Isolation of Lignin from Anaerobically Digested Unhydrolyzed Solids Produced in a Biorefinery
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass
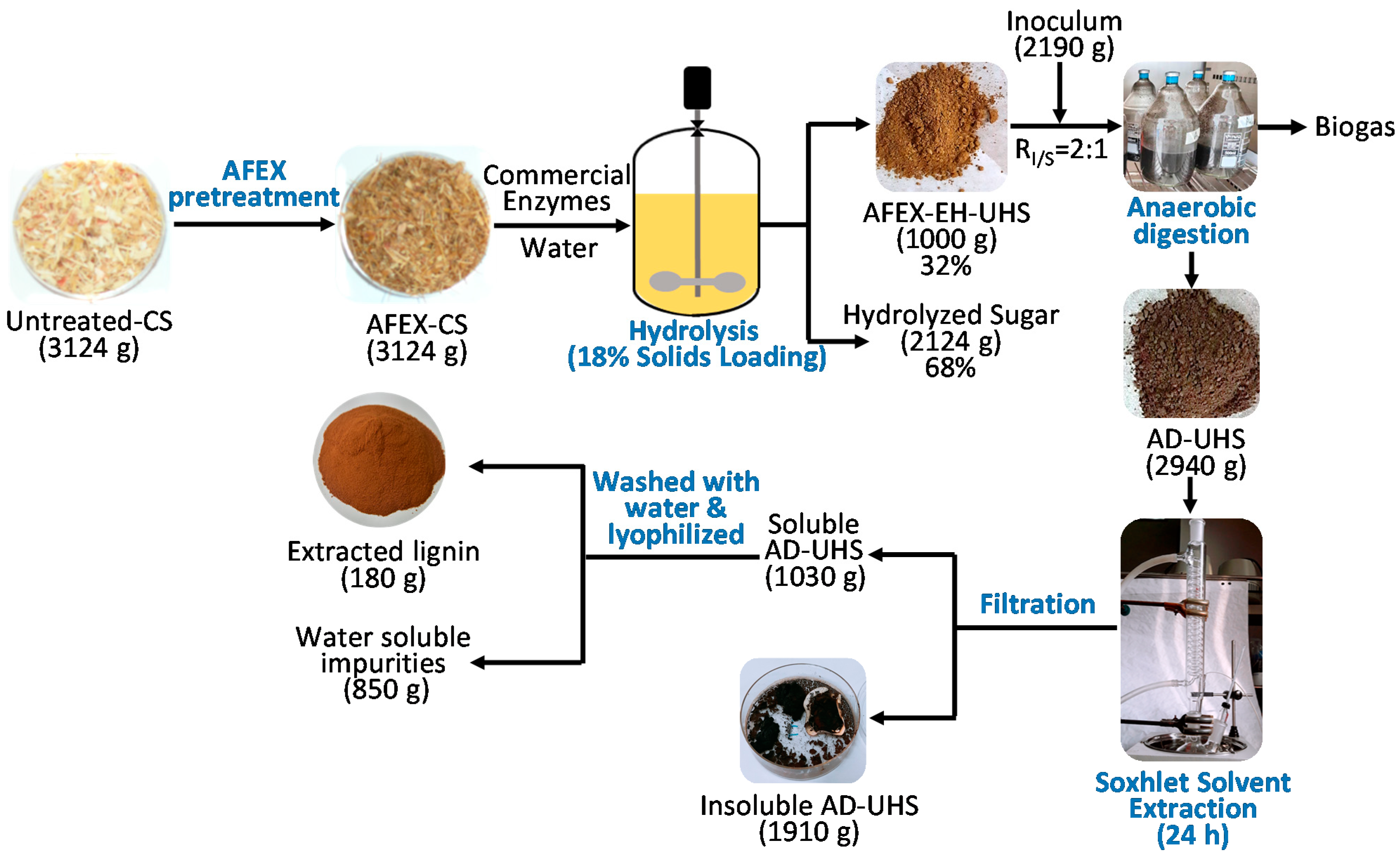
2.2. Preparation of AFEX-EH-UHS
2.3. AD of UHS
2.4. Isolation of Extracted Materials from ADUHS
2.5. Isolation of Standard Lignin
2.6. Analytical Methods
2.6.1. Compositional Analysis of Solids Sample
2.6.2. Gel permeation Chromatography (GPC) Analysis
2.6.3. Thermogravimetric Analysis (TGA)
2.6.4. NMR Analysis
3. Results and Discussion
3.1. The Effect of AD on UHS Composition
3.2. Mass Yield from Solvent Extraction of ADUHS
3.3. Compositional Analysis of Extracts and Solid Residues
3.4. Molecular Weight Analysis
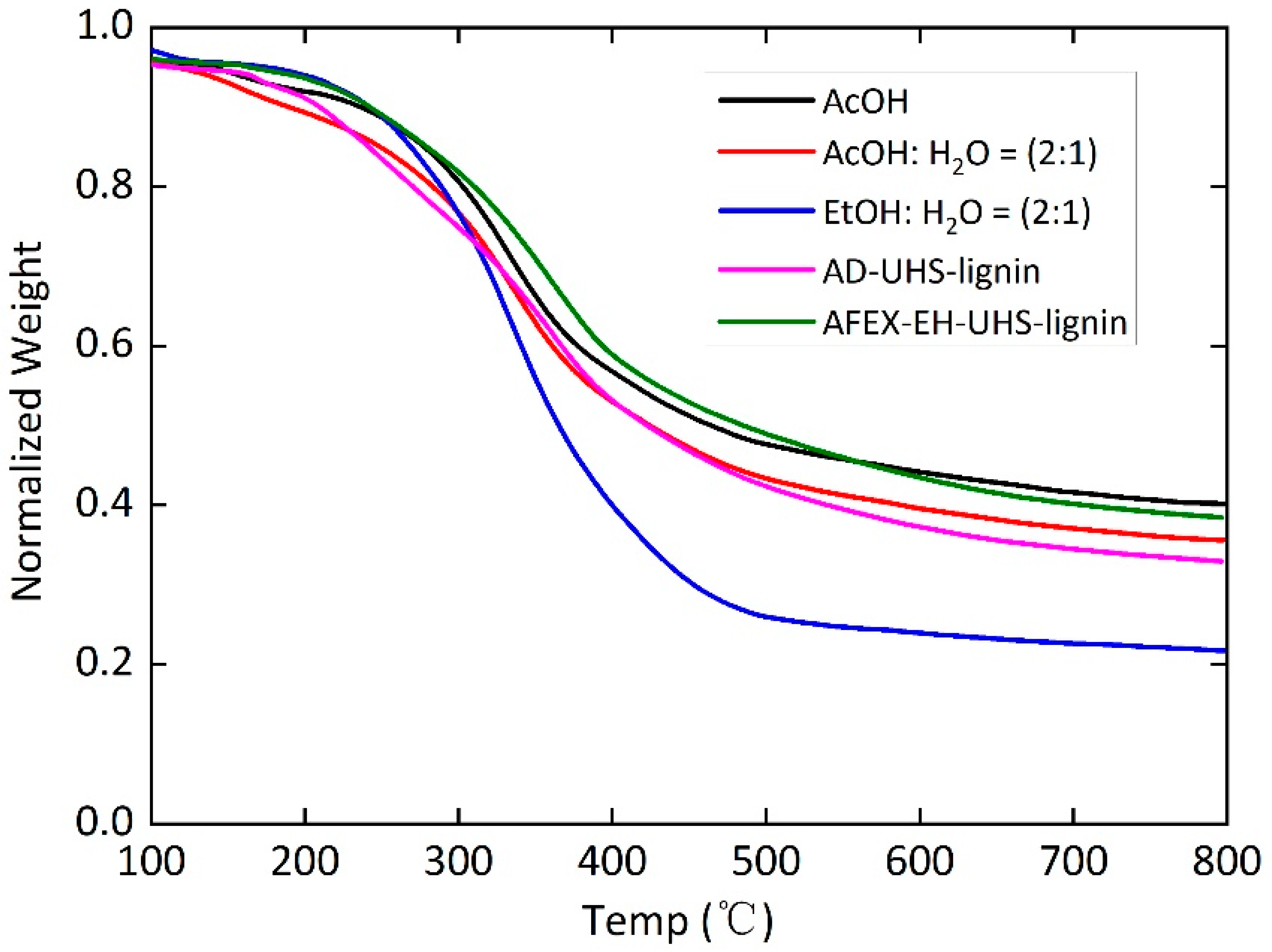
3.5. Thermogravimetric Analysis (TGA)
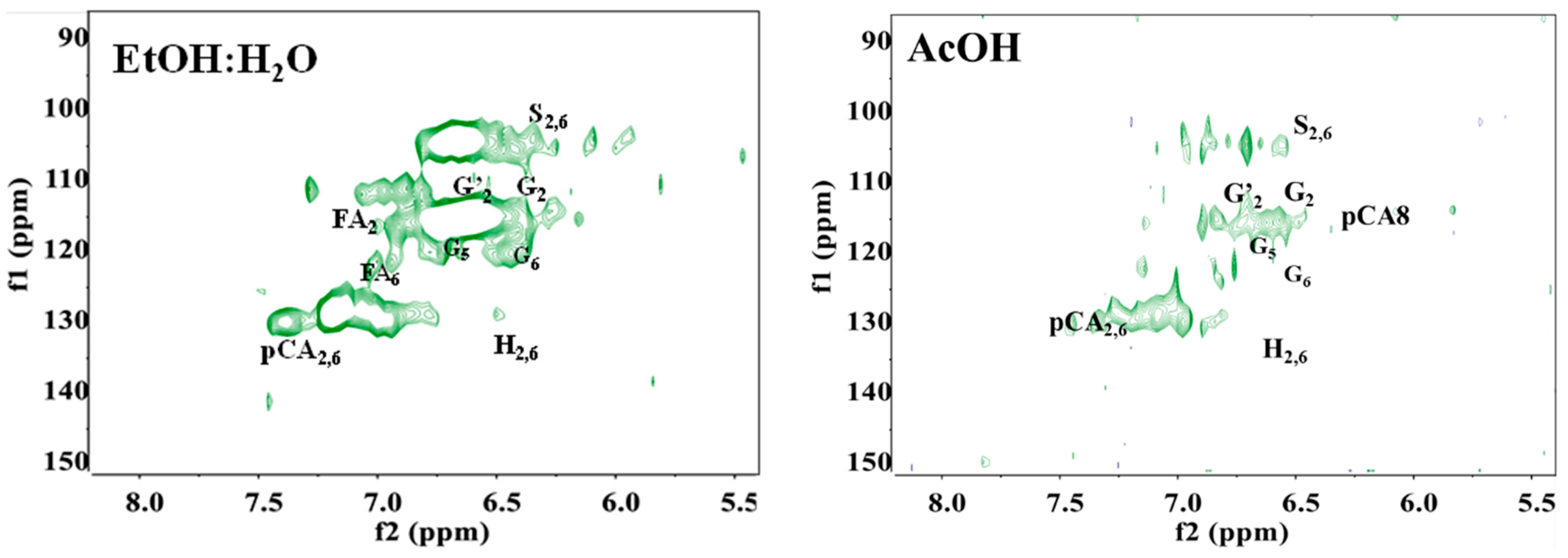
3.6. Nuclear Magnetic Resonance Analysis (NMR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Becker, J.; Wittmann, C. A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, J.; Erdocia, X.; Sánchez, C.; Alriols, M.G.; Labidi, J. Lignin depolymerization for phenolic monomers production by sustainable processes. J. Energy Chem. 2017, 26, 622–631. [Google Scholar] [CrossRef]
- Parsell, T.; Yohe, S.; Degenstein, J.; Jarrell, T.; Klein, I.; Gencer, E.; Hewetson, B.; Hurt, M.; Kim, J.I.; Choudhari, H.; et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem. 2015, 17, 1492–1499. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.; Lopes, A.; Lukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the way for lignin valorisation: Recent advances in bioengineering, biorefining and catalysis. Angew. Chem. 2016, 128, 8296–8354. [Google Scholar] [CrossRef]
