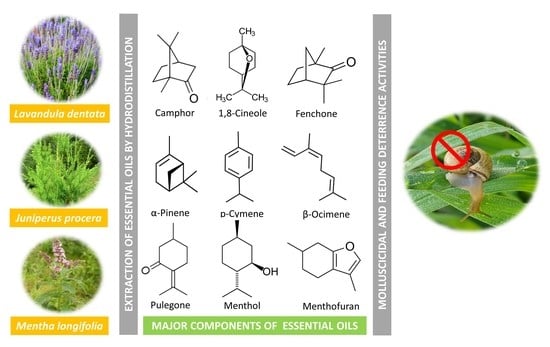
Fumigant Toxicity and Feeding Deterrent Activity of Essential Oils from Lavandula dentata, Juniperus procera, and Mentha longifolia against the Land Snail Monacha obstructa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Maintenance of Snails
2.2. Collection of Plants
2.3. Isolation of Essential Oils
2.4. Analysis of Essential Oils
2.5. Fumigant Toxicity Assay
2.6. Feeding Deterrence Assay
2.7. Data Analysis
3. Results
3.1. Chemical Composition of Essential Oils
3.2. Fumigant Toxicity
3.3. Feeding Deterrent Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godan, D. Pest Slugs and Snails; Springer: Berlin, Germany, 1983; p. 445. [Google Scholar]
- Barker, G.M. Mollusks as Crop Pests; CABI Publishing: Wallingford, UK, 2002; p. 441. [Google Scholar]
- Iglesias, J.; Castillejo, J.; Castro, R. The effects of repeated applications of the molluscicide metaldehyde and the biocontrol nematode Phasmarhabditis hermaphrodita on mollusks, earth worms, nematodes, acarids and collembolans: A two-years study in north–west Spain. Pest Manag. Sci. 2003, 59, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Borkakati, R.N.; Gogoi, R.; Borah, B.K. Snail: From present perspective to the history of Assam. Asian Agri-Hist. 2009, 13, 227–234. [Google Scholar]
- Kozłowski, J.; Kozłowski, R.J. Expansion of the invasive slug species Arion lusitanicus Mabille, 1868 (Gastropoda: Pulmonata: Stylommatophora) and dangers to garden crops–a literature review with some new data. Folia Malacol. 2011, 19, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Iwanowicz, D.D.; Sanders, L.R.; Schill, W.B.; Xayavong, M.V.; da Silva, A.J.; Qvarnstrom, Y.; Smith, T. Spread of the rat lungworm (Angiostrongylus cantonensis) in giant African land snails (Lissachatina fulica) in Florida, USA. J. Wildl. Dis. 2015, 51, 749–753. [Google Scholar] [CrossRef]
- Taofiq, S.; Bunza, M.D.A.; Majeed, Q.; Abubakar, M.B.; Ladan, M.U. Studies on snail vectors of helminth disease agents along Rima River Valley at Kwalkwalawa Village, Wamakko Local Government Area, Sokoto State, Nigeria. SM Trop. Med. J. 2017, 2, 1011–1115. [Google Scholar]
- Syed, A.R. Pest and disease management for crop production inside greenhouses. Acta Hortic. 2006, 710, 89–102. [Google Scholar] [CrossRef]
- Abdul-Sahib, I.M. A new record of a white terrestrial snail Monacha obstructa (Pfeiffer,1842), (Gastropoda: Pulmonata) from the Iraqi marshes. J. Basrah Res. 2006, 32, 70–73. [Google Scholar]
- Ali, S.M.; Said, S.M. Histological and scanning electron microscopic study of the effect of UV-A radiation on the land snail Monacha obstructa. J. Basic Appl. Zool. 2019, 80, 8. [Google Scholar] [CrossRef]
- Rashed, A.A. A new parasitic metacercaria from the land snail Monacha obstructa Pfeiffer1842 with critical review on relevant metacercariae belonging to the genus Brachylaima Dujardin 1843. J. Egypt. Soc. Parasitol. 2008, 38, 483–500. [Google Scholar]
- Mc Donnell, R.J. Essential oils and their constituents as novel biorational molluscicides for terrestrial gastropod pests. In Green Pesticides Handbook: Essential Oils for Pest Control; Nollet, L.M.L., Rathore, H.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 479–486. [Google Scholar]
- Andreasen, J.R. Metaldehyde toxicosis in ducklings. J. Vet. Diagn. Investig. 1993, 5, 500–501. [Google Scholar] [CrossRef] [Green Version]
