Nitrogen and Phosphorus Concentrations and Their Ratios as Indicators of Water Quality and Eutrophication of the Hydro-System Danube–Tisza–Danube
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection and Analysis
2.3. Criteria for Water Quality Assessment
2.4. Statistical Data Analyses
3. Results and Discussion
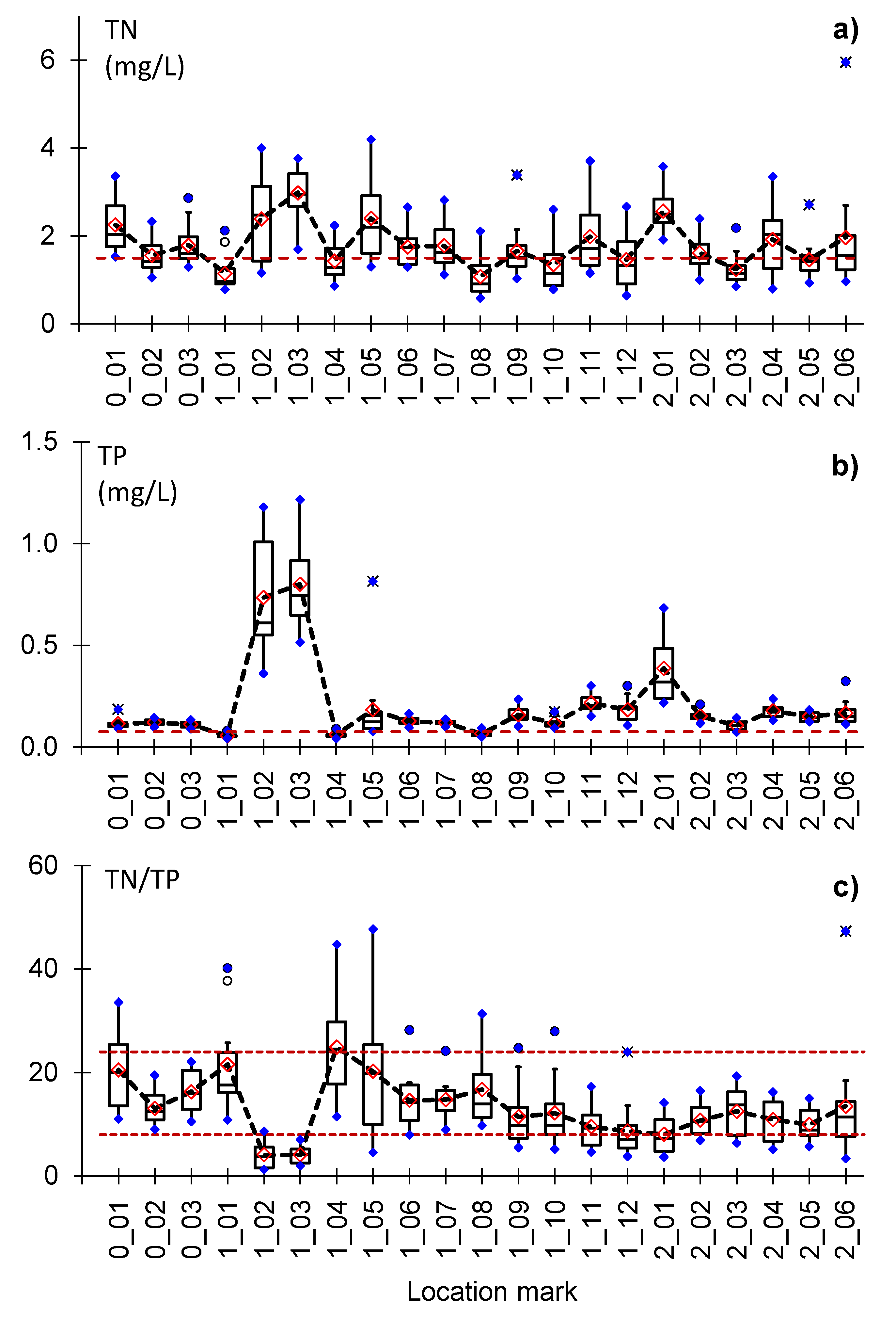
3.1. Nutrient Status in the Canal Network
3.2. Spatial Distribution of TN and TP
3.3. Seasonal Distribution of TN and TP
3.4. TN:TP Ratio
3.5. Correlation Analysis
3.6. Cluster Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hui, X.C.; Jie, L.; Xiuyun, H.; Chunlei, C.; Zhou, S.Y. Sediment-Water Interaction in Phosphorus Cycling as Affected by Trophic States in a Chinese Shallow Lake (Lake Donghu). Hydrobiologia 2016, 776, 19–33. [Google Scholar] [CrossRef]
- Glińska-Lewczuk, K.; Gołaś, I.; Koc, J.; Gotkowska-Płachta, A.; Harnisz, M.; Rochwerger, A. The Impact of Urban Areas on the Water Quality Gradient along a Lowland River. Environ. Monit. Assess. 2016, 188, 624. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Tyagi, S. Influences of Natural and Anthropogenic Factors on Surface and Groundwater Quality in Rural and Urban Areas. Front. Life Sci. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Mentzafou, A.; Panagopoulos, Y.; Dimitriou, E. Designing the National Network for Automatic Monitoring of Water Quality Parameters in Greece. Water 2019, 11, 1310. [Google Scholar] [CrossRef] [Green Version]
- Spänhoff, B.; Dimmer, R.; Friese, H.; Harnapp, S.; Herbst, F.; Jenemann, K.; Mickel, A.; Rohde, S.; Schönherr, M.; Ziegler, K.; et al. Ecological Status of Rivers and Streams in Saxony (Germany) According to the Water Framework Directive and Prospects of Improvement. Water 2012, 4, 887. [Google Scholar] [CrossRef]
- Ren, L.; Cui, E.; Sun, H. Temporal and Spatial Variations in the Relationship between Urbanization and Water Quality. Environ. Sci. Pollut. Res. 2014, 21, 13646–13655. [Google Scholar] [CrossRef]
- Savic, R.; Ondrasek, G.; Blagojevic, B.; Bubalo Kovacic, M.; Zemunac, R. Spatial Distribution and Temporal Variation of Chemical Properties of Drainage Watercourses in Rural and Peri-Urban Areas of Novi Sad (Serbia)—A Case Study. Environ. Monit. Assess. 2018, 190, 53. [Google Scholar] [CrossRef]
- Su, S.; Li, D.; Zhang, Q.; Xiao, R.; Huang, F.; Wu, J. Temporal Trend and Source Apportionment of Water Pollution in Different Functional Zones of Qiantang River, China. Water Res. 2011, 45, 1781–1795. [Google Scholar] [CrossRef]
- Yu, S.; Yu, G.B.; Liu, Y.; Li, G.L.; Feng, S.; Wu, S.C.; Wong, M.H. Urbanization Impairs Surface Water Quality: Eutrophication and Metal Stress in the Grand Canal of China. River Res. Appl. 2012, 28, 1135–1148. [Google Scholar] [CrossRef]
- Barakat, A.; El Baghdadi, M.; Rais, J.; Aghezzaf, B.; Slassi, M. Assessment of Spatial and Seasonal Water Quality Variation of Oum Er Rbia River (Morocco) Using Multivariate Statistical Techniques. Int. Soil Water Conserv. Res. 2016, 4, 284–292. [Google Scholar] [CrossRef]
- Bhateria, R.; Jain, D. Water Quality Assessment of Lake Water: A Review. Sustain. Water Resour. Manag. 2016, 2, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.A.; Johnson, M.V.V.; Morton, S.L.; Perkins, D.A.K.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are Harmful Algal Blooms Becoming the Greatest Inland Water Quality Threat to Public Health and Aquatic Ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef]