- Bourbiaux, D.; Pu, J.; Rataboul, F.; Djakovitch, L.; Geantet, C.; Laurenti, D. Reductive or oxidative catalytic lignin depolymerization: An overview of recent advances. Catal. Today 2021, 373, 24–37. [Google Scholar] [CrossRef]
- Crestini, C.; Crucianelli, M.; Orlandi, M.; Saladino, R. Oxidative strategies in lignin chemistry: A new environmental friendly approach for the functionalisation of lignin and lignocellulosic fibers. Catal. Today 2010, 156, 8–22. [Google Scholar] [CrossRef]
- Lignin and Its Derivaties. Available online: http://polymerdatabase.com/polymer%20classes/Lignin%20type.html#:~:text=COMMERCIAL%20Lignin%20Products&text=The%20most%20important%20types%20of,Weyerhaeuser%20among%20several%20other%20companies (accessed on 2 August 2022).
- Da Costa Sousa, L.; Foston, M.; Bokade, V.; Azarpira, A.; Lu, F.; Ragauskas, A.J.; Ralph, J.; Dale, B.; Balan, V. Isolation and characterization of new lignin streams derived from extractive-ammonia (EA) pretreatment. Green Chem. 2016, 18, 4205–4215. [Google Scholar] [CrossRef]
- Da Costa Sousa, L.; Jin, M.; Chundawat, S.P.S.; Bokade, V.; Tang, X.; Azarpira, A.; Lu, F.; Avci, U.; Humpula, J.; Uppugundla, N.; et al. Next-generation ammonia pretreatment enhances cellulosic biofuel production. Energy Environ. Sci. 2016, 9, 1215–1223. