Multi-Residue Determination of 244 Chemical Contaminants in Chicken Eggs by Liquid Chromatography-Tandem Mass Spectrometry after Effective Lipid Clean-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
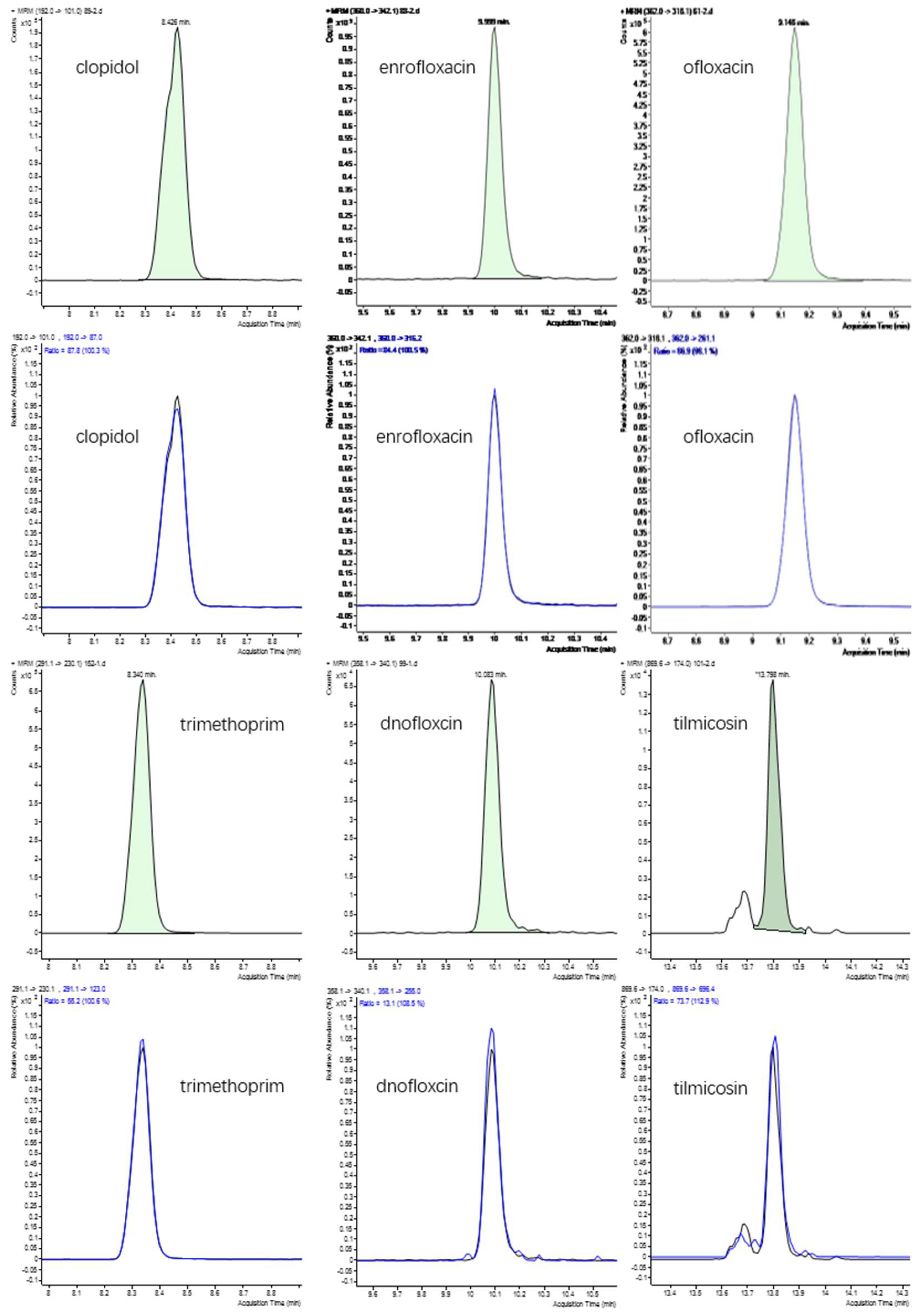
2.3. LC-MS/MS Analysis
2.4. Method Validation
3. Results and Discussion
3.1. Optimization of Pretreatment Methods
3.1.1. Selection of Extract Solvent
3.1.2. Selection of Clean-Up Method
3.2. Method Validation
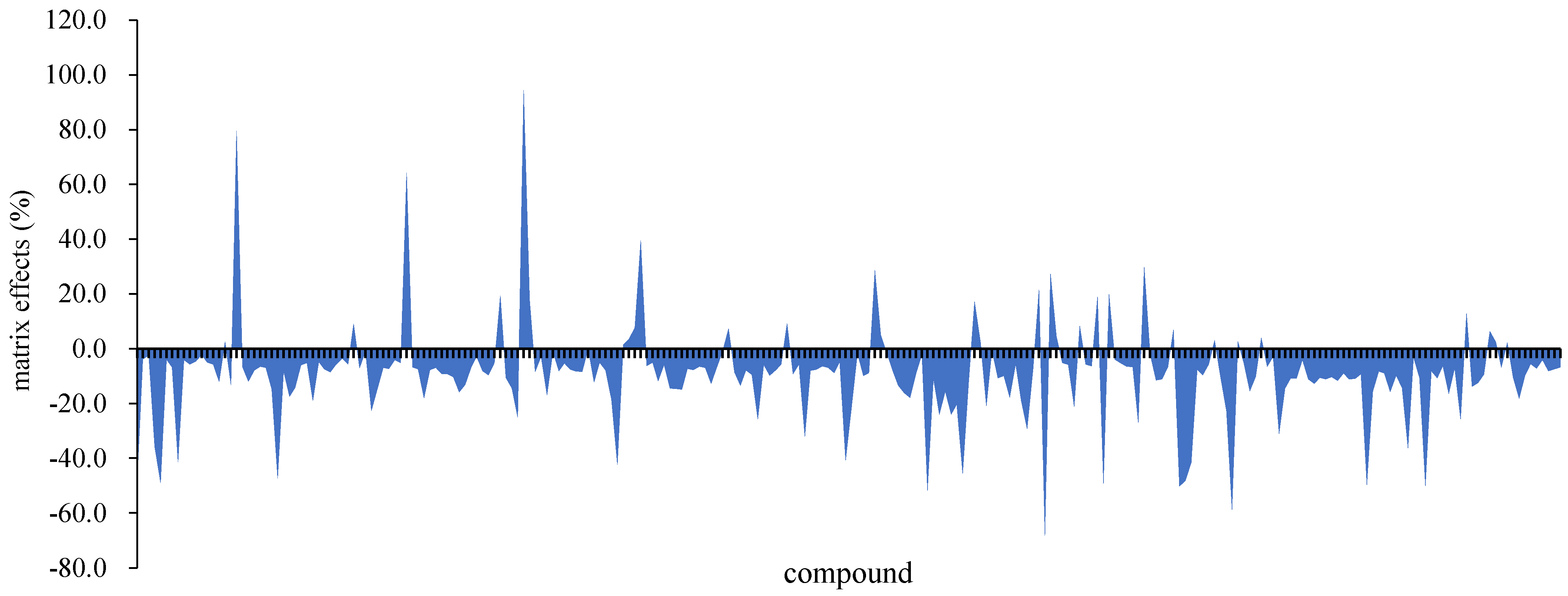
3.2.1. Matrix Effects
3.2.2. Linearity
3.2.3. LOQs and LODs
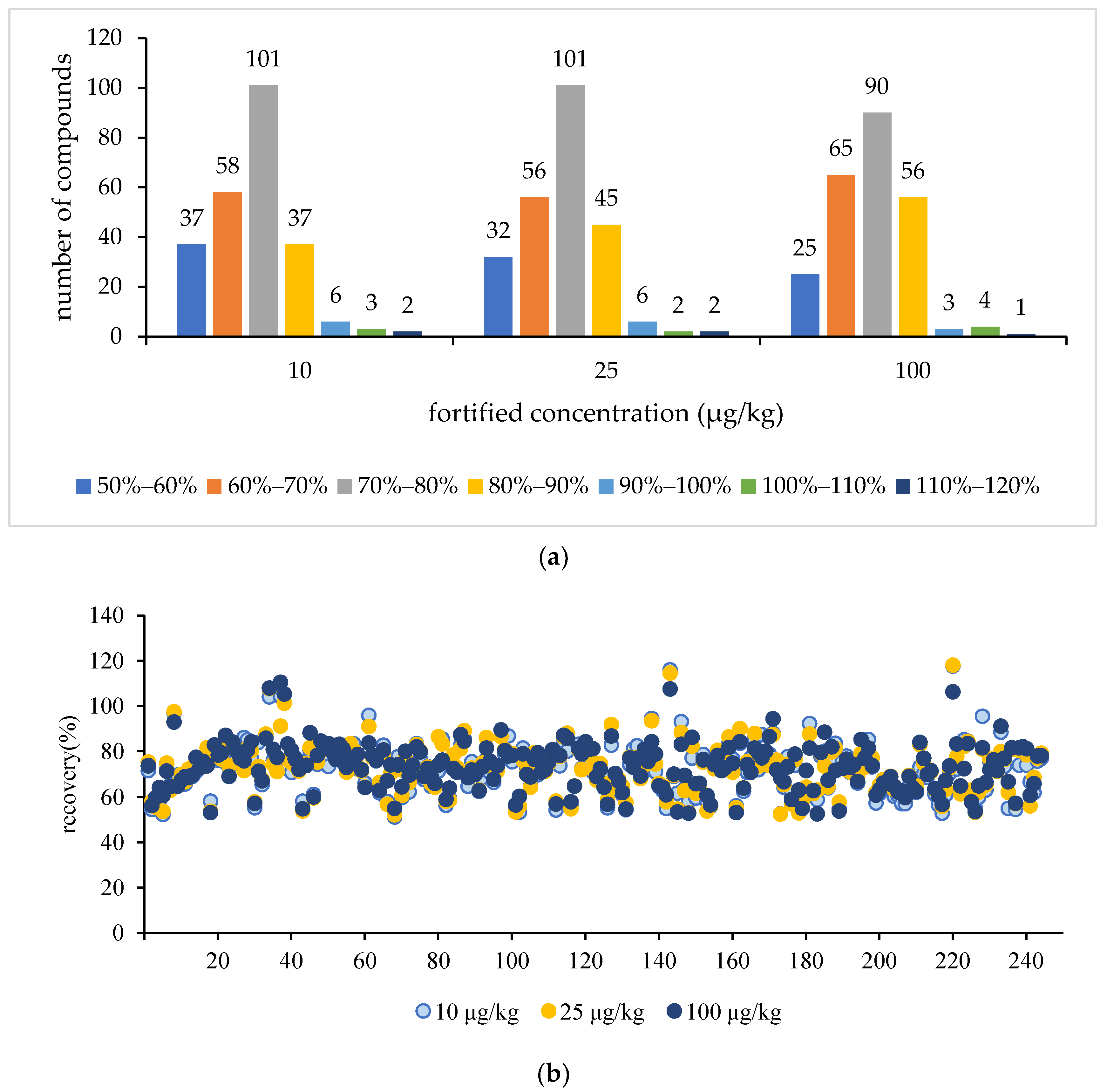
3.2.4. Accuracy and Precision
3.2.5. CCα and CCβ
3.3. Analysis of Real Egg Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food Chem. Toxicol. 2016, 88, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, A.E. Food Processing|Nutritional Influences. In Encyclopedia of Human Nutrition, 2nd ed.; Caballero, B., Ed.; Elsevier: Oxford, UK, 1998; pp. 894–899. [Google Scholar]
- Dasenaki, M.; Thomaidis, N.S. Multi-residue determination of 115 veterinary drugs and pharmaceutical residues in milk powder, butter, fish tissue and eggs using liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2015, 880, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, B.; Lehotay, S.J.; Mastovska, K.; Lightfield, A.R.; Furey, A.; Danaher, M. New method for the analysis of flukicide and other anthelmintic residues in bovine milk and liver using liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2009, 637, 196–207. [Google Scholar] [CrossRef]
- Moloney, M.; Clarke, L.; O’Mahony, J.; Gadaj, A.; O’Kennedy, R.; Danaher, M. Determination of 20 coccidiostats in egg and avian muscle tissue using ultra high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1253, 94–104. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Ma, W.; Guo, Z.; Li, X.; Song, S.; Tang, H.; Li, X.; Zhang, Q. Development of precise GC-EI-MS method to determine the residual fipronil and its metabolites in chicken egg. Food Chem. 2019, 281, 85–90. [Google Scholar] [CrossRef]
