Biomass Fatty Acid Profile and Fuel Property Prediction of Bagasse Waste Grown Nannochloropsis oculata
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sugarcane Bagasse Aqueous Extract (SBAE)
2.2. Experimental Design
2.3. Microalgal Growth Rate and Analysis
2.4. Lipid Extraction and Fatty Acid Analysis
2.5. Prediction of Biodiesel Properties
HHV = 49.43 − (0.041 ∗ S.N) − (0.015 ∗ IV); DU = (MUFA%) + 2 ∗ (PUFA%)
and LCSF = (0.1 ∗ C16) + (0.5 ∗ C18) + (1 ∗ C20) + (1.5 ∗ C22) + (2 ∗ C24)
CFPP = (3.1417 ∗ LCSF) − 16.477.
2.6. Statistical Analysis
3. Results
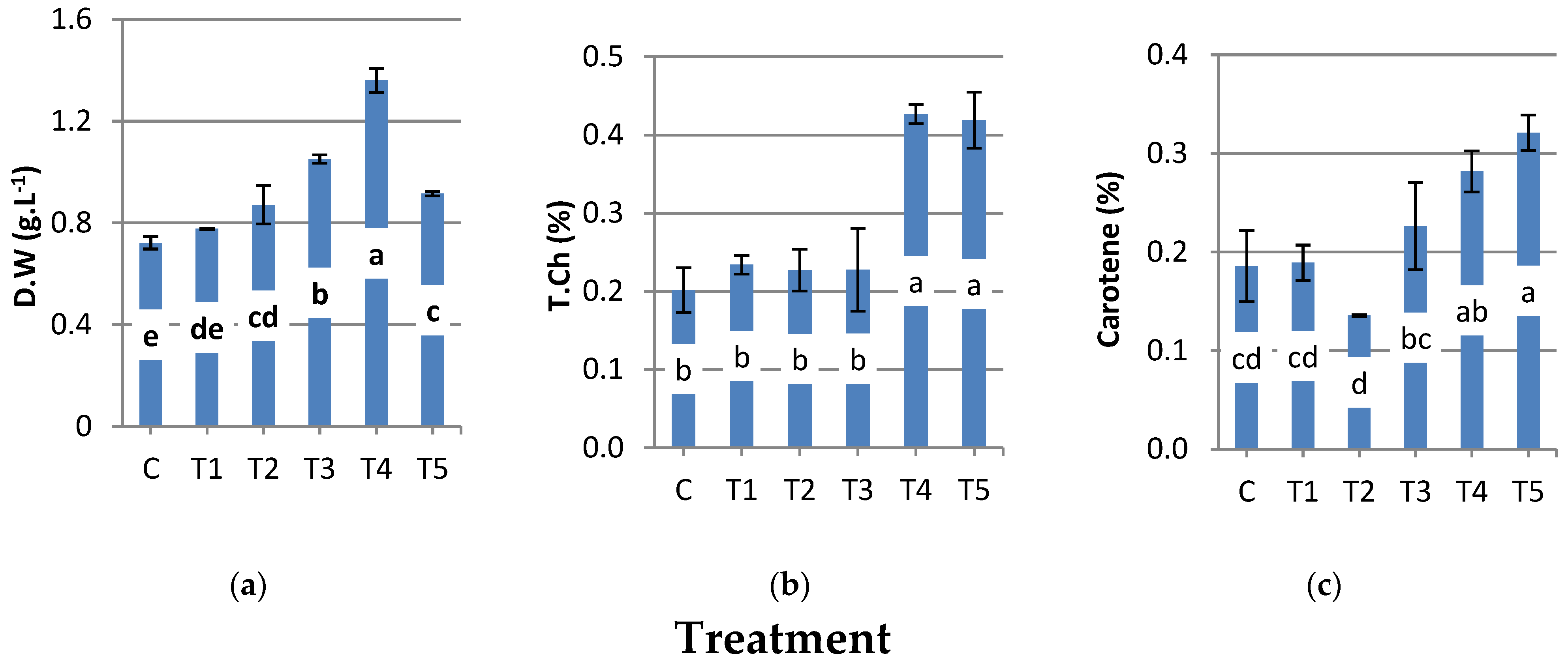
3.1. Effect of SBAE on N. oculata Growth under N Deficiency
3.2. Effect of Induction on N. oculata Growth
3.3. Effects of SBAE on Fatty Acid Profile of N. oculata
3.4. Prediction of Biodiesel Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, F.M.; Hayder, N.H.; Hammadi, S.S.F. Enhancement of Biodiesel Production from Local Isolates of Microalgae. Mesop. Environ. J. 2015, 1, 66–81. [Google Scholar]
- Rivera-Jaimes, J.A.; Postigo, C.; Melgoza, A.R.M.; Aceñ, A.; Barceló, D.; Ópez de Alda, M. Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico. Occurrence and environmental risk assessment. Sci. Total Environ. 2018, 613, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, Y.; Fan, C.; Hong, H.; Wu, W.; Zhang, W.; Wang, Y. Production, Processing, and Protection of Microalgal n-3 PUFA-Rich Oil. Foods 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A Review on the Use of Microalgae for Sustainable Aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Jones, C.S.; Mayfield, S.P. Algae Biofuels: Versatility for the Future of Bioenergy. Curr. Opin. Biotech. 2012, 23, 346–351. [Google Scholar] [CrossRef]
- Battah, M.G.; El-Sayed, A.B.; El-Sayed, E.W. Growth of the green alga Chlorella vulgaris as affected by different carbon sources. Life Sci. J. 2013, 10, 2075–2081. [Google Scholar]
- El-Sayed, A.B.; Battah, M.G.; El-Sayed, E.W. Utilization efficiency of artificial carbon dioxide and corn steam liquor by Chlorella vulgaris. Biolife 2015, 3, 391–403. [Google Scholar] [CrossRef][Green Version]
- El-Sayed, A.B. Carotenoids accumulation in the green alga Scenedesmus sp. incubated with industrial citrate waste and different inductions stress. Nat. Sci. 2010, 8, 34–40. [Google Scholar]
- El-Sayed, A.B.; Hoballah, E.M.; Khalafallah, M.A. Utilization of citrate wastes by Scenedesmus sp. I-Enhancement of Vegetative Growth. J. Appl. Sci. Res. 2012, 8, 739–745. [Google Scholar]
- Sheraz, M.K.; El-Sayed, A.B.; Hassan, A.A.; El-Shazly, H.A.M.; Ibrahim, M.T. Use of okara waste for algae nutrition. Arab. Univ. J. Agric. Sci. 2017, 25, 271–279. [Google Scholar]