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of second generation bioethanol production from residual biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7475–7485. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Holtman, K.M.; Chang, H.; Kadla, J.F. Solution-state nuclear magnetic resonance study of the similarities between milled wood lignin and cellulolytic enzyme lignin. J. Agric. Food Chem. 2004, 52, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Trajano, H.L.; Engle, N.L.; Foston, M.; Ragauskas, A.J.; Tschaplinski, T.J.; Wyman, C.E. The fate of lignin during hydrothermal pretreatment. Biotechnol. Biofuels 2013, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Van Aelst, K.; Van Sinay, E.; Vangeel, T.; Cooreman, E.; Van den Bossche, G.; Renders, T.; Van Aelst, J.; Van den Bosch, S.; Sels, B.F. Reductive catalytic fractionation of pine wood: Elucidating and quantifying the molecular structures in the lignin oil. Chem. Sci. 2020, 11, 11498–11508. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Y.; Yoo, C.G.; Meng, X.; Chen, X.; Ragauskas, A.J.; Yao, L. Physico-chemical properties of lignin fractions from acid pretreated corn stover and their effects on enzymatic hydrolysis of microcrystalline cellulose. BioResources 2020, 15, 4898–4911. [Google Scholar]
- Yadav, M.; Balan, V.; Varjani, S.; Tyagi, V.K.; Chaudhary, G.; Pareek, N.; Vivekanand, V. Multidisciplinary pretreatment approaches to improve the bio-methane production from lignocellulosic biomass. BioEnergy Res. 2022. [Google Scholar] [CrossRef]