- World Health Organization. Tackling Antibiotic Resistance from a Food Safety Perspective in Europe. Tackling Antibiotic Resistance from a Food Safety Perspective in Europe; World Health Organization: Geneva, Switzerland, 2011; p. 88. [Google Scholar]
- Barlow, J. Antimicrobial Resistance and the Use of Antibiotics in the Dairy Industry: Facing Consumer Perceptions and Producer Realities. In Proceedings of the 29th Annual Western Canadian Dairy Seminar on Facing the Challenges of Modern Dairying, Red Deer, AB, Canada, 8–11 March 2011. [Google Scholar]
- Hoigné, R.; Schlumberger, H.P.; Vervloet, D.; Zoppi, M. Epidemiology of allergic drug reactions. Monogr. Allergy 1993, 31, 147–170. [Google Scholar]
- Bilandžić, N.; Božić, D.; Kolanović, B.S.; Varenina, I.; Cvetnić, L. Distribution of sulfamonomethoxine and trimethoprim in egg yolk and white. Food Chem. 2015, 178, 32–37. [Google Scholar] [CrossRef]
- Luo, P.; Liu, X.; Kong, F.; Chen, L.; Wang, Q.; Li, W.; Wen, S.; Tang, L.; Li, Y. Simultaneous determination of 169 veterinary drugs in chicken eggs with EMR-Lipid clean-up using ultra-high performance liquid chromatography tandem mass spectrometry. Anal. Methods 2019, 11, 1657–1662. [Google Scholar] [CrossRef]
- Regal, P.; Lamas, A.; Franco, C.M.; Cepeda, A. Veterinary Drugs: Progress in Multiresidue Technique. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 470–480. [Google Scholar]
- Jung, Y.S.; Kim, D.-B.; Nam, T.G.; Seo, D.; Yoo, M. Identification and quantification of multi-class veterinary drugs and their metabolites in beef using LC–MS/MS. Food Chem. 2022, 382, 132313. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Barceló, D. Multi-residue analytical methods using LC-tandem MS for the determination of pharmaceuticals in environmental and wastewater samples: A review. Anal. Bioanal. Chem. 2006, 386, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Bolck, Y.; Rutgers, P.; Stolker, A.; Nielen, M. Multi-residue screening of veterinary drugs in egg, fish and meat using high-resolution liquid chromatography accurate mass time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 8206–8216. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Thurman, E.M. Multi-residue method for the analysis of 101 pesticides and their degradates in food and water samples by liquid chromatography/time-of-flight mass spectrometry. J. Chromatogr. A 2007, 1175, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, E.; Oltramare, C.; de Alencastro, L.F. Development of a modified QuEChERS method for multi-class pesticide analysis in human hair by GC-MS and UPLC-MS/MS. Anal. Chim. Acta 2018, 999, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Szakas, T.; Churley, M.; Lucas, D. Multi-class multi-residue analysis of pesticides in edible oils by gas chromatography-tandem mass spectrometry using liquid-liquid extraction and enhanced matrix removal lipid cartridge cleanup. J. Chromatogr. A 2019, 1584, 1–12. [Google Scholar] [CrossRef]
- Jadhav, M.R.; Pudale, A.; Raut, P.; Utture, S.; Shabeer, T.A.; Banerjee, K. A unified approach for high-throughput quantitative analysis of the residues of multi-class veterinary drugs and pesticides in bovine milk using LC-MS/MS and GC–MS/MS. Food Chem. 2019, 272, 292–305. [Google Scholar] [CrossRef]
- York, J.L.; Magnuson, R.H.; Schug, K.A. On-line sample preparation for multiclass vitamin, hormone, and mycotoxin determination in chicken egg yolk using LC-MS/MS. Food Chem. 2020, 326, 126939. [Google Scholar] [CrossRef]
