos, cyhalothrin, and imidacloprid | Whitefly mortality of up to 88% was reported from the study. | [323] |
| Tomato | Dimethoate, imidacloprid, lambdacyhalothrin, novaluron, imidacloprid, indoxacarb, azadirachtin | Imidacloprid was found to have the lowest whitefly density (2.18 adults/leaf) compared to the control with 5.69 adult/leaf. | [324] |
| Eggplant | Buprofezin, imidacloprid, fipronil, spinosad, and emamectin benzoate | The use of imidacloprid at a rate of 100 mL/ha have the greatest effect in lowering the whitefly densities with 1.00/leaf 2 weeks after treatment. | [257] |
| Tomato | Thiocyclam (hydrogen oxalate), acetambrid, and imidacloprid | Abamectin and imidacloprid were the toxic pesticides with 86.98 ± 2.63 and 84.19± 1.56 mortality rate, respectively. | [325] |
| Okra | Imidacloprid | It was effective against the whiteflies having 3.90 whiteflies/15 leaves 2 weeks after treatment. | [326] |
| Potato | Emamectin, thiodicarb, diafenthiuron, chlorpyriphos, chlorfenapyr, cryantraniliprole, bifenthrin, and spiromesifen | It was discovered that the insecticide spiromesifen 22.9 SC @ 1.00 mL/l was highly effective against mites and whiteflies. | [30] |
| Eggplant | Lambda-cyhalothrin | When treated with lambda-cyhalothrin, whitefly average density was dramatically reduced (2.21/leaf 2 weeks after application). | [327] |
| Crop Name | Treatments Deployed | Results | References |
|---|---|---|---|
| Tomato | Plant extracts, tween 20, and biological agent (predator) | Leaf and fruit extracts + tween-20 resulted in death rates ranging from 34.6 to 67.9% for leaf and 53.5 to 74.1% for fruits, respectively. | [347] |
| Tomato and verbena | Chemical insecticides and entomopathogenic nematode | The combined effect of nematodes and imidacloprid caused 70.9% B. tabaci larval mortality. | [309] |
| Tomatoes | Parasitoids, predators, and insecticides | The most effective treatments were a mix between Eretmocerus mundus and Amblyseius swirskii with an average of 0.7 ± 0.18 whiteflies per leaf. | [77] |
| Tomatoes | Chemical insecticides and entopathogenic nematode | The use of nematodes + thiacloprid and spiromesifen resulted in a greater B. tabaci lethality (86.5 and 94.3%), compared to nematodes alone (75.2%). | [238] |
| French bean | Novel insecticides and fatty acids deposits | Fatty acid deposits caused 10.7% adult whitefly mortality, diafenthiuron caused 62.7%, and the combined effect led to 69.7% lethality. | [348] |
| Ash gourd | Synthetic chemicals, sticky traps, plant extracts, farmers practices, and micronutrients | After 18 days, 100% whitefly inhibition was recorded while the average number of whiteflies per plant was 1.86 after 60 days. | [349] |
| Tomatoes | Biopesticides and synthetic chemical | Cytraniliprole + lambda-cyhalothrin (50 + 30 g a.i. ha-1) reduced whitefly by 64%. 72% larval mortality was recorded using 0.5% flaxseed + 0.3% sodium bicarbonate. | [350] |
| Tomatoes | Metallic reflective mulches and insecticides and resistant cultivar | Metallic reflecting mulch drastically decreased the insect density as well as the disease symptoms on tomatoes. | [351] |
| Tomato | Intercropping and irrigation system | Intercropping along with sprinkling irrigation reduced tomato plants’ suitability for B. tabaci multiplication. | [110] |
| Potatoes | Mineral oils and synthetic chemicals | Imidacloprid + thiamethoxam + mineral oils resulted in decrease in B. tabaci population (74.5%) and disease incidence (93.0%). | [38] |
| Cucumber | Botanicals and synthetic insecticides | Thiacloprid + deltamethrin (73.42%), pyrethrum + lambda-cyhalothrin (89.57%), and thiamethoxam + lambda-cyhalothrin (90.29%) mortality were recoded. | [313] |
| Brinjal | Botanicals and synthetic insecticides | The use of 5% neem extract (NSKE) lowered the population (3.5 whiteflies/leaf) as compared to the control (8.0 whiteflies/leaf). | [142] |
| Eggplant | Entomoathogenic fungi and plant extracts | Neem (1%) along with B. bassiana had the highest effect against both eggs (88.25%) and adult whiteflies (80.15%). | [80] |