- Fetyan, N.A.H.; El-Sayed, A.B.; Ibrahim, F.M.; Sadik, M.W.; Attia, A.Y. Bioethanol production from defatted biomass of Nannochloropsis oculata microalgae grown under mixotrophic conditions. Environ. Sci. Pollut. Res. 2021, 29, 2588–2597. [Google Scholar] [CrossRef]
- Gimpel, A.; Stelzenmuller, V.; Grote, B.; Buck, B.H.; Floeter, J.; Núñez-Riboni, I. A GIS modelling framework to evaluate marine spatial planning scenarios: Co-location of offshore wind farms and aquaculture in the German EEZ. Mar. Policy 2015, 55, 102–115. [Google Scholar] [CrossRef]
- Merchant, S.S.; Kropat, J.; Liu, B.; Shaw, J.; Warakanont, J. TAG, you are it! Chlamydomonas as a reference organism for understanding algal triacylglycerol accumulation. Curr. Opin. Biotech. 2012, 23, 352–363. [Google Scholar] [CrossRef]
- Muthukrishnan, L. Bio-engineering of microalgae: Challenges and future prospects toward industrial and environmental applications. J. Basic Microbiol. 2022, 62, 310–329. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Wu, Q. Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. 2007, 98, 764–771. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Microalgae biofuels: A critical review of issues, problems and the way forward. Biotech. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef]
- Ben Moussa-Dahmen, I.; Chtourou, H.; Rezgui, F.; Sayadi, S.; Dhouib, A. Salinity stress increases lipid, secondary metabolites and enzyme activity in Amphora subtropica and Dunaliella sp. for biodiesel production. Bioresour. Technol. 2016, 218, 816–825. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Devi, M.P. Salinity stress induced lipid synthesis toharness biodiesel during dual mode cultivation of mixotrophic microalgae. Bioresour. Technol. 2014, 165, 288–294. [Google Scholar] [CrossRef]
- Ishika, T.; Moheimani, N.R.; Laird, D.W.; Bahri, P.A. Stepwise culture approach optimizes the biomass productivity of microalgae cultivated using an incremental salinity increase strategy. Biomass Bioenergy 2019, 127, 105274. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, Y.L.; Zhan, J.; He, C.L.; Wang, Q. Comparative metabolic profiling of the lipid-producing green microalga Chlorella reveals that nitrogen and carbon metabolic pathways contribute to lipid metabolism. Biotechnol. Biofuels 2017, 10, 10153. [Google Scholar] [CrossRef]
- Metting, F.B. Biodiversity and application of microalgae. J. Ind. Microbiol. Biotechnol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- El-Sayed, A.B.; Fetyan, N.A.; Ibrahim, F.; AFayed, S.; Sadik, M.W. Application of bagasse extract in economic Nannochloropsis oculata mass production. Egypt. J. Chem. 2020, 63, 5183–5192. [Google Scholar]
