Allelopathic Potential of Aqueous Extracts from Fleagrass (Adenosma buchneroides Bonati) against Two Crop and Three Weed Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Aqueous Extract
2.3. Widely Targeted Metabolomics Analysis
2.4. Seed Germination Bioassay
2.5. Seedling Growth Development
2.6. Data Measurement
2.7. Statistical Analysis
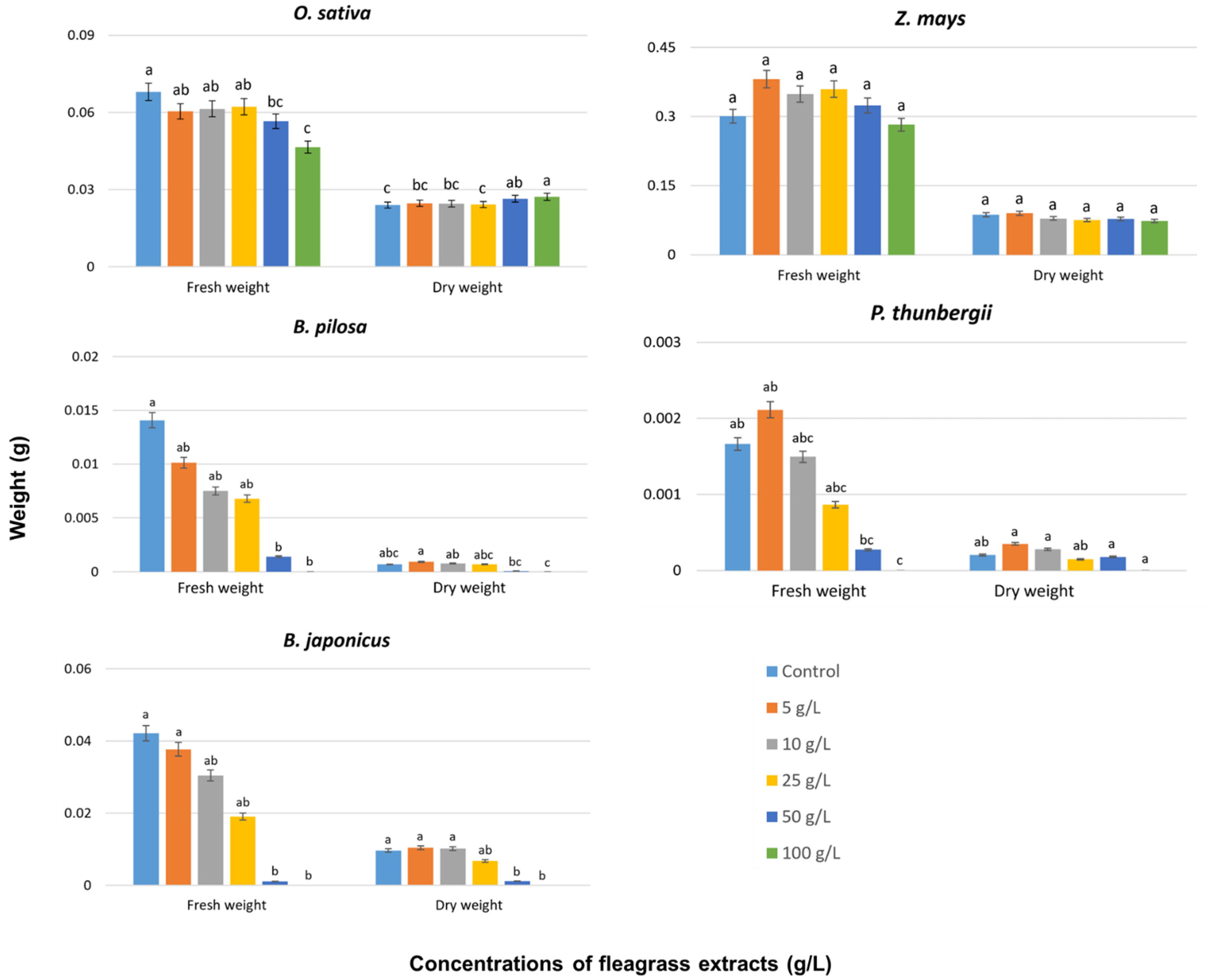
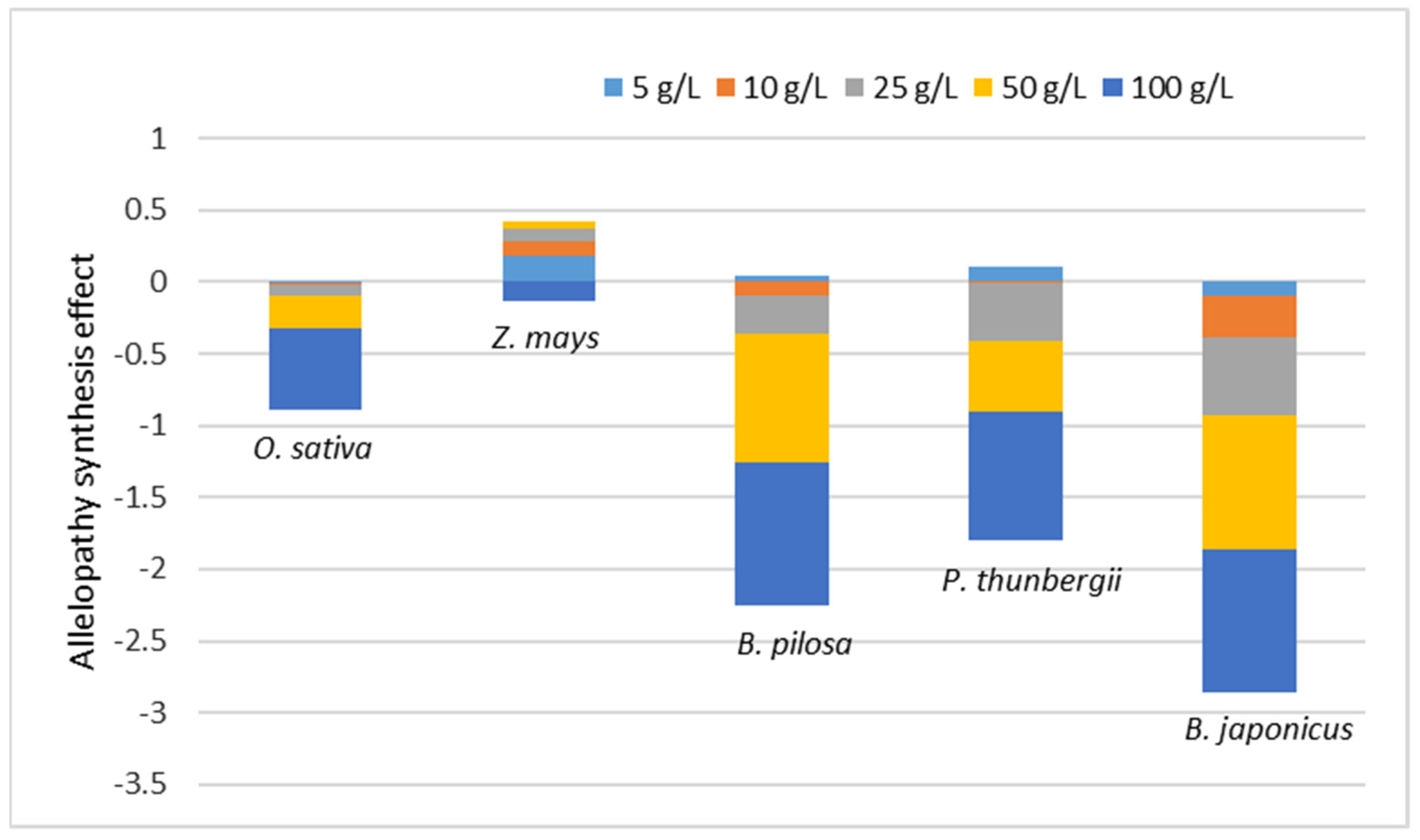
3. Results and Discussion
3.1. Qualitative Composition of the Aqueous Extract
3.2. Effects on Seed Germination
3.3. Effects on Seedling Growth
3.4. Allelopathy Synthesis Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Protect. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Kebaso, L.; Frimpong, D.; Iqbal, N.; Bajwa, A.A.; Namubiru, H.; Ali, H.H.; Ramiz, Z.; Hashim, S.; Manalil, S.; Chauhan, B.S. Biology, ecology and management of Raphanus raphanistrum L.: A noxious agricultural and environmental weed. Environ. Sci. Pollut. Res. 2020, 27, 17692–17705. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agr. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Abbas, T.; Zahir, Z.A.; Naveed, M.; Kremer, R.J. Chapter Five-Limitations of Existing Weed Control Practices Necessitate Development of Alternative Techniques Based on Biological Approaches. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: London, UK, 2018; Volume 147, pp. 239–280. [Google Scholar]
- Chauvel, B.; Guillemin, J.-P.; Gasquez, J.; Gauvrit, C. History of chemical weeding from 1944 to 2011 in France: Changes and evolution of herbicide molecules. Crop Protect. 2012, 42, 320–326. [Google Scholar] [CrossRef]
- Shad, R.A. Weeds and Weed Control; National Book Foundation: Islamabad, Pakistan, 2015. [Google Scholar]
- Carballido, J.; Rodriguez-Lizana, A.; Aguera, J.; Perez-Ruiz, M. Field sprayer for inter- and intra-row weed control: Performance and labor savings. Span. J. Agric. Res. 2013, 11, 642–651. [Google Scholar] [CrossRef]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed Management in 2050: Perspectives on the Future of Weed Science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef]
- Pan, L.; Guo, Q.S.; Wang, J.Z.; Shi, L.; Yang, X.; Zhou, Y.Y.; Yu, Q.; Bai, L.Y. CYP81A68 confers metabolic resistance to ALS and ACCase-inhibiting herbicides and its epigenetic regulation in Echinochloa crus-galli. J. Hazardous Mater. 2022, 428, 128225. [Google Scholar] [CrossRef]