- Chirania, P.; Holwerda, E.K.; Giannone, R.J.; Liang, X.; Poudel, S.; Ellis, J.C.; Bomble, Y.J.; Hettich, R.L.; Lynd, L.R. Metaproteomics reveals enzymatic strategies deployed by anaerobic microbiomes to maintain lignocellulose deconstruction at high solids. Nat. Commun. 2022, 13, 3870. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Kirk, T.K.; Farrell, R.L. Enzymatic “combustion”: The microbial degradation of lignin. Annu. Rev. Microbiol. 1987, 41, 465–505. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Cheng, G.; Sathitsuksanoh, N.; Wu, D.; Varanasi, P.; George, A.; Balan, V.; Gao, X.; Kumar, R.; Dale, B.E.; et al. Comparison of different biomass pretreatment techniques and their impact on chemistry and structure. Front. Energy Res. 2015, 2, 1–12. [Google Scholar] [CrossRef]
- Gunawan, C.; Xue, S.; Pattathil, S.; Da Costa Sousa, L.; Dale, B.E.; Balan, V. Comprehensive characterization of non-cellulosic recalcitrant cell wall carbohydrates in unhydrolyzed solids from AFEX-pretreated corn stover. Biotechnol. Biofuels 2017, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, X.; Xiao, Z.; Wang, W.; Yin, X.; Balan, V.; Wu, B.; Hu, Q. Biogas production of unhydrolyzed solid from corn stover hydrolysate by anaerobic digestion. J. Agro-Environ. Sci. 2016, 35, 584–589. [Google Scholar]
- Balan, V.; Bals, B.; Chundawat, S.P.S.; Marshall, D.; Dale, B.E. Lignocellulosic biomass pretreatment using AFEX. In Biofuels: Methods and Protocols; Mielenz, J.R., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 61–77. [Google Scholar]