- Krčmar, D.; Dubovina, M.; Grba, N.; Pešić, V.; Watson, M.; Tričković, J.; Dalmacija, B. Distribution of Organic and Inorganic Substances in the Sediments of the “Great Bačka Canal”, a European Environmental Hotspot. Sci. Total Environ. 2017, 601–602, 833–844. [Google Scholar] [CrossRef]
- Savić, R.; Radović, J.R.; Ondrašek, G.; Pantelić, S. Occurrence and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) in Drainage Channel Sediments in Vojvodina (Serbia). Environ. Eng. Manag. J. 2016, 15, 287–295. [Google Scholar] [CrossRef]
- Ustaoğlu, F.; Tepe, Y. Water Quality and Sediment Contamination Assessment of Pazarsuyu Stream, Turkey Using Multivariate Statistical Methods and Pollution Indicators. Int. Soil Water Conserv. Res. 2019, 7, 47–56. [Google Scholar] [CrossRef]
- Chen, D.; Lu, J.; Wang, H.; Shen, Y.; Kimberley, M.O. Seasonal Variations of Nitrogen and Phosphorus Retention in an Agricultural Drainage River in East China. Environ. Sci. Pollut. Res. 2010, 17, 312–320. [Google Scholar] [CrossRef]
- Newcomer Johnson, T.; Kaushal, S.; Mayer, P.; Smith, R.; Sivirichi, G. Nutrient Retention in Restored Streams and Rivers: A Global Review and Synthesis. Water 2016, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Scott, J.T.; McCarthy, M.J.; Newell, S.E.; Gardner, W.S.; Havens, K.E.; Hoffman, D.K.; Wilhelm, S.W.; Wurtsbaugh, W.A. It Takes Two to Tango: When and Where Dual Nutrient (N & P) Reductions Are Needed to Protect Lakes and Downstream Ecosystems. Environ. Sci. Technol. 2016, 50, 10805–10813. [Google Scholar] [CrossRef] [Green Version]
- Salas, E.A.L.; Subburayalu, S.K. Analysis of Phosphorus and Nitrogen Concentrations in the Great Miami and Little Miami Basins in Ohio, USA from 2015 to 2017. River Res. Appl. 2020, 36, 1345–1352. [Google Scholar] [CrossRef]
- Tekile, A.; Kim, I.; Kim, J. Mini-Review on River Eutrophication and Bottom Improvement Techniques, with Special Emphasis on the Nakdong River. J. Environ. Sci. 2015, 30, 113–121. [Google Scholar] [CrossRef]
- Niemistö, J.; Holmroos, H.; Pekcan-Hekim, Z.; Horppila, J. Interactions between Sediment Resuspension and Sediment Quality Decrease the TN: TP Ratio in a Shallow Lake. Limnol. Oceanogr. 2008, 53, 2407–2415. [Google Scholar] [CrossRef] [Green Version]
- Varol, M. Temporal and Spatial Dynamics of Nitrogen and Phosphorus in Surface Water and Sediments of a Transboundary River Located in the Semi-Arid Region of Turkey. CATENA 2013, 100, 1–9. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint Pollution of Surface Waters with Phosphorus and Nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Casquin, A.; Gu, S.; Dupas, R.; Petitjean, P.; Gruau, G.; Durand, P. River Network Alteration of C–N–P Dynamics in a Mesoscale Agricultural Catchment. Sci. Total Environ. 2020, 749, 141551. [Google Scholar] [CrossRef]
- Csathó, P.; Sisák, I.; Radimszky, L.; Lushaj, S.; Spiegel, H.; Nikolova, M.T.; Nikolov, N.; Čermák, P.; Klir, J.; Astover, A.; et al. Agriculture as a Source of Phosphorus Causing Eutrophication in Central and Eastern Europe. Soil Use Manag. 2007, 23, 36–56. [Google Scholar] [CrossRef]
- Dupas, R.; Delmas, M.; Dorioz, J.M.; Garnier, J.; Moatar, F.; Gascuel-Odoux, C. Assessing the Impact of Agricultural Pressures on N and P Loads and Eutrophication Risk. Ecol. Indic. 2015, 48, 396–407. [Google Scholar] [CrossRef]
- Stefanidis, K.; Christopoulou, A.; Poulos, S.; Dassenakis, E.; Dimitriou, E. Nitrogen and Phosphorus Loads in Greek Rivers: Implications for Management in Compliance with the Water Framework Directive. Water 2020, 12, 1531. [Google Scholar] [CrossRef]