- Xu, J.; Xu, S.; Xiao, Y.; Chingin, K.; Lu, H.; Yan, R.; Chen, H. Quantitative Determination of Bulk Molecular Concentrations of β-Agonists in Pork Tissue Samples by Direct Internal Extractive Electrospray Ionization-Mass Spectrometry. Anal. Chem. 2017, 89, 11252–11258. [Google Scholar] [CrossRef]
- Mastrianni, K.R.; Metavarayuth, K.; Brewer, W.E.; Wang, Q. Analysis of 10 β-agonists in pork meat using automated dispersive pipette extraction and LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1084, 64–68. [Google Scholar] [CrossRef]
- Zhang, M.; Bian, K.; Zhou, T.; Song, X.; Liu, Q.; Meng, C.; He, L. Determination of residual fipronil in chicken egg and muscle by LC–MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1014, 31–36. [Google Scholar] [CrossRef]
- Shendy, A.H.; Al-Ghobashy, M.A.; Alla, S.A.G.; Lotfy, H.M. Development and validation of a modified QuEChERS protocol coupled to LC–MS/MS for simultaneous determination of multi-class antibiotic residues in honey. Food Chem. 2016, 190, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, M.W.; Bargańska, Ż.; Marciniak, K.; Miedzianowska, E.; Kujawski, J.K.; Ślebioda, M.; Namieśnik, J. Determining pesticide contamination in honey by LC-ESI-MS/MS—Comparison of pesticide recoveries of two liquid–liquid extraction based approaches. LWT 2014, 56, 517–523. [Google Scholar] [CrossRef]
- Guidi, L.R.; Santos, F.A.; Ribeiro, A.C.S.R.; Fernandes, C.; da Silva, L.H.M.; Gloria, M.B.A. Quinolones and tetracyclines in aquaculture fish by a simple and rapid LC-MS/MS method. Food Chem. 2018, 245, 1232–1238. [Google Scholar] [CrossRef]
- Venkatesh, P.; Kumar, N.A.; Prasad, R.H.; Krishnamoorthy, K.; Prasath, K.H.; Soumya, V. LC–MS/MS analysis of tetracycline antibiotics in prawns (Penaeus monodon) from south India coastal region. J. Pharm. Res. 2013, 6, 48–52. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Zhao, S.; Zhang, J.; Qi, K.; Du, Z.; Shao, B. Determination of fipronil and its metabolites in chicken egg, muscle and cake by a modified QuEChERS method coupled with LC-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1543–1552. [Google Scholar] [CrossRef]