- Daniel, R.; Lewis, D.; Payne, J. Metaldehyde poisoning in a dairy herd. Vet. Rec. 2009, 165, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Bates, N.S.; Sutton, N.M.; Campbell, A. Suspected metaldehyde slug bait poisoning in dogs: A retrospective analysis of cases reported to the Veterinary Poisons Information Service. Vet. Rec. 2012, 171, 324. [Google Scholar] [CrossRef] [PubMed]
- Chase, R.; Croll, R.P. Tentacular function in snail olfactory orientation. J. Comp. Physiol. 1981, 143, 357–362. [Google Scholar] [CrossRef]
- Barone, M.; Frank, T. Effects of plant extracts on the feeding behavior of the slug Arion lusitanicus. Ann. Appl. Biol. 1999, 134, 341–345. [Google Scholar] [CrossRef]
- Rosenthal, G.A.; Berenbaum, M.R. Herbivores: Their Interactions with Secondary Plant Metabolites: Ecological and Evolutionary Processes, 2nd ed.; Academic Press: San Diego, CA, USA, 1992; Volume II. [Google Scholar]
- Sergeeva, V. Use of plant extracts and essential oils in modern plant protection. Acta Hortic. 2016, 1125, 361–368. [Google Scholar] [CrossRef]
- Upson, T. The Taxonomy of the Genus Lavandula L. Lavender: The Genus Lavandula; Taylor & Francis: London, UK, 2002; pp. 2–34. [Google Scholar]
- Muyima, N.Y.O.; Zulu, G.; Bhengu, T.; Popplewell, D. The potential application of some novel essential oils as natural cosmetic preservatives in an aqueous cream formulation. Flavour Fragr. J. 2002, 17, 258–266. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Al-Musayeib, N.M.; Mothana, R.A.; Matheeussen, A.; Cos, P.; Maes, L. In vitro antiplasmodial, antileishmanial and antitrypanosomal activities of selected medicinal plants used in the traditional Arabian Peninsular region. BMC Complement. Altern. Med. 2012, 12, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Aly, M.M.; Al-Ghamdi, M.; Bafeel, S.O.; Khedr, A.M. Antimicrobial activities and phytochemical analysis of the essential oil of Lavandula dentata and Plectranthus tenuiflorus, collected from Al Baha region, Saudi Arabia. Life Sci. J. 2013, 10, 3302–3309. [Google Scholar]
- Dammak, I.H.; Euchc, S.K.E.; Zemni, H.; Mliki, A.; Hassouna, M.; Lasram, S. Evaluation of antifungal and anti-ochratoxigenic activities of Salvia officinalis, Lavandula dentata and Laurus nobilis essential oils and a major monoterpene constituent 1,8-cineole against Aspergillus carbonarius. Ind. Crops Prod. 2019, 128, 85–93. [Google Scholar] [CrossRef]
- Tumen, I.; Süntar, I.; Eller, F.J.; Keleş, H.; Akkol, E.K. Topical wound-healing effects and phytochemical composition of heartwood essential oils of Juniperus virginiana L., Juniperus occidentalis Hook., and Juniperus ashei J. Buchholz. J. Med. Food 2013, 16, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eryiğit, T.; Okut, N.; Ekici, K.; Yildirim, B. Chemical composition and antibacterial activities of Juniperus horizontalis essential oil. Can. J. Plant Sci. 2014, 94, 323–327. [Google Scholar] [CrossRef]
- Karunamoorthi, K.; Girmay, A.; Fekadu, S. Larvicidal efficacy of Ethiopian ethnomedicinal plant Juniperus procera essential oil against Afrotropical malaria vector Anopheles arabiensis (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2014, 4, S99–S106. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, T.M.; Hassan, M.M.; El-Naggar, M.A.; El-Mongy, M.A. GC/MS analysis of Juniperus procera extract and its activity with silver nanoparticles against Aspergillus flavus growth and aflatoxins production. Biotechnol. Rep. 2020, 27, e00496. [Google Scholar] [CrossRef]