- Van Drecht, G.; Bouwman, A.F.; Harrison, J.; Knoop, J.M. Global Nitrogen and Phosphate in Urban Wastewater for the Period 1970 to 2050. Glob. Biogeochem. Cycles 2009, 23, GB0A03. [Google Scholar] [CrossRef] [Green Version]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, Eutrophication and Harmful Algal Blooms along the Freshwater to Marine Continuum. WIREs Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Dzialowski, A.R.; Wang, S.-H.; Lim, N.-C.; Spotts, W.W.; Huggins, D.G. Nutrient Limitation of Phytoplankton Growth in Central Plains Reservoirs, USA. J. Plankton Res. 2005, 27, 587–595. [Google Scholar] [CrossRef]
- Oppeltová, P.; Kasal, P.; Krátký, F.; Hajšlová, J. Analysis of Selected Water Quality Indicators from Runoff during Potato Cultivation after Natural Precipitation. Agriculture 2021, 11, 1220. [Google Scholar] [CrossRef]
- Smith, V.H.; Schindler, D.W. Eutrophication Science: Where Do We Go from Here? Trends Ecol. Evol. 2009, 24., 201–207. [Google Scholar] [CrossRef] [PubMed]
- Varol, M.; Şen, B. Assessment of Nutrient and Heavy Metal Contamination in Surface Water and Sediments of the Upper Tigris River, Turkey. CATENA 2012, 92, 1–10. [Google Scholar] [CrossRef]
- Yang, X.E.; Wu, X.; Hao, H.L.; He, Z.L. Mechanisms and Assessment of Water Eutrophication. J. Zhejiang Univ. Sci. B 2008, 9, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, X.; Liu, D.; Lang, C.; Shan, B. Temporal and Spatial Variation of Nitrogen and Phosphorus and Eutrophication Assessment for a Typical Arid River—Fuyang River in Northern China. J. Environ. Sci. 2017, 55, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Peng, B.; Hong, H.; Turyaheebwa, N.; Cui, S.; Mo, X. Nutrient Enrichment and N:P Ratio Decline in a Coastal Bay–River System in Southeast China: The Need for a Dual Nutrient (N and P) Management Strategy. Ocean Coast. Manag. 2013, 81, 7–13. [Google Scholar] [CrossRef]
- Savic, R.; Ondrasek, G.; Letic, L.; Nikolic, V.; Tanaskovik, V. Nutrients Accumulation in Drainage Channel Sediments. Int. J. Sediment Res. 2017, 32, 180–185. [Google Scholar] [CrossRef]
- Savic, R.; Ondrasek, G.; Zemunac, R.; Bubalo Kovacic, M.; Kranjcec, F.; Nikolic Jokanovic, V.; Bezdan, A. Longitudinal Distribution of Macronutrients in the Sediments of Jegricka Watercourse in Vojvodina, Serbia. Sci. Total Environ. 2021, 754, 142138. [Google Scholar] [CrossRef]
- Voutsa, D.; Manoli, E.; Samara, C.; Sofoniou, M.; Stratis, I. A Study of Surface Water Quality in Macedonia, Greece: Speciation of Nitrogen and Phosphorus. Water Air. Soil Pollut. 2001, 129, 13–32. [Google Scholar] [CrossRef]
- Bechmann, M.; Greipsland, I.; Øgaard, A.F. Implementation of Mitigation Measures to Reduce Phosphorus Losses: The Vestre Vansjø Pilot Catchment. Agriculture 2019, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Guignard, M.S.; Leitch, A.R.; Acquisti, C.; Eizaguirre, C.; Elser, J.J.; Hessen, D.O.; Jeyasingh, P.D.; Neiman, M.; Richardson, A.E.; Soltis, P.S.; et al. Impacts of Nitrogen and Phosphorus: From Genomes to Natural Ecosystems and Agriculture. Front. Ecol. Evol. 2017, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Ondrasek, G.; Bakić Begić, H.; Zovko, M.; Filipović, L.; Meriño-Gergichevich, C.; Savić, R.; Rengel, Z.; Ondrašek, G.; Bakić Begić, H.; Zovko, M.; et al. Biogeochemistry of Soil Organic Matter in Agroecosystems & Environmental Implications. Sci. Total Environ. 2019, 658, 1559–1573. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and Eutrophication: Where Do We Go from Here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef] [Green Version]