- Song, N.-E.; Lee, J.Y.; Mansur, A.R.; Jang, H.W.; Lim, M.-C.; Lee, Y.; Yoo, M.; Nam, T.G. Determination of 60 pesticides in hen eggs using the QuEChERS procedure followed by LC-MS/MS and GC-MS/MS. Food Chem. 2019, 298, 125050. [Google Scholar] [CrossRef]
- Lozano, A.; Rajski, Ł.; Uclés, S.; Belmonte-Valles, N.; Mezcua, M.; Fernández-Alba, A.R. Evaluation of zirconium dioxide-based sorbents to decrease the matrix effect in avocado and almond multiresidue pesticide analysis followed by gas chromatography tandem mass spectrometry. Talanta 2014, 118, 68–83. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Lehotay, S.J. Multi-class, multi-residue analysis of pesticides, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, polybrominated diphenyl ethers and novel flame retardants in fish using fast, low-pressure gas chromatography–tandem mass spectrometry. Anal. Chim. Acta 2013, 758, 80–92. [Google Scholar] [CrossRef]
- Rejczak, T.; Tuzimski, T. Recent Trends in Sample Preparation and Liquid Chromatography/Mass Spectrometry for Pesticide Residue Analysis in Food and Related Matrixes. J. AOAC Int. 2015, 98, 1143–1162. [Google Scholar] [CrossRef]
- Zhao, L.; Lucas, D.; Long, D.; Richter, B.; Stevens, J. Multi-class multi-residue analysis of veterinary drugs in meat using enhanced matrix removal lipid cleanup and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2018, 1549, 14–24. [Google Scholar] [CrossRef] [PubMed]
- SANTE. Eueopean Union Guidance Document on Analytical Quality Control and Validation Procedures for Pesticide Residues Analysis in Food and Feed; Document No. SANTE/11945/2015. Available online: https://www.eurl-pesticides.eu/library/docs/allcrl/AqcGuidance_SANTE_2015_11945.pdf (accessed on 14 March 2022).