- Schulz, R.; Bub, S.; Petschick, L.L.; Stehle, S.; Wolfram, J. Applied pesticide toxicity shifts toward plants and invertebrates, even in GM crops. Science 2021, 372, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Anaya, A.L. Allelopathy as a tool in the management of biotic resources in agroecosystems. Crit. Rev. Plant Sci. 1999, 18, 697–739. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Swanton, C.J.; Weise, S.F. Integrated Weed Management: The Rationale and Approach. Weed Technol. 1991, 5, 657–663. [Google Scholar] [CrossRef]
- Knörzer, H.; Graeff-Hönninger, S.; Guo, B.; Wang, P.; Claupein, W. The Rediscovery of Intercropping in China: A Traditional Cropping System for Future Chinese Agriculture–A Review. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms: Climate Change, Intercropping, Pest Control and Beneficial Microorganisms; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 13–44. [Google Scholar] [CrossRef]
- Farooq, M.; Bajwa, A.A.; Cheema, S.A.; Cheema, Z.A. Application of Allelopathy in Crop Production. Int. J. Agric. Biol. 2013, 15, 1367–1378. [Google Scholar]
- Farooq, N.; Iqbal, M.; Zahir, Z.A.; Farooq, M. Integration of Allelopathic Crop Residues and Npk Fertilizer to Mitigate Residue-Phytotoxicity, Improve Soil Fertility and Wheat Growth Under Different Moisture Conditions. Planta Daninha 2018, 36, 102. [Google Scholar] [CrossRef]
- Mattner, S.W. The Impact of Pathogens on Plant Interference and Allelopathy; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Li, Z.R.; Amist, N.; Bai, L.Y. Allelopathy in sustainable weeds management. Allelopathy J. 2019, 48, 109–138. [Google Scholar] [CrossRef]
- Macías, F.A.; Durán, A.G.; Molinillo, J.M.G. Allelopathy: The Chemical Language of Plants. In Progress in the Chemistry of Organic Natural Products 112; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J.I., Asakawa, Y., Liu, J.-K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–84. [Google Scholar] [CrossRef]
- Shen, P.Q.; Sun, H.D.; Pei, S.J. Ethnobotany of fleagrass (Adenosma buchneroides bonati), a traditional cultivated plant of the Hani people, Xishuangbanna, Yunnan, China. In Proceedings of the First International Congress of Ethnobiology, Belém, Brazil, 19–22 July 1988; pp. 305–309. [Google Scholar]