- OECD (Organisation for Economic Cooperation and Development). Environmental Performance of Agriculture in OECD Countries Since 1990; OECD: Paris, France, 2008; Volume 9789264040854. [Google Scholar]
- Ondrasek, G.; Bakić Begić, H.; Romić, D.; Brkić, Ž.; Husnjak, S.; Bubalo Kovačić, M. A Novel LUMNAqSoP Approach for Prioritising Groundwater Monitoring Stations for Implementation of the Nitrates Directive. Environ. Sci. Eur. 2021, 33, 23. [Google Scholar] [CrossRef]
- Parris, K. Impact of Agriculture on Water Pollution in OECD Countries: Recent Trends and Future Prospects. Int. J. Water Resour. Dev. 2011, 27, 33–52. [Google Scholar] [CrossRef] [Green Version]
- Gramlich, A.; Stoll, S.; Stamm, C.; Walter, T.; Prasuhn, V. Effects of Artificial Land Drainage on Hydrology, Nutrient and Pesticide Fluxes from Agricultural Fields—A Review. Agric. Ecosyst. Environ. 2018, 266, 84–99. [Google Scholar] [CrossRef]
- Olaoye, I.A.; Confesor, R.B.; Ortiz, J.D. Impact of Agricultural Practices on Water Quality of Old Woman Creek Watershed, Ohio. Agriculture 2021, 11, 426. [Google Scholar] [CrossRef]
- Downing, J.A.; Mccauley, E. The Nitrogen: Phosphorus Relationship in Lakes. Limnol. Oceanogr. 1992, 37, 936–945. [Google Scholar] [CrossRef] [Green Version]
- Jarvie, H.P.; Whitton, B.A.; Neal, C. Nitrogen and Phosphorus in East Coast British Rivers: Speciation, Sources and Biological Significance. Sci. Total Environ. 1998, 210–211, 79–109. [Google Scholar] [CrossRef]
- Stelzer, R.S.; Lamberti, G.A. Effects of N:P Ratio and Total Nutrient Concentration on Stream Periphyton Community Structure, Biomass, and Elemental Composition. Limnol. Oceanogr. 2001, 46, 356–367. [Google Scholar] [CrossRef]
- Fujimotol, N.; Sudo, R.; Sugiura, N.; Inamori, Y. Nutrient-Limited Growth of Microcystis Aeruginosa and Phomzidium Tenue and Competition under Various N:P Supply Ratios and Temperatures. Limnol. Oceanogr. 1997, 42, 250–256. [Google Scholar] [CrossRef]
- Kilic Taseli, B. Water Quality Changes in Uzungol Lake Due to Influent Tributaries and Land-Based Fish-Farms. Carpathian J. Earth Environ. Sci. 2013, 8, 125–136. [Google Scholar]
- Seip, K.L. Phosphorus and Nitrogen Limitation of Algal Biomass across Trophic Gradients. Aquat. Sci. 1994, 56, 16–28. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Muneoka, T.; Okazawa, H.; Kimura, M.; Tsuji, O. Influence of the Agricultural Land Agglomeration to the Nutrients of the River Water in the Tokachi River Basin. Paddy Water Environ. 2017, 15, 277–290. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Shan, B. Spatial and Temporal Variations of Nutrition in Representative River Networks in Southwest China. Environ. Monit. Assess. 2018, 190, 707. [Google Scholar] [CrossRef]
- Redfield, A.C. The Biological Control of Chemical Factors in the Environment. Sci. Prog. 1960, 11, 150–170. [Google Scholar]
- Dević, G.; Sakan, S.; Đorđević, D. Assessment of the Environmental Significance of Nutrients and Heavy Metal Pollution in the River Network of Serbia. Environ. Sci. Pollut. Res. 2016, 23, 282–297. [Google Scholar] [CrossRef]
- Dzigurski, D.; Stojanovic, S.; Knezevic, A.; Nikolic, L.; Ljevnaic-Masic, B. Vegetation of the Classes Hydrochari-Lemnetea Oberd. 1967 and Potametea Tx. et Prsg. 1942 in the Jegrička Watercourse (The Province of Vojvodina, Serbia). Zb. Matice Srp. Za Prir. Nauk. 2010, 118, 99–106. [Google Scholar] [CrossRef]
- Istvánovics, V.; Honti, M. Efficiency of Nutrient Management in Controlling Eutrophication of Running Waters in the Middle Danube Basin. Hydrobiologia 2012, 686, 55–71. [Google Scholar] [CrossRef]