- European Commission. Commission Decision 2002/657/EC of 12 August 2002; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- Choi, S.; Kim, S.; Shin, J.Y.; Kim, M.; Kim, J.-H. Development and verification for analysis of pesticides in eggs and egg products using QuEChERS and LC–MS/MS. Food Chem. 2015, 173, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, J.-J.; Cong, J.-M.; Cai, Z.-X.; Zhang, J.-S.; Wang, J.-L.; Ren, Y.-P. Optimization for quick, easy, cheap, effective, rugged and safe extraction of mycotoxins and veterinary drugs by response surface methodology for application to egg and milk. J. Chromatogr. A 2018, 1532, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, A.; Fan, K.; Minh Tien, M.; Savoy, M.-C.; Tarres, A.; Fuger, D.; Bessaire, T.; Mottier, P. Determination of 105 antibiotic, anti-inflammatory, antiparasitic agents and tranquilizers by LC-MS/MS based on an acidic QuEChERS-like extraction. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.; Reyes, R.C.; Romero-González, R.; Frenich, A.G.; Vidal, J.L.M. Development and validation of a multiclass method for the determination of veterinary drug residues in chicken by ultra high performance liquid chromatography–tandem mass spectrometry. Talanta 2012, 89, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, Y.; Jia, Q.; Zhang, L.; Zhang, W.; Mu, P.; Jia, Y.; Qian, Y.; Qiu, J. Simultaneous determination of 58 pesticides and relevant metabolites in eggs with a multi-functional filter by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1593, 81–90. [Google Scholar] [CrossRef]
- Freitas, A.; Barbosa, J.; Ramos, F. Multi-residue and multi-class method for the determination of antibiotics in bovine muscle by ultra-high-performance liquid chromatography tandem mass spectrometry. Meat Sci. 2014, 98, 58–64. [Google Scholar] [CrossRef]
- Song, S.; Zhu, K.; Han, L.; Sapozhnikova, Y.; Zhang, Z.; Yao, W. Residue Analysis of 60 Pesticides in Red Swamp Crayfish Using QuEChERS with High-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 5031–5038. [Google Scholar] [CrossRef]
- Rejczak, T.; Tuzimski, T. QuEChERS-based extraction with dispersive solid phase extraction clean-up using PSA and ZrO2-based sorbents for determination of pesticides in bovine milk samples by HPLC-DAD. Food Chem. 2017, 217, 225–233. [Google Scholar] [CrossRef]
- Asteggiante, L.G.; Lehotay, S.J.; Lightfield, A.R.; Dutko, T.; Ng, C.; Bluhm, L. Ruggedness testing and validation of a practical analytical method for >100 veterinary drug residues in bovine muscle by ultrahigh performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1258, 43–54. [Google Scholar] [CrossRef]