| Soybeans | Different chemical pesticides | There was reduced level of egg hatching greatly to about 4.35% compared to 95% in the control. | [352] |
| Tomato | Botanical oils and chemicals | Mortality rate of up to 80.5% was reported in the study. | [195] |
| Tomato | Physical method (use of kaolin, a clay mineral) | 90.1–91.6% drop in whitefly number was reported at 5% while 89% and 85.7% were reported for nymphs at 5% w/v. | [353] |
| Cotton | Entomopathogenic fungi and insecticides | A greater death rate (96.78%) was seen when matrine was combined with L. muscarium with LC50 values of 0.034, 0.063, and 0.21 mg/L. | [354] |
| Sweet potato | Entomopathogenic fungi and aqueous plant extracts | NATURALIS + Calotropis procera had highest mortality rate on eggs (62.6%), nymphs (67%), and adult whiteflies (65.2%). | [355] |
| Cucumber | Plant extracts and commercial insecticides | The use of the extracts along with the pesticides resulted in up to 80% whitefly mortality. | [193] |
| Eggplant | Biopesticides and synthetic insecticides | In comparison to the control (11.04), the overall mean number of whiteflies per leaf was significantly lower (3.20 to 5.49) across all treated crops. | [104] |
| Bt cotton | Chemicals, plant extracts, and entomopathogenic fungi | Spiromesifen had the greatest reduction in whitefly numbers (82.27%, 80.57), then imidacloprid (82.27%, 80.57%). | [37] |
| Eggplant | Synthetic chemicals, biopesticides | The field treated with imidacloprid 17.8 SL @ 100 mL/ha had the lowest whitefly density (2.40 whiteflies/leaf). | [256] |
| Tomatoes | Plant elicitors (methyl salicylate) and volatile organic compounds | The plant elicitor was reported to effectively limit whitefly population and enhance production by 11% when used on healthy tomato plants. | [356] |
| Crop plants | Mixture of cow urine with nettle leaves, wild azadirachta, and holy basil | The concoction was very effective in controlling crop pests at nearly no costs. | [39] |
| Orange | Different organic pesticides | None of the substances tested resulted in a significant fatality of any of the orange spiny whitefly instars. | [357] |
| Cotton | Three biopesticides along with synthetic insecticides | Eco-Bb® treated plots caused 60% mortality while Karate® led to 67% whitefly mortality. | [358] |
| Poinsettia | Integrated using systemic and trans laminar insecticides | Lowest nymph density (1.0 + 0.5) was reported using imidcloprid. | [359] |
| Tomato | Plant derivatives with the neonicotinoid insecticide | Up to 94.4% mortality rate was recorded. | [202] |
| Eggplant | Botanicals and synthetic insecticides | The average number of whiteflies was higher in integrated treatments (2.37) and lower in the lambda cyhalothrin treatment (2.21). | [327] |
| Okra | Biopesticides and synthetic insecticides | On average, there were 3.90 whiteflies per 15 leaves when imdacloprid 17.8% (0.3 mL per liter) was applied to the plants. Beauveria bassiana and M. anisopliae were found to be less efficient, but still more potent than the control. | [326] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abubakar, M.; Koul, B.; Chandrashekar, K.; Raut, A.; Yadav, D. Whitefly (Bemisia tabaci) Management (WFM) Strategies for Sustainable Agriculture: A Review. Agriculture 2022, 12, 1317. https://doi.org/10.3390/agriculture12091317
Abubakar M, Koul B, Chandrashekar K, Raut A, Yadav D. Whitefly (Bemisia tabaci) Management (WFM) Strategies for Sustainable Agriculture: A Review. Agriculture. 2022; 12(9):1317. https://doi.org/10.3390/agriculture12091317
Chicago/Turabian StyleAbubakar, Mustapha, Bhupendra Koul, Krishnappa Chandrashekar, Ankush Raut, and Dhananjay Yadav. 2022. "Whitefly (Bemisia tabaci) Management (WFM) Strategies for Sustainable Agriculture: A Review" Agriculture 12, no. 9: 1317. https://doi.org/10.3390/agriculture12091317
APA StyleAbubakar, M., Koul, B., Chandrashekar, K., Raut, A., & Yadav, D. (2022). Whitefly (Bemisia tabaci) Management (WFM) Strategies for Sustainable Agriculture: A Review. Agriculture, 12(9), 1317. https://doi.org/10.3390/agriculture12091317