- Ljevnaić-Mašić, B.; Džigurski, D.; Nikolić, L.; Brdar-Jokanović, M.; Čabilovski, R.; Ćirić, V.; Petrović, A. Assessment of the Habitat Conditions of a Rare and Endangered Inland Saline Wetland Community with Bolboschoenus maritimus (L.) Palla Dominance in Southeastern Europe: The Effects of Physical–Chemical Water and Soil Properties. Wetl. Ecol. Manag. 2020, 28, 421–438. [Google Scholar] [CrossRef]
- Peršić, V.; Horvatić, J.; Has-Schön, E.; Bogut, I. Changes in N and P Limitation Induced by Water Level Fluctuations in Nature Park Kopački Rit (Croatia): Nutrient Enrichment Bioassay. Aquat. Ecol. 2009, 43, 27–36. [Google Scholar] [CrossRef]
- Savic, R.; Belic, A.; Pantelic, S. Comparative Review of Sediment Properties from Drainage Canals. Pol. J. Environ. Stud. 2013, 22, 849–859. [Google Scholar]
- Svirčev, Z.; Simeunović, J.; Subakov-Simić, G.; Krstić, S.; Pantelić, D.; Dulić, T. Cyanobacterial Blooms and Their Toxicity in Vojvodina Lakes, Serbia. Int. J. Environ. Res. 2013, 7, 745–758. [Google Scholar]
- McDowell, R.W.; Sharpley, A.N. Atmospheric Deposition Contributes Little Nutrient and Sediment to Stream Flow from an Agricultural Watershed. Agric. Ecosyst. Environ. 2009, 134, 19–23. [Google Scholar] [CrossRef]
- Neal, C.; Davies, H.; Neal, M. Water Quality, Nutrients and the Water Framework Directive in an Agricultural Region: The Lower Humber Rivers, Northern England. J. Hydrol. 2008, 350, 232–245. [Google Scholar] [CrossRef]
- Rozpondek, K.; Rozpondek Rafałand Pachura, P. Characteristics of Spatial Distribution of Phosphorus and Nitrogen in the Bottom Sediments of the Water Reservoir Poraj. J. Ecol. Eng. 2017, 18, 178–184. [Google Scholar] [CrossRef]
- Kadović, R.; Belanović, S.; Ristić, R.; Knežević, M.; Kostadinov, S.; Beloica, J.; Radić, B.; Dragović, N.; Milijić, S.; Miljanović, D.; et al. Deposol Reclamation along a Canal of the Danube-Tisza-Danube Hydro System. Pol. J. Environ. Stud. 2014, 23, 1185–1194. [Google Scholar]
- Milanović, A.; Milijašević, D.; Brankov, J. Assessment of Polluting Effects and Surface Water Quality Using Water Pollution Index: A Case Study of Hydro–System Danube–Tisa–Danube, Serbia. Carpathian J. Earth Environ. Sci. 2011, 6, 269–277. [Google Scholar]
- Zemunac, R.; Savic, R.; Blagojevic, B.; Benka, P.; Bezdan, A.; Salvai, A. Assessment of Surface and Groundwater Quality for Irrigation Purposes in the Danube-Tisa-Danube Hydrosystem Area (Serbia). Environ. Monit. Assess. 2021, 193, 519. [Google Scholar] [CrossRef]
- Josimov-Dundjerski, J.; Savić, R.; Belić, A.; Salvai, A.; Grabić, J. Sustainability of the Constructed Wetland Based on the Characteristics in Effluent. Soil Water Res. 2015, 10, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Official Gazette, Republic of Serbia. Regulation on the Parameters of Ecological and Chemical Status of Surface Waters and Parameters of Chemical Status and Quantitative Status of Groundwaters; Serbian Legislation, Official Gazette of the RS, 74/2011; Ministry of Agriculture and Environmental Protection: Belgade, Serbia, 2011. (In Serbian) [Google Scholar]
- Diatta, J.; Waraczewska, Z.; Grzebisz, W.; Niewiadomska, A.; Tatuśko-Krygier, N. Eutrophication Induction Via N/P and P/N Ratios Under Controlled Conditions-Effects of Temperature and Water Sources. Water Air Soil Pollut. 2020, 231, 149. [Google Scholar] [CrossRef] [Green Version]