- Gulluce, M.; Shain, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiouf, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. spp. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Anwar, F.; Abbas, A.; Mehmood, T.; Gilani, A.H.; Rehman, N. Mentha: A genus rich in vital nutra-pharmaceuticals—A review. Phytother. Res. 2019, 33, 2548–2570. [Google Scholar] [CrossRef]
- Radwan, M.A.; Gad, A.F. Essential oils and their components as promising approach for gastropod mollusc control: A review. J. Plant Dis. Prot. 2021, 128, 923–949. [Google Scholar] [CrossRef]
- Finney, D.N. Probit Analysis; Cambridge University Press: London, UK, 1971. [Google Scholar]
- Ikawati, S.; Himawan, T.; Abadi, A.L.; Tarno, H. Fumigant and feeding deterrent activity of essential oils against Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae). Biodiversitas 2020, 21, 4301–4308. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Alsaid, M.S.; Hasoon, S.S.; Al-Mosaiyb, N.M.; Al-Rehaily, A.J.; Al-Yahya, M.A. Antimicrobial and antioxidant activities and gas chromatography mass spectrometry (GC/MS) analysis of the essential oils of Ajuga bracteosa Wall. ex Benth. and Lavandula dentata L. growing wild in Yemen. J. Med. Plants Res. 2012, 6, 3066–3071. [Google Scholar]
- Boubaker, H.; Karim, H.; Msanda, F.; Boudyach, E.H.; Aoumar, A.A.B. Study of essential oil composition and antifungal activity of Lavandula mairei, L. dentata and Tetraclinis articulate. J. Appl. Sci. 2019, 19, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Bousmaha, L.; Bekkara, F.A.; Tomi, F.; Casanova, J. Advances in the chemical composition of Lavandula dentata L. essential oil from Algeria. J. Essent. Oil Res. 2005, 17, 292–295. [Google Scholar] [CrossRef]
- Dob, T.; Dahmane, D.; Tayeb, B.; Chelghoum, C. Chemical composition of the essential oil of Lavandula dentata L. from Algeria. Int. J. Aromather. 2005, 15, 110–114. [Google Scholar] [CrossRef]
- Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Milani, F.; Papini, A.; Flamini, G.; Fico, G. Lavandula dentata L. from Italy: Analysis of trichomes and volatiles. Chem. Biodivers. 2020, 17, e2000532. [Google Scholar] [CrossRef] [PubMed]
- Mostefa, M.B.; Kabouche, A.; Abaza, I.; Aburjai, T.; Touzani, R.; Kabouche, Z. Chemotypes investigation of Lavandula essential oils growing at different North African soils. J. Mater. Environ. Sci. 2014, 5, 1896–1901. [Google Scholar]
- Eshra, E.H.; Abobakr, Y.; Abddelgalil, G.M.; Ebrahim, E.; Hussein, H.I.; Al-Sarar, A.S. Fumigant toxicity and antiacetylcholinesterase activity of essential oils against the land snail, Theba pisana (Müller). Egypt Sci. J. Pestic. 2016, 2, 91–95. [Google Scholar]
- Bedini, S.; Flamini, G.; Ascrizzi, R.; Venturi, F.; Ferroni, G.; Bader, A.; Girard, J.; Conti, B. Essential oils sensory quality and their bioactivity against the mosquito Aedes albopictus. Sci. Rep. 2018, 8, 17857. [Google Scholar] [CrossRef] [Green Version]
- Msaada, K.; Salem, N.; Tammar, S.; Hammami, M.; Saharkhiz, M.J.; Debiche, N.; Liman, F.; Marzouk, B. Essential oil composition of Lavandula dentata, L. stoechas and L. multifida cultivated in Tunisia. J. Essent. Oil Bear. Plants 2012, 15, 1030–1039. [Google Scholar] [CrossRef]
- Dris, D.; Tine-Djebbar, F.; Soltani, N. Lavandula dentata essential oils: Chemical composition and larvicidal activity against Culiseta longiareolata and Culex pipiens (Diptera: Culicidae). Afr. Entomol. 2017, 25, 387–394. [Google Scholar] [CrossRef]
- Adams, R.P. Juniperus procera of East Africa: Volatile leaf oil composition and putative relationship to J. excels. Biochem. Syst. Ecol. 1990, 18, 207–210. [Google Scholar] [CrossRef]