- Uppugundla, N.; Da Costa Sousa, L.; Chundawat, S.P.; Yu, X.; Simmons, B.; Singh, S.; Gao, X.; Kumar, R.; Wyman, C.E.; Dale, B.E.; et al. A comparative study of ethanol production using dilute acid, ionic liquid and AFEX™ pretreated corn stover. Biotechnol. Biofuels 2014, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Gao, X.; Zhu, Q.; Tang, X. Effect of anaerobic digestion on lignin derived from unhydrolyzed solid of corn stover. J. Agro-Environ. Sci. 2020, 39, 2661–2667. [Google Scholar]
- Gao, X.; Tang, X.; Zhao, K.; Balan, V.; Zhu, Q. Biogas production from anaerobic co-digestion of spent mushroom substrate with different livestock manure. Energies 2021, 14, 570. [Google Scholar] [CrossRef]
- Meyer, J.R.; Waghmode, S.B.; He, J.; Gao, Y.; Hoole, D.; Da Costa Sousa, L.; Balan, V.; Foston, M.B. Isolation of lignin from ammonia fiber expansion (AFEX) pretreated biorefinery waste. Biomass Bioenergy 2018, 119, 446–455. [Google Scholar] [CrossRef]
- Guerra, A.; Mendonça, R.; Ferraz, A.; Lu, F.; Ralph, J. Structural characterization of lignin during Pinus taeda wood treatment with Ceriporiopsis subvermispora. Appl. Environ. Microbiol. 2004, 70, 4073–4078. [Google Scholar] [CrossRef]
- Holtman, K.M.; Chang, H.M.; Jameel, H.; Kadla, J.F. Quantitative 13C NMR characterization of milled wood lignins isolated by different milling techniques. J. Wood Chem. Technol. 2006, 26, 21–34. [Google Scholar] [CrossRef]
- Biomass Compositional Analysis Laboratory Procedures. Available online: https://www.nrel.gov/bioenergy/biomass-compositional-analysis.html (accessed on 17 July 2022).