- Burnison, K. Modified dimethyl sulfoxide (DMSO) extraction for chlorophyll analysis of phytoplankton. Can. J. Fish. Aquat. Sci. 1980, 37, 729–733. [Google Scholar] [CrossRef]
- Boussiba, S.; Fan, L.; Vonshak, A. Enhancement and determination of astaxanthin accumulation in green alga Haematococcus pluvialis. In Methods in Enzymology, Carotenoids Part A; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1992; Volume 213, pp. 386–391. [Google Scholar]
- Davies, H. Carotenoids. In Chemistry and Biochemistry of Plant Pigments, 2nd ed.; Goodwin, T.W., Ed.; Academic Press: New York, NY, USA, 1976; Volume 2, pp. 38–165. [Google Scholar]
- Arora, N.; Patel, A.; Pruthi, P.A.; Pruthi, V. Boosting TAG Accumulation with Improved Biodiesel Production from Novel Oleaginous Microalgae Scenedesmus sp. IITRIND2 Utilizing Waste Sugarcane Bagasse Aqueous Extract (SBAE). Appl. Bioch. Biotech. 2016, 180, 109–121. [Google Scholar] [CrossRef]
- El-Sayed, A.B. Some Physiological Studies on Green Algae. Ph.D. Thesis, Plant Physiology Department, Faculty of Agriculture Cairo University, Cairo, Egypt, 1999. [Google Scholar]
- Almutairi, A.W.; El-sayed, A.B.; Reda, M.M. Combined effect of salinity and pH on lipid content and fatty acid composition of Tisochrysis lutea. Saudi J. Biol. Sci. 2020, 27, 3553–3558. [Google Scholar] [CrossRef]
- El-Shafey, Y.H.; El-Fouly, M.M.; Khalil, M.M.; Abdallah, F.E.; El-Sayed, A.B. Secondary carotenoids accumulation by some green algae species. In Proceedings of the First Congress on the Recent Technologies in Agriculture, Faculty of Agriculture, Cairo University, Cairo, Egypt, 27–29 November 1999. [Google Scholar]
- Talebi, A.F.; Tabatabaei, M.; Mohtashami, S.K.; Tohidfar, M.; Moradi, F. Comparative salt stress study on intracellular ion concentration in marine and saltadapted freshwater strains of microalgae. Not. Sci. Biol. 2013, 5, 309–315. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Gheda, S.F.; El-Sayed, A.E.-K.B.; Shady, A.M.A.; El-Sheikh, M.E.; Schagerl, M. Outdoor cultivation of the green microalga Chlorella vulgaris under stress conditions as a feedstock for biofuel. Environ. Sci. Pollut. Res. 2019, 26, 18520–18532. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Borghi, M.D. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Dang, N.M.; Lee, K. Decolorization of organic fertilizer using advanced oxidation process and its application for microalgae cultivation. J. Ind. Eng. Chem. 2018, 59, 297–303. [Google Scholar] [CrossRef]