- El-Said, H.; Ashgar, S.S.; Bader, A.; AlQathama, A.; Halwani, M.; Ascrizzi, R.; Flamini, G. Essential oil analysis and antimicrobial evaluation of three aromatic plant species growing in Saudi Arabia. Molecules 2021, 26, 959. [Google Scholar] [CrossRef]
- Almadiy, A.A. Chemical composition, insecticidal and biochemical effects of two plant oils and their major fractions against Aedes aegypti, the common vector of dengue fever. Heliyon 2020, 6, e04915. [Google Scholar] [CrossRef] [PubMed]
- Al-Busafi, S.N.; Al-Saidi, S.H.; Al-Riyami, A.I.; Al-Manthary, N.S. Comparison of chemical composition and antioxidant activity of four essential oils extracted from different parts of Juniperus excels. SQU J. Sci. 2016, 21, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hameed, E.S.S.; Salman, M.S.; Fadl, M.A.; Elkhateeb, A.; Hassan, M.M. Chemical composition and biological activity of Mentha longifolia L. essential oil growing in Taif, KSA extracted by hydrodistillation, solvent free microwave and microwave hydrodistillation. J. Essent. Oil Bear. Plants 2018, 21, 1–14. [Google Scholar] [CrossRef]
- Murad, H.A.S.; Abdallah, H.M.; Ali, S.S. Mentha longifolia protects against acetic-acid induced colitis in rats. J. Ethnopharmacol. 2016, 190, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Nazem, V.; Sabzalian, M.R.; Saeidi, G.; Rahimmalek, M. Essential oil yield and composition and secondary metabolites in self- and open-pollinated populations of mint (Mentha spp.). Ind. Crops Prod. 2019, 130, 332–340. [Google Scholar] [CrossRef]
- Okut, N.; Yagmur, M.; Selcuk, N.; Yildirim, B. Chemical composition of essential oil of Mentha longifolia L. subsp. longifolia growing wild. Pak. J. Bot. 2017, 49, 525–529. [Google Scholar]
- Soilhi, Z.; Rhimi, A.; Heuskin, S.; Fauconnier, M.L.; Mekki, M. Essential oil chemical diversity of Tunisian Mentha spp. collection. Ind. Crops Prod. 2019, 131, 330–340. [Google Scholar] [CrossRef] [Green Version]
- Asghari, B.; Zengin, G.; Bahadoric, M.B.; Abbas-Mohammadid, M.; Dinparaste, L. Amylase, glucosidase, tyrosinase, and cholinesterases inhibitory, antioxidant effects, and GC-MS analysis of wild mint (Mentha longifolia var. calliantha) essential oil: A natural remedy. Eur. J. Integr. Med. 2018, 22, 44–49. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Al-Sarar, A.S.; Hussein, H.I.; Abobakr, Y.; Bayoumi, A.E.; Al-Otaibi, M.T. Fumigant toxicity and antiacetylcholinesterase activity of Saudi Mentha longifolia and Lavandula dentata species against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Turk. Entomoloji Derg 2014, 38, 11–18. [Google Scholar]
- El-Zemity, S.R.; Mohamed, S.A.; Radwan, M.A.; Sherby, S.M. Molluscicidal efficacy and repellency of some naturally occurring monoterpenoids against the land snail, Helix aspersa, Müller (Mollusca: Pulmonata). Ann. Agric. Sci. Ain Shams Univ. Cairo 2001, 46, 339–346. [Google Scholar]
- Abdelgaleil, S.A.M. Molluscicidal and insecticidal potential of monoterpenes on the white garden snail, Theba pisana (Müller) and the cotton leafworm, Spodoptera littoralis (Boisduval). Appl. Entomol. Zool. 2010, 45, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.I.; Eshra, E.H.; Abobakr, Y. Molluscicidal activity and biochemical effects of certain monoterpenoids against land snails. J. Adv. Agric. Res. 2007, 12, 679–693. [Google Scholar]
- Hussein, H.I. Composition of essential oils isolated from three plant species and their molluscicidal activity against Theba pisana snails. J. Pest Control Environ. Sci. 2005, 13, 15–24. [Google Scholar]