- ICPDR (International Commission for the Protection of the Danube River). Joint Danube Survey. Technical Report of the International Commission for the Protection of the Danube River; ICPDR: Vienna, Austria, 2002; 30p. [Google Scholar]
- Liu, X.; Zhang, G.; Sun, G.; Wu, Y.; Chen, Y. Assessment of Lake Water Quality and Eutrophication Risk in an Agricultural Irrigation Area: A Case Study of the Chagan Lake in Northeast China. Water 2019, 11, 2380. [Google Scholar] [CrossRef] [Green Version]
- OECD (Organisation for Economic Cooperation and Development). Eutrophication of Waters: Monitoring, Assessment and Control; No. 42077; OECD: Paris, France, 1982. [Google Scholar]
- Poikane, S.; Kelly, M.G.; Salas Herrero, F.; Pitt, J.A.; Jarvie, H.P.; Claussen, U.; Leujak, W.; Lyche Solheim, A.; Teixeira, H.; Phillips, G. Nutrient Criteria for Surface Waters under the European Water Framework Directive: Current State-of-the-Art, Challenges and Future Outlook. Sci. Total Environ. 2019, 695, 133888. [Google Scholar] [CrossRef]
- Wojtkowska, M.; Bojanowski, D. Assessing Trophic State of Surface Waters of Służewiecki Stream (Warsaw). Appl. Water Sci. 2021, 11, 118. [Google Scholar] [CrossRef]
- Abell, J.M.; Özkundakci, D.; Hamilton, D.P. Nitrogen and Phosphorus Limitation of Phytoplankton Growth in New Zealand Lakes: Implications for Eutrophication Control. Ecosystems 2010, 13, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Søndergaard, M.; Lauridsen, T.L.; Johansson, L.S.; Jeppesen, E. Nitrogen or Phosphorus Limitation in Lakes and Its Impact on Phytoplankton Biomass and Submerged Macrophyte Cover. Hydrobiologia 2017, 795, 35–48. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, G.; Lei, K.; Li, Y. Multivariate Analysis of Surface Water Quality in the Three Gorges Area of China and Implications for Water Management. J. Environ. Sci. 2011, 23, 1460–1471. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Bai, Y.; Tian, Z.; Li, J.; Shao, X.; Mustavich, L.F.; Li, B.L. Assessment of Surface Water Quality via Multivariate Statistical Techniques: A Case Study of the Songhua River Harbin Region, China. J. Hydro-Environ. Res. 2013, 7, 30–40. [Google Scholar] [CrossRef]
- Voza, D.; Vukovic, M.; Takic, L.; Nikolic, D.; Mladenovic-Ranisavljevic, I. Application of Multivariate Statistical Techniques in the Water Quality Assessment of Danube River, Serbia. Arch. Environ. Prot. 2015, 41, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Matijevic, B.M.; Vastag, D.D.; Becelic-Tomin, M.R.; Dalmacija, B.D.; Apostolov, S.L. Interpretation of the Results of Surface Water Quality Applying Multivariate Analysis. Hem. Ind. 2015, 69, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.P.; Malik, A.; Mohan, D.; Sinha, S. Multivariate Statistical Techniques for the Evaluation of Spatial and Temporal Variations in Water Quality of Gomti River (India)—A Case Study. Water Res. 2004, 38, 3980–3992. [Google Scholar] [CrossRef]
- Singh, G.; Patel, N.; Jindal, T.; Srivastava, P.; Bhowmik, A. Assessment of Spatial and Temporal Variations in Water Quality by the Application of Multivariate Statistical Methods in the Kali River, Uttar Pradesh, India. Environ. Monit. Assess. 2020, 192, 394. [Google Scholar] [CrossRef]
- Chounlamany, V.; Tanchuling, M.A.; Inoue, T. Spatial and Temporal Variation of Water Quality of a Segment of Marikina River Using Multivariate Statistical Methods. Water Sci. Technol. 2017, 76, 1510–1522. [Google Scholar] [CrossRef]