- Lubián, M.L.; Montero, O.; Moreno-Garrido, I.; Huertas, E.; Sobrino, C.; del Valle, M.G.; Parés, G. Nannochloropsis (Eustigmatophyceae) as source of commercially pigments. J. Appl. Phycol. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Shtaida, N.; Khozin-Goldberg, I.; Solovchenko, A.; Chekanov, K.; Didi-Cohen, S.; Leu, S.; Cohen, Z.; Boussiba, S. Down regulation of a putative plastid PDC E1α subunit impairs photosynthetic activity and triacylglycerol accumulation in nitrogen-starved photoautotrophic Chlamydomonas reinhardtii. J. Exp. Bot. 2014, 65, 6563–6576. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.P.; Williams, E.; Wang, D.Z.; Xie, Z.X.; Hsia, R.C.; Jenck, A.; Halden, R.; Li, J.; Chen, F.; Place, A.R. Responses of Nannochloropsis oceanica IMET1 to long-term nitrogen starvation and recovery. Plant Physiol. 2013, 162, 1110–1126. [Google Scholar] [CrossRef]
- Cerón Garcıía, M.C.; Mirón, A.S.; Sevilla, J.F.; Grima, E.M.; Camacho, F.G. Mixotrophic growth of the microalga Phaeodactylum tricornutum. Influence of diferent nitrogen and organic carbon source on productivity and biomass composition. Proc. Biochem. 2005, 40, 297–305. [Google Scholar] [CrossRef]
- Tittel, J.; Bissinger, V.; Gaedke, U.; Kamjunke, N. Inorganic carbon limitation and mixotrophic growth in Chlamydomonas from an acidic mining lake. Protist 2005, 156, 63–750. [Google Scholar] [CrossRef]
- Kaye, Y.; Grundman, O.; Leu, S.; Zarka, A.; Zorin, B.; Didi-Cohen, S.; Goldberg, K.I.; Sammy, B. Metabolic engineering toward enhanced LC-PUFA biosynthesis in Nannochloropsis oceanica: Overexpression of endogenous 12 desaturase driven by stress-inducible promoter leads to enhanced deposition of polyunsaturated fatty acids in TAG. Algal Res. 2015, 2, 387–398. [Google Scholar] [CrossRef]
- Ma, X.N.; Liu, J.; Liu, B.; Chen, T.P.; Yang, B.; Chen, F. Physiological and biochemical changes reveal stress-associated photosynthetic carbon partitioning into triacylglycerol in the oleaginous marine alga Nannochloropsis oculata. Algal Res. 2016, 16, 18–35. [Google Scholar] [CrossRef]
- Wei, L.; Huang, X. Long-duration effect of multi-factor stresses on the cellular biochemistry, oil-yielding performance and morphology of Nannochloropsis oculata. PLoS ONE 2017, 12, e0174646. [Google Scholar] [CrossRef]
- Hamza, H.A.; Hamouda, R.A.; Husein, M.H.; Abd-Elwahid, S.S. The characteristics of biomass production and lipid accumulation of Chlorella kessleri growth under mixotrophic and heterotrophic conditions. Egypt. J. Exp. Biol. (Bot.) 2013, 9, 19–26. [Google Scholar]
- Wang, Y.; Chen, T.; Qin, S. Differential fatty acid profiles of Chlorella kessleri grown with organic materials. J. Chem. Technol. Biotechnol. 2012, 88, 651–657. [Google Scholar] [CrossRef]
- Francisco, E.C.; Neves, D.B.; Jacob-Lopes, E.; Franco, T.T. Microalgae as feedstock for biodiesel production: Carbon dioxide sequestration, lipid production and biofuel quality. J. Chem. Technol. Biotech. 2010, 85, 395–403. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Yang, S.-Y.; Song, H.-S.; Park, J.Y.; Park, Y.-L.; Han, Y.-H.; Choi, Y.-K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef]