- Gou, Y.; Fan, R.; Pei, S.; Wang, Y. Before it disappeared: Ethnobotanical study of fleagrass (Adenosma buchneroides), a traditional aromatic plant used by the Akha people. J. Ethnobiol. Ethnomed. 2018, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, M.; Zhang, H.; Sun, H.; Su, H.; Wang, Y.; Du, Z. Bioassay-guided isolation of active compounds from Adenosma buchneroides essential oil as mosquito repellent against Aedes albopictus. J. Ethnopharmacol. 2019, 231, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Shuang, C.; Zhu, X. Invasion mechanisms, control and utilization of alien plant species Bidens pilosa. Pratacult. Sci. 2019, 36, 47–60. [Google Scholar]
- Li, Q.; Tan, J.N.; Li, W.; Yuan, G.H.; Du, L.; Ma, S.; Wang, J.X. Effects of Environmental Factors on Seed Germination and Emergence of Japanese Brome (Bromus japonicus). Weed Sci. 2015, 63, 641–646. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.L.; Wang, W.S.; Zhang, H.Y.; Liu, X.Q.; Yu, S.B.; Xiong, L.Z.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Yang, M.; Yang, J.; Su, L.; Sun, K.; Li, D.X.; Liu, Y.Z.; Wang, H.; Chen, Z.Q.; Guo, T. Metabolic profile analysis and identification of key metabolites during rice seed germination under low-temperature stress. Plant Sci. 2019, 289, 110282. [Google Scholar] [CrossRef]
- Bruce Williamson, G.; Richardson, D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, M.D.; Pendery, B.M. Germination Salt Resistance of Alfalfa (Medicago-Sativa L.) Germplasm in Relation to Subspecies and Centers of Diversity. Plant Soil 1990, 124, 47–51. [Google Scholar] [CrossRef]
- Bao, S.; Miao, Y.; Deng, S.; Xu, Y. Allelopathic effects of alfalfa (Medicago sativa) in the seedling stage on seedgermination and growth of Elymus nutans in different area. Acta Ecol. Sinica 2019, 39, 1475–1483. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 July 2022).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Ma, Y.; Zhang, H.; Sun, G.; Du, Z. Chemical Constituents from Adenosma buchneroides. Nat. Prod. Res. Dev. 2018, 30, 1376–1381, 1398. [Google Scholar]
- Ming, Y.; Zhu, Z.-J.; Li, J.; Hu, G.-X.; Fan, X.-M.; Yuan, D.-Y. Allelopathic Effects of Castanea henryi Aqueous Extracts on the Growth and Physiology of Brassica pekinensis and Zea mays. Chem. Biodivers. 2020, 17, e2000135. [Google Scholar] [CrossRef]