- Zhu, T.; Zhu, Q.; Li, Q.; Tang, X.; Zhao, K.; Wu, B. Anaerobic degradation of recalcitrant carbohydrates in lignocellulose hydrolysis residues. China Biogas 2018, 36, 43–47. [Google Scholar]
- Khan, M.U.; Ahring, B.K. Lignin degradation under anaerobic digestion: Influence of lignin modifications—A review. Biomass Bioenergy 2019, 128, 105325. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.; Sun, R.; Fowler, P.; Baird, M.S. Comparative study of organosolv lignins from wheat straw. Ind. Crop. Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Valencia, C.; Ibarra, D.; Ballesteros, I.; Franco, J.M. Lignin-enriched residues from bioethanol production: Chemical characterization, isocyanate functionalization and oil structuring properties. Int. J. Biol. Macromol. 2022, 195, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Wang, H.H.; Zhang, G.C.; Fowler, P.; Rajaratnam, M. Extraction and characterization of lignins from maize stem and sugarcane bagasse. J. Appl. Polym. Sci. 2011, 120, 3587–3595. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, P.; Li, M.; Bian, J.; Peng, F. γ-valerolactone/water system for lignin fractionation to enhance antibacterial and antioxidant capacities. Sep. Purif. Technol. 2021, 279, 119780. [Google Scholar] [CrossRef]
- Macala, G.S.; Matson, T.D.; Johnson, C.L.; Lewis, R.S.; Iretskii, A.V.; Ford, P.C. Hydrogen transfer from supercritical methanol over a solid base catalyst: A model for lignin depolymerization. ChemSusChem 2009, 2, 215–217. [Google Scholar] [CrossRef]


| Sample | Composition (wt.%) | |||
|---|---|---|---|---|
| Glucan | Xylan | AIL | Ash | |
| Inoculum | 2.95 ± 0.04 | 2.13 ± 0.04 | 23.04 ± 0.43 | 31.16 ± 0.16 |
| AFEX-EH-UHS | 8.44 ± 0.08 | 4.18 ± 0.09 | 64.68 ± 0.44 | 4.44 ± 0.01 |
| AFEX-EH-UHS + INC | 4.46 ± 0.23 | 2.48 ± 0.15 | 34.79 ± 0.73 | 20.95 ± 0.10 |
| AD-UHS-day20 | 2.83 ± 0.02 | 1.79 ± 0.33 | 39.58 ± 1.55 | 22.27 ± 0.13 |
| Solvent | Refluxing Temp (°C) | Time (h) | Mass Yield (%) |
|---|---|---|---|
| AcOH | 105 | 24 | 35.20 |
| AcOH: H2O ratio (2:1) | 105 | 24 | 37.37 |
| EtOH: H2O ratio (2:1) | 85 | 24 | 21.57 |
| Solvent | Glucan (wt.%) | Xylan (wt.%) | AIL (wt.%) | Ash (wt.%) |
|---|---|---|---|---|
| AcOH | 0.76 ± 0.01 | 1.52 ± 0.02 | 94.59 ± 0.01 | 2.44 ± 0.30 |
| AcOH: H2O = (2:1) | 0.95 ± 0.15 | 0.90 ± 0.01 | 60.92 ± 0.55 | 5.43 ± 0.59 |
| EtOH: H2O = (2:1) | 0.79 ± 0.00 | 1.83 ± 0.01 | 79.41 ± 1.46 | 3.36 ± 0.20 |
| Glucan (wt.%) | Xylan (wt.%) | AIL (wt.%) | Ash (wt.%) | |
|---|---|---|---|---|
| AcOH | 2.26 ± 0.46 | 1.47 ± 0.31 | 54.88 ± 2.00 | 18.78 ± 0.14 |