- Banani, R.; Youssef, S.; Bezzarga, M.; Abderrabba, M. Waste frying oil with high levels of free fatty acids as one of the prominent sources of biodiesel production. J. Mater. Environ. Sci. 2015, 6, 1178–1185. [Google Scholar]
- Nascimento, I.A.; Izabel-Marques, S.S.; Dominguez-Cabanelas, I.T. Screening microalgae strains for biodiesel production: Lipid productivity an estimation of fuel quality base on fatty acids profiles as selective criteria. Bioenergy Res. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Tang, H.; Chen, M.; Garcia, M.E.D.; Abunasser, N.; Ng, K.S.; Salley, S.O. Culture of microalgae Chlorella minutissima for biodiesel feedstock production. Biotechnol. Bioeng. 2011, 108, 2280–2287. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.; Wu, Q. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotech. 2006, 126, 499–507. [Google Scholar] [CrossRef]
- Francisco, E.C.; Jacob-Lopes, E.; Vieira, K.R.; Franco, T.T. Nitrogen Starvation of Assessment in the Production of Single Cell Oils and Biodiesel Quality in Heterotrophic Cultures of Cyanobacteria Phormidium autumnale. J. Adv. Chem. Eng. 2019, 9, 192. [Google Scholar] [CrossRef]
- Masojidek, J.; Koblizek, M.; Torzillo, G. Photosynthesis in Microalgae. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 20–39. [Google Scholar]
- Rai, H. The influence of photon flux density (PFD) on short term 14 C incorporation into proteins, carbohydrates and lipids in freshwater algae. Hydrobiologia 1995, 308, 51–59. [Google Scholar] [CrossRef]
- Rai, H.; ARTS, M.; Wainman, B.; Dockal, N.; Krambeck, H. Lipid production innatural phytoplankton communities in a small freshwater Baltic Lake, LakeSchöhsee, Germany. Freshw. Biol. 1997, 38, 581–590. [Google Scholar] [CrossRef]
- Al-Mutairi, A.W.; Toulibah, H.E. Effect of Salinity and pH on Fatty Acid Profile of the Green Algae Tetraselmis suecica. J. Pet. Environ. Biotech. 2017, 8, 333. [Google Scholar]
- Stansell, G.R.; Gray, V.M.; Sym, S.D. Microalgal fatty acid composition: Implications for biodiesel quality. J. Appl. Phycol. 2012, 24, 791–801. [Google Scholar] [CrossRef]
- Yeesang, C.; Cheirsilp, B. Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour. Technol. 2011, 102, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis, C.C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioengin. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Li, Z.-R.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef]

| Cu | Mn | Zn | Fe | Na | Mg | Ca | K | P | N | O.C |