- Naghmouchi, S.; Alsubeie, M. Biochemical profile, antioxidant capacity and allelopathic effects from five Ziziphyus spina-christi (L.) provenances growing wild in Saudi Arabia. Not. Bot. Hort. Agrobot. Cluj-Napoca 2020, 48, 1600–1612. [Google Scholar] [CrossRef]
- Gomaa, N.H.; Hassan, M.O.; Fahmy, G.M.; Gonzalez, L.; Hammouda, O.; Atteya, M.A. Allelopathic effects of Sonchus oleraceus L. on the germination and seedling growth of crop and weed species. Acta Bot. Brasil. 2014, 28, 408–416. [Google Scholar] [CrossRef]
- Synowiec, A.; Kalemba, D.; Drozdek, E.; Bocianowski, J. Phytotoxic potential of essential oils from temperate climate plants against the germination of selected weeds and crops. J. Pest Sci. 2017, 90, 407–419. [Google Scholar] [CrossRef]
- Pula, J.; Barabasz-Krasny, B.; Mozdzen, K.; Soltys-Lelek, A.; Lepiarczyk, A. Effect of Aqueous Extracts of Sticky Willy (Galium aparine L.) on the Growth of Seedlings of Selected Maize Varieties (Zea mays L.). Not. Bot. Hort. Agrobot. Cluj-Napoca 2016, 44, 518–524. [Google Scholar] [CrossRef][Green Version]
- Khan, M.; Hussain, F.; Musharaf, S.; Imdadullah. Allelopathic effects of Rhazya stricta decne on seed germination and seedling growth of maize. Afr. J. Agric. Res. 2011, 6, 6391–6396. [Google Scholar] [CrossRef]
- Findura, P.; Kocira, S.; Hara, P.; Pawlowska, A.; Szparaga, A.; Kangalov, P. Extracts from Artemisia vulgaris L. in Potato Cultivation-Preliminary Research on Biostimulating Effect. Agriculture 2020, 10, 356. [Google Scholar] [CrossRef]
- Pannacci, E.; Masi, M.; Farneselli, M.; Tei, F. Evaluation of Mugwort (Artemisia vulgaris L.) Aqueous Extract as a Potential Bioherbicide to Control Amaranthus retroflexus L. in Maize. Agriculture 2020, 10, 642. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathy in Agroecosystems. J. Crop Product. 2001, 4, 1–41. [Google Scholar] [CrossRef]
- Belz, R.G. Allelopathy in crop/weed interactions-an update. Pest Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Alsaadawi, I.S.; Baerson, S.R. Sorghum Allelopathy-From Ecosystem to Molecule. J. Chem. Ecol. 2013, 39, 142–153. [Google Scholar] [CrossRef]
- Weston, L.A.; Ryan, P.R.; Watt, M. Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J. Exp. Bot. 2012, 63, 3445–3454. [Google Scholar] [CrossRef]