| AcOH: H2O = (2:1) | 2.56 ± 0.07 | 1.75 ± 0.14 | 58.14 ± 0.59 | 19.87 ± 0.85 |
| EtOH: H2O = (2:1) | 3.25 ± 0.37 | 2.35 ± 0.26 | 34.03 ± 0.65 | 24.11 ± 0.50 |
| Unextracted AD-UHS | 2.83 ± 0.02 | 1.79 ± 0.33 | 39.58 ± 1.55 | 22.27 ± 0.13 |
| Mn (g/mol) | Mw (g/mol) | Mw/Mn | |
|---|---|---|---|
| AFEX-EH-UHS | 2315 | 6408 | 2.77 |
| AD-UHS | 2260 | 4429 | 1.95 |
| AcOH | 1860 | 2808 | 1.51 |
| AcOH: H2O = (2:1) | 1740 | 2679 | 1.54 |
| EtOH: H2O = (2:1) | 1900 | 2964 | 1.56 |
| Name | δC/δH | Assignments |
|---|---|---|
| pCA7 | 144.5/7.54 | Cα-Hα in p-coumarate (pCA) |
| pCA2,6 | 129.6/7.48 | C2,6-H2,6 in p-coumarate (pCA) |
| H2,6 | 127.8/7.25 | C2,6-H2,6 in 4-hydroxycinnamyl units (H) |
| FA6 | 123.1/7.17 | C6-H6 in ferulate (FA) |
| G’6 | 122.8/7.12 | C6-H6 in oxidized (C=O) guaiacyl units (G’) |
| G6 | 118.8/6.75 | C6-H6 in guaiacyl units (G) |
| G5 | 115.2/6.75 | C5-H5 in guaiacyl units (G) |
| pCA8 | 114.0/6.28 | Cβ-Hβ in p-coumarate (PCA) |
| FA2 | 110.8/6.25 | C2-H2 in ferulate (FA) |
| G2 | 110.1/6.92 | C2-H2 in guaiacyl units (G) |
| S′2,6 | 106.1/7.28 | C2,6-H2,6 in oxidized syringyl units(S′) |
| S2,6 | 103.7/6.69 | C2,6-H2,6 in syringyl units (S) |
| Cα | 87.6/5.50 | Cα-Hα in phenylcoumaran © |
| Aβ(S) | 85.8/4.10 | Cβ-Hβ in β-O-4 linked to S(A) |
| Aβ(G) | 83.4/4.42 | Cβ-Hβ in β-O-4 linked to G (A) |
| Aα | 72.0/4.85 | Cα-Hα in β-O-4 unit (A) |
| Bγ | 71.8/3.81 | Cγ-Hγ in β-β resinol (B) |
| A’γ | 63.4/4.16 | Cγ-Hγ in γ-acylated β-O-4(A′) |
| Aγ | 62.53/4.40 | Cγ-Hγ in β-O-4 substructures (A) |
| OCH3 | 55.9/3.72 | C-H in methoxyl group (OMe) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Zhao, K.; Gao, C.; Gao, X.; Balan, V.; Wang, W. Isolation of Lignin from Anaerobically Digested Unhydrolyzed Solids Produced in a Biorefinery. Agriculture 2022, 12, 1621. https://doi.org/10.3390/agriculture12101621
Tang X, Zhao K, Gao C, Gao X, Balan V, Wang W. Isolation of Lignin from Anaerobically Digested Unhydrolyzed Solids Produced in a Biorefinery. Agriculture. 2022; 12(10):1621. https://doi.org/10.3390/agriculture12101621
Chicago/Turabian StyleTang, Xiaoyu, Kunyang Zhao, Chunlin Gao, Xionghui Gao, Venkatesh Balan, and Wenguo Wang. 2022. "Isolation of Lignin from Anaerobically Digested Unhydrolyzed Solids Produced in a Biorefinery" Agriculture 12, no. 10: 1621. https://doi.org/10.3390/agriculture12101621
APA StyleTang, X., Zhao, K., Gao, C., Gao, X., Balan, V., & Wang, W. (2022). Isolation of Lignin from Anaerobically Digested Unhydrolyzed Solids Produced in a Biorefinery. Agriculture, 12(10), 1621. https://doi.org/10.3390/agriculture12101621