- Pepeljnjak, S.; Kosalec, I.; Kalodera, Z.; Blazević, N. Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae). Acta Pharm. 2005, 55, 417–422. [Google Scholar]
- Al-Zanbagi, N.A. Noticeable effect of Juniperus procera as Toxoplasma gondii tachyzoites inhibitor in vivo. Int. J. Health Wellness Soc. 2011, 1, 197–204. [Google Scholar] [CrossRef]
- Teixeira, T.; Rosa, J.S.; Rainha, N.; Baptista, J.; Rodrigues, A. Assessment of molluscicidal activity of essential oils from five Azorean plants against Radix peregra. Chemosphere 2012, 87, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Abdelgaleil, S.A.M.; Saad, M.M.G. The synergistic effect of piper onyl butoxide on the molluscicidal potential of monoterpenes and phenylpropenes against Theba pisana. J. Plant Prot. Res. 2018, 58, 381–386. [Google Scholar]
- Lee, S.E.; Lee, B.H.; Choi, W.S.; Park, B.S.; Kim, J.G.; Campbell, B.C. Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil Sitophilus oryzae (L.). Pest Manag. Sci. 2001, 57, 548–553. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, S.E.; Annis, P.C.; Pratt, S.J.; Park, B.S.; Tumaalii, F. Fumigant toxicity of essential oils and monoterpenes against the red flour beetle, Tribolium castaneum Herbst. J. Asia-Pac. Entomol. 2002, 5, 237–240. [Google Scholar] [CrossRef]
- Yang, P.; Ma, Y.; Zheng, S. Adulticidal activity of five essential oils against Culex pipiens quinquefasciatus. J. Pestic. Sci. 2005, 30, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Abdelgaleil, S.A.M.; Badawy, M.E.I. Acaricidal and molluscicidal potential of three essential oils isolated from Egyptian plants. J. Pest Control Environ. Sci. 2006, 14, 35–46. [Google Scholar]
- Mohamed, M.I.E.; Abdelgaleil, S.A.M. Chemical composition and insecticidal potential of essential oils from Egyptian plants against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Appl. Entomol. Zool. 2008, 43, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Biert, K.; Speiser, B. Feeding deterrent effect of carvone, a compound from caraway seeds, on the slug Arion lusitanicus. Ann. Appl. Biol. 2002, 141, 93–100. [Google Scholar] [CrossRef]
- O’Reilly-Wapstra, J.M.; Iason, G.R.; Thoss, V. The role of genetic and chemical variation of Pinus sylvestris seedlings in influencing slug herbivory. Oecologia 2007, 152, 82–91. [Google Scholar] [CrossRef]
- Fritz, R.S.; Hochwender, C.G.; Lewkiewicz, D.A.; Bothwell, S.; Orians, C.M. Seedling herbivory by slugs in a willow hybrid system: Developmental changes in damage, chemical defense, and plant performance. Oecologia 2001, 129, 87–97. [Google Scholar] [CrossRef]
- Watkins, R.W.; Mosson, H.J.; Gurney, J.E.; Cowan, D.P.; Edwards, J.P. Cinnamic acid derivatives: Novel repellent seed dressings for the protection of wheat seed against damage by the field slug, Deroceras reticulatum. Crop. Prot. 1996, 15, 77–83. [Google Scholar] [CrossRef]
- El-Zemity, S.R.; Radwan, M.A. Molluscicidal and antifeedant activity of some essential oils and their major chemical constituents against Theba pisana snails. Arab. Univ. J. Agric. Sci. 2001, 9, 483–493. [Google Scholar]
- Tomas, J.; Gil, L.; Llorens-Molina, J.A.; Cardona, C.; García, M.T.; Llorens, L. Biogenic volatiles of rupicolous plants act as direct defenses against mollusks: The case of the endangered Clinopodium rouyanum. Flora 2019, 258, 151428. [Google Scholar] [CrossRef]