- Sheheryar; Khan, E.A.; Hussain, I. Integrated Control of Jungle Rice in Hybrid Maize through Sorghum Allelopathy. Pak. J. Bot. 2019, 51, 499–503. [Google Scholar] [CrossRef]
- Hussain, M.I.; Danish, S.; Sanchez-Moreiras, A.M.; Vicente, O.; Jabran, K.; Chaudhry, U.K.; Branca, F.; Reigosa, M.J. Unraveling Sorghum Allelopathy in Agriculture: Concepts and Implications. Plants 2021, 10, 1795. [Google Scholar] [CrossRef]
- Haramoto, E.R.; Gallandt, E.R. Brassica cover cropping: II. Effects on growth and interference of green bean (Phaseolus vulgaris) and redroot pigweed (Amaranthus retroflexus). Weed Sci. 2005, 53, 702–708. [Google Scholar] [CrossRef]
- Haramoto, E.R.; Gallandt, E.R. Brassica cover cropping: I. Effects on weed and crop establishment. Weed Sci. 2005, 53, 695–701. [Google Scholar] [CrossRef]
- Pittman, K.B.; Cahoon, C.W.; Bamber, K.W.; Rector, L.S.; Flessner, M.L. Herbicide selection to terminate grass, legume, and brassica cover crop species. Weed Technol. 2020, 34, 48–54. [Google Scholar] [CrossRef]
- Ahn, J.K.; Hahn, S.J.; Kim, J.T.; Khanh, T.D.; Chung, I.M. Evaluation of allelopathic potential among rice (Oryza sativa L.) germplasm for control of Echinochloa crusgalli P. Beauv in the field. Crop Protect. 2005, 24, 413–419. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.T.; Kim, S.H. Evaluation of allelopathic potential and quantification of momilactone A,B from rice hull extracts and assessment of inhibitory bioactivity on paddy field weeds. J. Agric. Food Chem. 2006, 54, 2527–2536. [Google Scholar] [CrossRef]
- Berendji, S.; Asghari, J.B.; Matin, A.A. Allelopathic potential of rice (Oryza sativa) varieties on seedling growth of barnyardgrass (Echinochloa crusgalli). J. Plant Interact. 2008, 3, 175–180. [Google Scholar] [CrossRef]
- Thi, H.L.; Lin, C.H.; Smeda, R.J.; Leigh, N.D.; Wycoff, W.G.; Fritschi, F.B. Isolation and identification of an allelopathic phenylethylamine in rice. Phytochemistry 2014, 108, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Alsaadawi, I.S.; Sarbout, A.K.; Al-Shamma, L.M. Differential allelopathic potential of sunflower (Helianthus annuus L.) genotypes on weeds and wheat (Triticum aestivum L.) crop. Arch Agron. Soil Sci. 2012, 58, 1139–1148. [Google Scholar] [CrossRef]