- Mgelwa, A.S.; Hu, Y.L.; Ngaba, M.J.Y. Patterns of Nitrogen Concentrations and Their Controls in Two Southern China Urban River Ecosystems. Glob. Ecol. Conserv. 2020, 23, e01112. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Qiao, X.; Zheng, B.; Chang, S.; Fu, Q. Investigation of Nitrogen and Phosphorus Contents in Water in the Tributaries of Danjiangkou Reservoir. R. Soc. Open Sci. 2018, 5, 170624. [Google Scholar] [CrossRef] [Green Version]
- Vrzel, J.; Ogrinc, N. Nutrient Variations in the Sava River Basin. J. Soils Sediments 2015, 15, 2380–2386. [Google Scholar] [CrossRef] [Green Version]
- Correll, D.L. The Role of Phosphorus in the Eutrophication of Receiving Waters: A Review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Dodds, W.K.; Smith, V.H.; Lohman, K. Nitrogen and Phosphorus Relationships to Benthic Algal Biomass in Temperate Streams. Can. J. Fish. Aquat. Sci. 2002, 59, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Kolzau, S.; Wiedner, C.; Rücker, J.; Köhler, J.; Köhler, A.; Dolman, A.M. Seasonal Patterns of Nitrogen and Phosphorus Limitation in Four German Lakes and the Predictability of Limitation Status from Ambient Nutrient Concentrations. PLoS ONE 2014, 9, e96065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havens, K.E.; James, R.T.; East, T.L.; Smith, V.H. N:P Ratios, Light Limitation, and Cyanobacterial Dominance in a Subtropical Lake Impacted by Non-Point Source Nutrient Pollution. Environ. Pollut. 2003, 122, 379–390. [Google Scholar] [CrossRef]
- Maberly, S.C.; Pitt, J.A.; Davies, P.S.; Carvalho, L. Nitrogen and Phosphorus Limitation and the Management of Small Productive Lakes. Inland Waters 2020, 10, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Saito, M.A.; Goepfert, T.J.; Ritt, J.T. Some Thoughts on the Concept of Colimitation: Three Definitions and the Importance of Bioavailability. Limnol. Oceanogr. 2008, 53, 276–290. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.H.; Bennett, S.J. Nitrogen:Phosphorus Supply Ratios and Phytoplankton Community Structure in Lakes. Arch. Fur Hydrobiol. 1999, 146, 37–53. [Google Scholar] [CrossRef]
- Guildford, S.J.; Hecky, R.E. Total Nitrogen, Total Phosphorus, and Nutrient Limitation in Lakes and Oceans: Is There a Common Relationship? Limnol. Oceanogr. 2000, 45, 1213–1223. [Google Scholar] [CrossRef] [Green Version]
- Nikolai, S.J.; Dzialowski, A.R. Effects of Internal Phosphorus Loading on Nutrient Limitation in a Eutrophic Reservoir. Limnologica 2014, 49, 33–41. [Google Scholar] [CrossRef]
- Quirós, R. The Relationship between Nitrate and Ammonia Concentrations in the Pelagic Zone of Lakes. Limnetica 2003, 22, 37–50. [Google Scholar] [CrossRef]
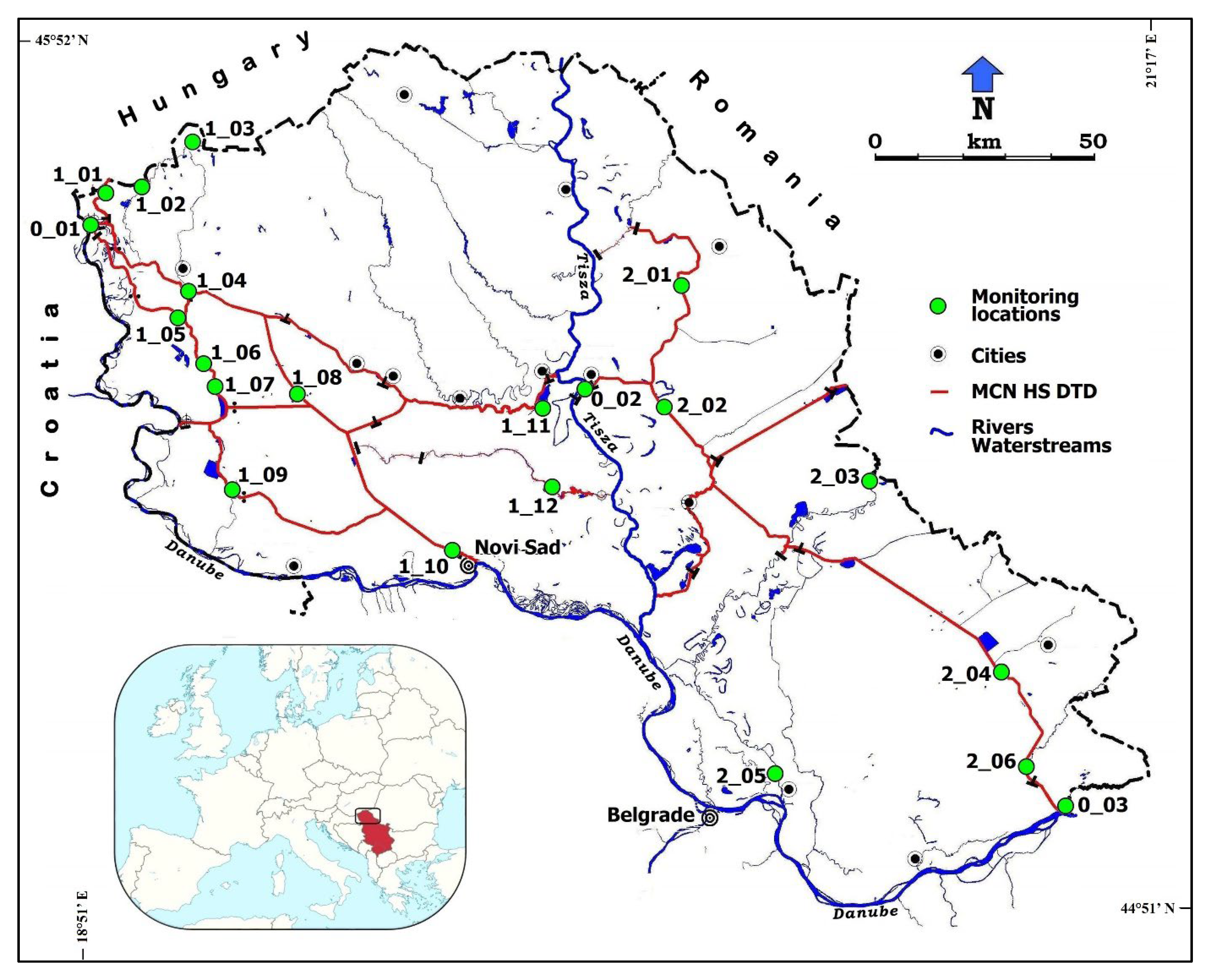





| No. | Code | Location Name | Latitude N | Longitude E | Water Course/ Canal Name |
|---|---|---|---|---|---|
| 1 | 0_01 | Bezdan | 45.8598 | 18.8564 | Danube River |
| 2 | 0_02 | Novi Becej | 45.5848 | 20.1384 | Tisa (Tisza) River |
| 3 | 0_03 | Ban. Palanka | 44.8261 | 21.3436 | Danube River |
| 4 | 1_01 | Backi Breg 1 | 45.9081 | 18.8989 | Bajski Canal |
| 5 | 1_02 | Backi Breg 2 | 45.9230 | 18.9852 | Plazovic |
| 6 | 1_03 | Ridjica | 46.0194 | 19.1350 | Plazovic |
| 7 | 1_04 | Sombor | 45.7408 | 19.1109 | DTD Canal |
| 8 | 1_05 | Prigrevica | 45.6910 | 19.0944 | DTD Canal |
| 9 | 1_06 | Doroslovo | 45.6042 | 19.1746 | DTD Canal |
| 10 | 1_07 | Srpski Miletic | 45.5586 | 19.1927 | DTD Canal |
| 11 | 1_08 | Ruski Krstur | 45.5605 | 19.4006 | DTD Canal |
| 12 | 1_09 | Bac | 45.3879 | 19.2320 | DTD Canal |
| 13 | 1_10 | Novi Sad 1 | 45.2841 | 19.8065 | DTD Canal |
| 14 | 1_11 | Backo Gradiste | 45.5365 | 20.0227 | DTD Canal |
| 15 | 1_12 | Zabalj | 45.3914 | 20.0646 | Jegricka |