- Powell, A.L.; Bowen, I.D. The screening of naturally-occurring compounds for use as seed treatments for the protection of wheat against slug damage. In Slug and Snail Pests in Agriculture; Henderson, I.F., Ed.; BCPC Proceedings No. 66; BCPC: Farnham, UK, 1996; pp. 231–236. [Google Scholar]
- Dawson, G.W.; Henderson, I.F.; Martin, A.P.; Pye, B.J. Physiochemical barriers as plant protectants against slugs (Gastropoda, Pulmonata). In Slug and Snail Pests in Agriculture; Henderson, I.F., Ed.; BCPC Proceedings No. 66; BCPC: Farnham, UK, 1996; pp. 439–444. [Google Scholar]
- Sinclair, A.R.E.; Jogia, M.K.; Andersen, R.J. Camphor from juvenile white spruce as an antifeedant for snowshoe hares. J. Chem. Ecol. 1988, 14, 1505–1514. [Google Scholar] [CrossRef]
- Ali, A.M.; Ibrahim, A.M. Castor and camphor essential oils alter hemocyte populations and induce biochemical changes in larvae of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). J. Asia-Pac. Entomol. 2018, 21, 631–637. [Google Scholar] [CrossRef]
- Huang, Y.; Hee, S.K.; Ho, S.H. Antifeedant and growth inhibitory effects of α-pinene on the stored-product insects, Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Int. Pest Control 1998, 40, 18–20. [Google Scholar]
- Tripathi, A.K.; Prajapati, V.; Aggarwal, K.K.; Kumar, S. Toxicity, feeding deterrence, and effect of activity of 1,8-cineole from Artemisia annua on progeny production of Tribolium castanaeum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2001, 94, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Colorado, B.E.; Pino-Benitez, N.; Gonzalez-Coloma, A. Volatile composition and biocidal (antifeedant and phytotoxic) activity of the essential oils of four Piperaceae species from Choco-Colombia. Ind. Crop. Prod. 2019, 138, 111463. [Google Scholar] [CrossRef]
| No. | Compound | Chemical Class | Molecular Formula | RT (min) | Area (%) | RI |
|---|---|---|---|---|---|---|
| 1 | α-Pinene | Bicyclic monoterpene | C10H16 | 8.78 | 0.86 | 937 |
| 2 | β-Myrecene | Acyclic monoterpene | C10H16 | 11.997 | 9.02 | 992 |
| 3 | Limonene | Cyclohexane monoterpene | C10H16 | 13.26 | 1.56 | 1026 |
| 4 | 1,8-Cineole | Oxygenated cyclic monoterpene | C10H18O | 14.00 | 18.63 | 1032 |
| 5 | γ-Terpinene | Cyclohexane monoterpene | C10H16 | 16.22 | 0.91 | 1060 |
| 6 | Fenchone | Oxygenated bicyclic monoterpene | C10H16O | 16.70 | 18.06 | 1096 |
| 7 | cis-Verbinol | Oxygenated bicyclic monoterpene | C10H16O | 16.99 | 4.38 | 1141 |
| 8 | Camphor | Oxygenated bicyclic monoterpene | C10H16O | 17.17 | 45.74 | 1145 |
| Total identified | 99.16 | |||||
| Grouped components (%) | ||||||
| Monoterpene hydrocarbons | 12.35 | |||||
| Oxygenated monoterpenes | 86.81 | |||||
| Others | 0.84 |
| No. | Compound | Chemical Class | Molecular Formula | RT (min) | Area (%) | RI |
|---|---|---|---|---|---|---|
| 1 | α-Pinene | Bicyclic monoterpene | C10H16 | 8.7809 | 53.70 | 937 |
| 2 | p-Cymene | Cyclic monoterpene | C10H14 | 12.0992 | 24.83 | 1014 |
| 3 | β-Ocimene | Acyclic monoterpene | C10H16 | 12.7914 | 10.78 | 1048 |
| 4 | Thymol | Oxygenated cyclic monoterpene | C10H14O | 24.9006 | 3.31 | 1290 |
| 5 | γ-Elemene | Cyclic Sesquiterpene | C15H24 | 25.7675 | 5.25 | 1342 |
| Total identified | 97.87 | |||||
| Grouped components (%) | ||||||
| Monoterpene hydrocarbons | 89.31 | |||||
| Oxygenated monoterpenes | 3.31 | |||||
| Sesquiterpene hydrocarbons | 5.25 | |||||
| Others | 2.13 |
| No. | Compound | Chemical Class | Molecular Formula | RT (min) | Area (%) | RI |
|---|---|---|---|---|---|---|