- Barney, J.N.; Hay, A.G.; Weston, L.A. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Amosse, C.; Jeuffroy, M.H.; Celette, F.; David, C. Relay-intercropped forage legumes help to control weeds in organic grain production. Eur. J. Agron. 2013, 49, 158–167. [Google Scholar] [CrossRef]

| Target Plant | Index | Concentration of Aqueous Extracts (g/L) | IC50 | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 5 | 10 | 25 | 50 | 100 | |||
| O. sativa | GP | 100 ± 0 a | 99 ± 1 a | 100 ± 0 a | 99 ± 1 a | 98 ± 1.225 a | 25 ± 6.325 b | 82.298 |
| RI | −0.01 | 0 | −0.01 | −0.0101 | −0.75 | |||
| Z. mays | GP | 93.33 ± 4.41 a | 96.67 ± 1.67 a | 93.33 ± 3.33 a | 81.67 ± 3.33 b | 80 ± 5.77 c | 68.33 ± 1.67 d | 255.240 |
| RI | 0.03445 | −0.03575 | −0.125 | −0.1724 | −0.1964 | |||
| B. pilosa | GP | 80 ± 6.45 ab | 91.25 ± 4.27 a | 77.5 ± 4.33 b | 68.75 ± 6.57 b | 2.5 ± 1.44 c | 0 c | 21.638 |
| RI | 0.1233 | −0.0313 | −0.1406 | −0.9726 | −1 | |||
| P. thunbergii | GP | 57.5 ± 4.33 a | 53.33 ± 6.67 a | 50 ± 9.35 a | 31.25 ± 7.47 b | 7.5 ± 4.33 c | 2.5 ± 2.5 c | 7.794 |
| RI | −0.0725 | −0.1304 | −0.4565 | −0.8594 | −0.95 | |||
| B. japonicus | GP | 45 ± 6.45 a | 47.5 ± 11.09 a | 27.5 ± 10.31 ab | 15 ± 9.57 bc | 2.5 ± 2.5 c | 0 c | 4.930 |
| RI | 0.0526 | −0.3889 | −0.6667 | −0.9474 | −1 | |||
| Target Plant | Index | Concentration of Aqueous Extracts (g/L) | |||||
|---|---|---|---|---|---|---|---|
| Control | 5 | 10 | 25 | 50 | 100 | ||
| O. sativa | GI | 17.78 ± 0.31 a | 17.34 ± 0.36 a | 17.54 ± 0.26 a | 16.21 ± 0.28 b | 9.66 ± 0.47 c | 0.95 ± 0.3 d |
| RI | −0.0247 | −0.0135 | −0.0883 | −0.4429 | −0.9458 | ||
| Z. mays | GI | 19.22 ± 1.74 ab | 20.84 ± 1.34 ab | 15.38 ± 1.29 bc | 14.32 ± 1.07 c | 13.26 ± 1.72 c | 8.33 ± 1.81 d |
| RI | 0.0777 | −0.1998 | −0.2549 | −0.3637 | −0.4584 | ||
| B. pilosa | GI | 13.2 ± 1.47 a | 13.51 ± 0.26 a | 9.76 ± 0.89 b | 3.83 ± 0.43 c | 0.05 ± 0.03 d | 0d |
| RI | 0.0229 | −0.2606 | −0.7099 | −0.9963 | −1 | ||
| P. thunbergii | GI | 2.67 ± 0.08 a | 2.3 ± 0.36 a | 2.38 ± 0.67 a | 0.89 ± 0.21 b | 0.24 ± 0.16 b | 0.05 ± 0.06 b |
| RI | −0.1386 | −0.1086 | −0.6667 | −0.8957 | −0.979 | ||
| B. japonicus | GI | 8.32 ± 1.47 a | 8.26 ± 0.26 a | 3.2 ± 0.89 b | 1.58 ± 0.43 b | 0.05 ± 0.03 b | 0 b |
| RI | −0.0072 | −0.6154 | −0.8101 | −0.9939 | −1 | ||
| Target Plants | RI | Concentration of Aqueous Extracts (g/L) | ||||