| 16 | 2_01 | Novo Milosevo | 45.7687 | 20.3671 | DTD Canal, Kikinda |
| 17 | 2_02 | Melenci | 45.5432 | 20.3372 | DTD Canal |
| 18 | 2_03 | Jasa Tomic | 45.4317 | 20.8616 | Tamis |
| 19 | 2_04 | Vlajkovac | 45.0742 | 21.1842 | DTD Canal |
| 20 | 2_05 | Pancevo | 44.8708 | 20.6311 | Tamis |
| 21 | 2_06 | Kajtasovo | 44.9026 | 21.2458 | DTD Canal |
| Parameters | NH4-N | NO2-N | NO3-N | Total N | PO4-P | Total P |
|---|---|---|---|---|---|---|
| Limit values (mg/L) | 0.2 | 0.03 | 3.0 | 4.0 | 0.2 | 0.3 |
| Parameter | NO3-N (mg/L) | NH4-N (mg/L) | NO2-N (mg/L) | DON (mg/L) | TN (mg/L) | PO4-P (mg/L) | TP (mg/L) | TN/TP (Mass) |
|---|---|---|---|---|---|---|---|---|
| Min | 0.04 | 0.01 | 0.002 | 0.17 | 0.59 | 0.009 | 0.041 | 1.24 |
| Max | 3.21 | 2.00 | 0.425 | 3.98 | 5.95 | 1.165 | 1.215 | 47.73 |
| Avg | 0.92 | 0.17 | 0.025 | 0.69 | 1.80 | 0.124 | 0.205 | 13.25 |
| STD | 0.57 | 0.26 | 0.033 | 0.40 | 0.80 | 0.191 | 0.218 | 8.18 |
| Cv | 0.62 | 1.50 | 1.33 | 0.58 | 0.45 | 1.54 | 1.07 | 0.62 |
| Skewness | 0.69 | 3.41 | 7.86 | 3.42 | 1.25 | 3.06 | 2.78 | 1.49 |
| Kurtosis | 0.52 | 14.49 | 84.77 | 20.96 | 2.43 | 9.74 | 7.69 | 3.11 |
| TN/TP (Mass) | Spring–Summer (%) | Fall–Winter (%) | Total (%) | Limitation |
|---|---|---|---|---|
| <8 | 22.2 | 4.8 | 27.0 | N-limit. |
| 8–24 | 26.6 | 37.3 | 63.9 | N and P-co-limit. |
| >24 | 1.2 | 7.9 | 9.1 | P-limit. |
| Per | Variables | TN | TP |
|---|---|---|---|
| Locations | TN | 1 | |
| TP | 0.76 * (p < 0.0001) | 1 | |
| TN:TP | −0.40 (p = 0.0700) | −0.71 * (p = 0.0003) | |
| Months | TN | 1 | |
| TP | −0.55 (p = 0.0631) | 1 | |
| TN:TP | 0.96 * (p < 0.0001) | −0.74 * (p = 0.0065) | |
| Per | Variables | TN | TP |
| Locations | TN | 1 | |
| TP | 0.76 * (p < 0.0001) | 1 | |
| TN:TP | −0.40 (p = 0.0700) | −0.71 * (p = 0.0003) | |
| Months | TN | 1 | |
| TP | −0.55 (p = 0.0631) | 1 | |
| TN:TP | 0.96 * (p < 0.0001) | −0.74 * (p = 0.0065) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savic, R.; Stajic, M.; Blagojević, B.; Bezdan, A.; Vranesevic, M.; Nikolić Jokanović, V.; Baumgertel, A.; Bubalo Kovačić, M.; Horvatinec, J.; Ondrasek, G. Nitrogen and Phosphorus Concentrations and Their Ratios as Indicators of Water Quality and Eutrophication of the Hydro-System Danube–Tisza–Danube. Agriculture 2022, 12, 935. https://doi.org/10.3390/agriculture12070935
Savic R, Stajic M, Blagojević B, Bezdan A, Vranesevic M, Nikolić Jokanović V, Baumgertel A, Bubalo Kovačić M, Horvatinec J, Ondrasek G. Nitrogen and Phosphorus Concentrations and Their Ratios as Indicators of Water Quality and Eutrophication of the Hydro-System Danube–Tisza–Danube. Agriculture. 2022; 12(7):935. https://doi.org/10.3390/agriculture12070935
Chicago/Turabian StyleSavic, Radovan, Milica Stajic, Boško Blagojević, Atila Bezdan, Milica Vranesevic, Vesna Nikolić Jokanović, Aleksandar Baumgertel, Marina Bubalo Kovačić, Jelena Horvatinec, and Gabrijel Ondrasek. 2022. "Nitrogen and Phosphorus Concentrations and Their Ratios as Indicators of Water Quality and Eutrophication of the Hydro-System Danube–Tisza–Danube" Agriculture 12, no. 7: 935. https://doi.org/10.3390/agriculture12070935
APA StyleSavic, R., Stajic, M., Blagojević, B., Bezdan, A., Vranesevic, M., Nikolić Jokanović, V., Baumgertel, A., Bubalo Kovačić, M., Horvatinec, J., & Ondrasek, G. (2022). Nitrogen and Phosphorus Concentrations and Their Ratios as Indicators of Water Quality and Eutrophication of the Hydro-System Danube–Tisza–Danube. Agriculture, 12(7), 935. https://doi.org/10.3390/agriculture12070935