|---|---|---|---|---|---|---|---|---|---|---|
| ppm | % | |||||||||
| 0.03 ± 0.021 | 0.42 ± 0.033 | 1.02 ± 0.371 | 1.15 ± 0.46 | 32.5 ± 2.657 | 33.75 ± 1.247 | 44 ± 1.421 | 51 ± 2.301 | 7.0 ± 1.021 | 0.04 ± 0.0012 | 98.9 ± 3.221 |
| Fatty Acid (% Total FA) | Control | * Vegetative | ** MFS |
|---|---|---|---|
| Caprylic acid (C8:0) | 3.83 a | 3.98 a | 0.894 b |
| Capric acid (C10:0) | 7.30 a | 7.150 a | 1.876 b |
| Lauric (C12:0) | 5.91 a | 5.500 a | 2.650 b |
| Myristic acid (C14:0) | 1.96 b | 3.0400 b | 11.095 a |
| Myristoleic acid (C14:1) | 5.37 a | 4.410 a | 1.226 b |
| Palmitic (C16:0) | 14.91 b | 17.270 b | 31.300 a |
| Palmitolecic (C16:1) | 3.08 b | 3.470 b | 10.064 a |
| Stearic (C18:0) | 6.88 a | 5.570 a | 2.505 b |
| Oleic (C18:1) | 10.33 c | 13.32 b | 26.700 a |
| Linoleic (C18:2) | 18.12 a | 17.490 a | 3.70 b |
| Linolenic (C18:3) | 12.11 a | 9.7 b | 1.05 c |
| Arachidonic acid (C20:4) | 10.21 a | 9.110 a | 6.94 b |
| Σ C16 | 17.39 c | 20.74 b | 41.368 a |
| Σ C18 | 47.44 a | 46.08 a | 34.01 b |
| Σ C20 | 10.21 c | 9.11 c | 6.94 c |
| Σ MUSFA | 18.78 c | 21.2 b | 37.99 a |
| Σ PUSFA | 40.44 a | 36.3 a b | 11.69 c |
| TUSFA | 59.22 a | 57.5 a | 49.68 a |
| TSFA | 40.78 a | 42.5 a | 50.32 a |
| Parameter | DU | SV | IV | CN | LCSF | CEPP | Vis | Oil% |
|---|---|---|---|---|---|---|---|---|
| Vegetative | 93.8 | 248.29 | 111.05 | 43.27 | 4.51 | −2.30 | 3.31 | 6.19 |
| MFS | 63.37 | 223.02 | 71.04 | 54.79 | 3.31 | −2.79 | 3.40 | 11.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, A.E.-K.B.; Fetyan, N.A.; Moghanm, F.S.; Elbagory, M.; Ibrahim, F.M.; Sadik, M.W.; Shokr, M.S. Biomass Fatty Acid Profile and Fuel Property Prediction of Bagasse Waste Grown Nannochloropsis oculata. Agriculture 2022, 12, 1201. https://doi.org/10.3390/agriculture12081201
El-Sayed AE-KB, Fetyan NA, Moghanm FS, Elbagory M, Ibrahim FM, Sadik MW, Shokr MS. Biomass Fatty Acid Profile and Fuel Property Prediction of Bagasse Waste Grown Nannochloropsis oculata. Agriculture. 2022; 12(8):1201. https://doi.org/10.3390/agriculture12081201
Chicago/Turabian StyleEl-Sayed, Abo El-Khair B., Nashwa A. Fetyan, Farahat S. Moghanm, Mohssen Elbagory, Fatma M. Ibrahim, Mahmoud W. Sadik, and Mohamed S. Shokr. 2022. "Biomass Fatty Acid Profile and Fuel Property Prediction of Bagasse Waste Grown Nannochloropsis oculata" Agriculture 12, no. 8: 1201. https://doi.org/10.3390/agriculture12081201
APA StyleEl-Sayed, A. E.-K. B., Fetyan, N. A., Moghanm, F. S., Elbagory, M., Ibrahim, F. M., Sadik, M. W., & Shokr, M. S. (2022). Biomass Fatty Acid Profile and Fuel Property Prediction of Bagasse Waste Grown Nannochloropsis oculata. Agriculture, 12(8), 1201. https://doi.org/10.3390/agriculture12081201