|---|---|---|---|---|---|---|
| 5 | 10 | 25 | 50 | 100 | ||
| O. sativa | SH | −0.0331 | −0.0125 | −0.0246 | −0.3334 | −0.6169 |
| RL | 0.079 | 0.0297 | −0.2641 | −0.5024 | −0.8598 | |
| FW | −0.1117 | −0.0972 | −0.085 | −0.1676 | −0.3161 | |
| DW | 0.0291 | 0.0224 | 0.0091 | 0.0937 | 0.119 | |
| Z. mays | SH | 0.2748 | 0.3992 | 0.3738 | 0.3405 | −0.1904 |
| RL | 0.4791 | 0.3971 | 0.5134 | 0.4753 | 0.2864 | |
| FW | 0.211 | 0.1378 | 0.163 | 0.0714 | −0.0615 | |
| DW | 0.0347 | −0.0925 | −0.1327 | −0.1026 | −0.1545 | |
| B. pilosa | SH | −0.053 | −0.0728 | −0.2139 | −1 | −1 |
| RL | 0.1817 | 0.1625 | −0.03 | −0.65 | −1 | |
| FW | −0.2811 | −0.4677 | −0.5188 | −0.8999 | −1 | |
| DW | 0.2581 | 0.1154 | 0.0143 | −0.8841 | −1 | |
| P. thunbergii | SH | 0.0245 | −0.0241 | −0.3935 | −0.5474 | −0.7694 |
| RL | 0.2213 | 0.0614 | −0.5275 | −0.9013 | −0.9065 | |
| FW | 0.2131 | −0.1017 | −0.1356 | 0.3867 | −1 | |
| DW | 0.4167 | 0.2671 | −0.2709 | −0.1133 | −0.7734 | |
| B. japonicus | SH | −0.2389 | −0.2596 | −0.4486 | −0.9193 | −1 |
| RL | −0.3203 | −0.3244 | −0.4245 | −0.8916 | −1 | |
| FW | −0.1043 | −0.2771 | −0.5474 | −0.9739 | −1 | |
| DW | 0.0738 | 0.0529 | −0.3002 | −0.8768 | −1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Qi, J.; Liu, Q.; Wang, Y.; Wang, H. Allelopathic Potential of Aqueous Extracts from Fleagrass (Adenosma buchneroides Bonati) against Two Crop and Three Weed Species. Agriculture 2022, 12, 1103. https://doi.org/10.3390/agriculture12081103
Wang C, Qi J, Liu Q, Wang Y, Wang H. Allelopathic Potential of Aqueous Extracts from Fleagrass (Adenosma buchneroides Bonati) against Two Crop and Three Weed Species. Agriculture. 2022; 12(8):1103. https://doi.org/10.3390/agriculture12081103
Chicago/Turabian StyleWang, Chen, Jinfeng Qi, Qing Liu, Yuhua Wang, and Hongbin Wang. 2022. "Allelopathic Potential of Aqueous Extracts from Fleagrass (Adenosma buchneroides Bonati) against Two Crop and Three Weed Species" Agriculture 12, no. 8: 1103. https://doi.org/10.3390/agriculture12081103
APA StyleWang, C., Qi, J., Liu, Q., Wang, Y., & Wang, H. (2022). Allelopathic Potential of Aqueous Extracts from Fleagrass (Adenosma buchneroides Bonati) against Two Crop and Three Weed Species. Agriculture, 12(8), 1103. https://doi.org/10.3390/agriculture12081103