- Guo, C.; Wang, M.; Xiao, H.; Huai, B.; Wang, F.; Pan, G.; Liao, X.; Liu, Y. Development of a modified QuEChERS method for the determination of veterinary antibiotics in swine manure by liquid chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1027, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Bousova, K.; Senyuva, H.; Mittendorf, K. Quantitative multi-residue method for determination antibiotics in chicken meat using turbulent flow chromatography coupled to liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2013, 1274, 19–27. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Decision (EU) No 37/2010 of December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Bogialli, S.; D’Ascenzo, G.; Di Corcia, A.; Laganà, A.; Tramontana, G. Simple assay for monitoring seven quinolone antibacterials in eggs: Extraction with hot water and liquid chromatography coupled to tandem mass spectrometry: Laboratory validation in line with the European Union Commission Decision 657/2002/EC. J. Chromatogr. A 2009, 1216, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Hildmann, F.; Gottert, C.; Frenzel, T.; Kempe, G.; Speer, K. Pesticide residues in chicken eggs–A sample preparation methodology for analysis by gas and liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2015, 1403, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dubreil-Cheneau, E.; Bessiral, M.; Roudaut, B.; Verdon, E.; Sanders, P. Validation of a multi-residue liquid chromatography-tandem mass spectrometry confirmatory method for 10 anticoccidials in eggs according to Commission Decision 2002/657/EC. J. Chromatogr. A 2009, 1216, 8149–8157. [Google Scholar] [CrossRef]
- Galarini, R.; Fioroni, L.; Moretti, S.; Pettinacci, L.; Dusi, G. Development and validation of a multi-residue liquid chromatography-tandem mass spectrometry confirmatory method for eleven coccidiostats in eggs. Anal. Chim. Acta 2011, 700, 167–176. [Google Scholar] [CrossRef]
- Barreto, F.; Ribeiro, C.; Hoff, R.B.; Dalla Costa, T. A simple and high-throughput method for determination and confirmation of 14 coccidiostats in poultry muscle and eggs using liquid chromatography–quadrupole linear ion trap–tandem mass spectrometry (HPLC-QqLIT-MS/MS): Validation according to European Union 2002/657/EC. Talanta 2017, 168, 43–51. [Google Scholar]