| 1 | α-Pinene | Bicyclic monoterpene | C10H16 | 8.7809 | 1.23 | 937 |
| 2 | p-Cymene | Cyclic monoterpene | C10H14 | 12.0992 | 1.15 | 1014 |
| 3 | p-Mentha-3,8-diene | Cyclic monoterpene | C10H16 | 14.9971 | 3.99 | 1074 |
| 4 | 1,8-Cineole | Oxygenated cyclic monoterpene | C10H18O | 17.0024 | 0.889 | 1032 |
| 5 | Menthofuran | Oxygenated bicyclic monoterpene | C10H14O | 17.4746 | 7.748 | 1150 |
| 6 | Borneol | Oxygenated cyclic monoterpene | C10H18O | 17.8174 | 4.303 | 1161 |
| 7 | Menthol | Oxygenated cyclic monoterpene | C10H20O | 19.4928 | 18.257 | 1169 |
| 8 | Benzofuran 4,7-dimethyl | Benzofuran | C10H10O | 19.8227 | 5.011 | 1222 |
| 9 | Pulegone | Oxygenated cyclic monoterpene | C10H16O | 20.0232 | 56.53 | 1235 |
| 10 | Neryl acetate | Oxygenated acyclic monoterpene | C12H20O2 | 22.8371 | 0.712 | 1367 |
| Total identified | 99.82 | |||||
| Grouped components (%) | ||||||
| Monoterpene hydrocarbons | 6.37 | |||||
| Oxygenated monoterpenes | 93.45 | |||||
| Others | 0.18 |
| Essential Oil | Exposure Time (h) | LC50 (μL/L Air) | 95% Fiducial Limits | LC95 (μL/L Air) | 95% Fiducial Limits | Slope ± SE | χ2 | R2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||||
| Lavandula dentata | 24 | 8.68 | 8.24 | 9.23 | 13.79 | 12.42 | 16.03 | 8.19 ± 0.83 | 0.59 | 0.997 |
| 48 | 7.24 | 6.99 | 7.49 | 10.44 | 9.84 | 11.30 | 10.34 ± 0.86 | 2.58 | 0.995 | |
| Juniperus procera | 24 | 25.63 | 24.34 | 26.88 | 42.53 | 39.49 | 46.86 | 7.48 ± 0.61 | 2.98 | 0.994 |
| 48 | 20.11 | 19.06 | 21.09 | 30.24 | 28.19 | 33.32 | 9.29 ± 0.93 | 1.78 | 0.98 | |
| Mentha longifolia | 24 | >50 | - | - | >50 | - | - | - | - | - |
| 48 | >50 | - | - | >50 | - | - | - | - | - | |
| Essential Oil | Concentration (µL/L Air) | Feeding Deterrence Index (FDI) % ± SD | |
|---|---|---|---|
| 24 h | 48 h | ||
| Lavandula dentata | 0.75 | 86.20 ± 1.42 a | 89.83 ± 1.33 a |
| 1.25 | 91.53 ± 1.36 b | 92.33 ± 0.81 a | |
| 2.5 | 93.37 ± 2.41 b | 97.10 ± 2.90 b | |
| Juniperus procera | 5 | 87.13 ± 4.58 a | 87.60 ± 3.38 a |
| 10 | 89.03 ± 6.11 a | 92.97 ± 2.95 a | |
| 15 | 93.97 ± 1.53 a | 95.63 ± 0.70 a | |
| Mentha longifolia | 5 | 79.77 ± 2.38 a | 84.33 ± 3.30 a |
| 10 | 92.47 ± 1.94 b | 93.70 ± 1.85 b | |
| 15 | 94.13 ± 0.59 b | 98.40 ± 0.41 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abobakr, Y.; Al-Sarar, A.S.; Abdel-Kader, M.S. Fumigant Toxicity and Feeding Deterrent Activity of Essential Oils from Lavandula dentata, Juniperus procera, and Mentha longifolia against the Land Snail Monacha obstructa. Agriculture 2022, 12, 934. https://doi.org/10.3390/agriculture12070934
Abobakr Y, Al-Sarar AS, Abdel-Kader MS. Fumigant Toxicity and Feeding Deterrent Activity of Essential Oils from Lavandula dentata, Juniperus procera, and Mentha longifolia against the Land Snail Monacha obstructa. Agriculture. 2022; 12(7):934. https://doi.org/10.3390/agriculture12070934
Chicago/Turabian StyleAbobakr, Yasser, Ali S. Al-Sarar, and Maged S. Abdel-Kader. 2022. "Fumigant Toxicity and Feeding Deterrent Activity of Essential Oils from Lavandula dentata, Juniperus procera, and Mentha longifolia against the Land Snail Monacha obstructa" Agriculture 12, no. 7: 934. https://doi.org/10.3390/agriculture12070934
APA StyleAbobakr, Y., Al-Sarar, A. S., & Abdel-Kader, M. S. (2022). Fumigant Toxicity and Feeding Deterrent Activity of Essential Oils from Lavandula dentata, Juniperus procera, and Mentha longifolia against the Land Snail Monacha obstructa. Agriculture, 12(7), 934. https://doi.org/10.3390/agriculture12070934