- Chen, D.; Cao, X.; Tao, Y.; Wu, Q.; Pan, Y.; Peng, D.; Liu, Z.; Huang, L.; Wang, Y.; Wang, X.; Yuan, Z. Development of a liquid chromatography-tandem mass spectrometry with ultrasound-assisted extraction and auto solid-phase clean-up method for the determination of Fusarium toxins in animal derived foods. J. Chromatogr. A 2013, 1311, 21–29. [Google Scholar] [CrossRef]
- Chen, D.; Yu, J.; Tao, Y.; Pan, Y.; Xie, S.; Huang, L.; Peng, D.; Wang, X.; Wang, Y.; Liu, Z.; Yuan, Z. Qualitative screening of veterinary anti-microbial agents in tissues, milk, and eggs of food-producing animals using liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2016, 1017, 82–88. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, V.G.; Oliveira, M.S.; de Almeida, C.A.A.; Hoff, R.B.; Mallmann, C.A. Liquid Chromatography-Tandem Mass Spectrometry Determination and Depletion Profile of Chlortetracycline, Doxycycline, and Oxytetracycline in Broiler Chicken Muscle After Oral Administration. Food Anal. Methods 2018, 11, 2181–2194. [Google Scholar] [CrossRef]

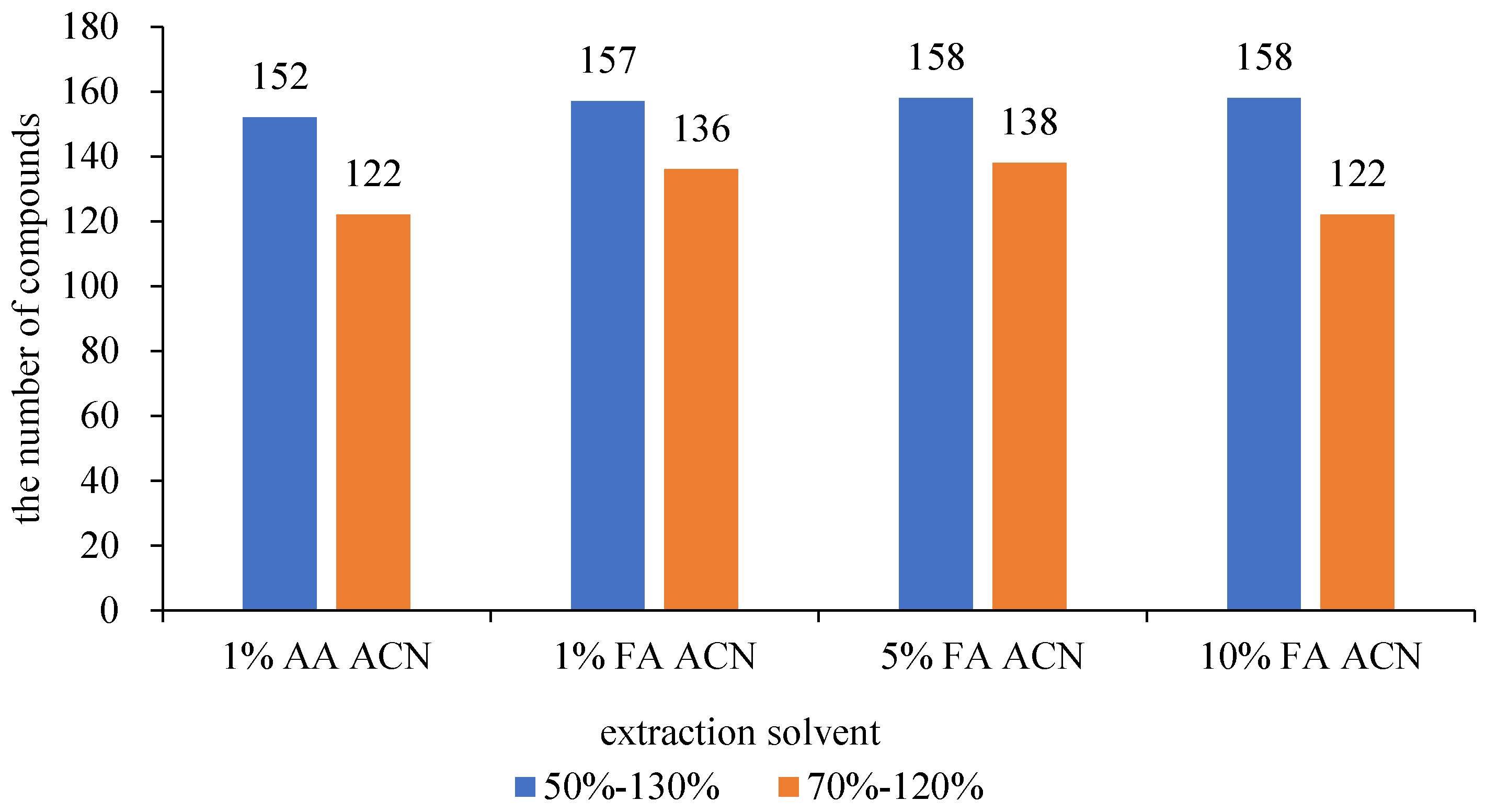

| Compound Name | Fortified Concentration (n = 6) | LOQ | LOD | CCα | CCβ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 μg kg−1 | 50 μg kg−1 | 100 μg kg−1 | |||||||||||
| Recovery | RSDr | RSDwR | Recovery | RSDr | RSDwR | Recovery | RSDr | RSDwR | |||||
| Unit | % | μg kg−1 | |||||||||||
| 2-Aminobenzimidazole | 71.8 | 3.7 | 3.8 | 75.5 | 5.5 | 6.0 | 73.9 | 2.1 | 3.0 | 5.0 | 2.0 | 11.2 | 17.4 |
| 2-Mercaptobenzimidazole | 54.7 | 9.8 | 11.7 | 58.2 | 9.4 | 11.5 | 56.4 | 7.0 | 10.4 | 5.0 | 2.0 | 16.1 | 27.2 |
| 2-Methyl-4 (5)-nitroimidazole | 56.9 | 4.0 | 6.7 | 55.6 | 5.5 | 6.1 | 59.8 | 3.8 | 4.0 | 2.0 | 1.0 | 5.1 | 8.2 |
| 2-Methyl-5-nitroimidazole | 58.8 | 1.6 | 1.7 | 61.0 | 2.2 | 2.4 | 64.4 | 1.5 | 1.6 | 0.5 | 0.2 | 12.7 | 24.9 |
| 3-Methoxytyramine (3-MT) | 52.5 | 1.9 | 2.0 | 53.8 | 2.7 | 3.0 | 60.8 | 1.4 | 1.9 | 0.5 | 0.2 | 11.4 | 22.4 |
| 4-Acetamido antipyrine | 72.3 | 2.5 | 3.2 | 75.0 | 4.0 | 6.4 | 71.5 | 2.3 | 2.4 | 0.5 | 0.2 | 15.5 | 30.5 |
| 4-Formylamino antipyrine | 65.5 | 2.1 | 2.4 | 63.1 | 4.1 | 4.3 | 64.7 | 1.6 | 1.8 | 0.5 | 0.2 | 4.5 | 8.6 |
| 4-Nitroimidazole | 97.0 | 2.0 | 2.5 | 97.6 | 4.7 | 5.9 | 93.1 | 6.0 | 8.6 | 0.5 | 0.2 | 22.9 | 45.3 |
| 4-Nitrophenol | 69.8 | 9.1 | 9.2 | 65.9 | 6.7 | 6.7 | 64.9 | 3.3 | 4.0 | 5.0 | 2.0 | 11.6 | 18.1 |
| 5-Hydroxymebendazole | 67.4 | 3.1 | 3.9 | 70.0 | 3.9 | 4.6 | 67.6 | 4.9 | 7.8 | 0.5 | 0.2 | 5.3 | 10.0 |
| 5-Hydroxy-thiabendazole | 65.9 | 1.5 | 1.8 | 66.9 | 2.3 | 2.6 | 69.1 | 1.9 | 2.4 | 0.5 | 0.2 | 12.2 | 23.9 |
| 5-Nitrobenzimidazole | 69.4 | 2.7 | 2.7 | 72.4 | 1.2 | 2.1 | 68.8 | 5.0 | 7.8 | 2.0 | 1.0 | 5.1 | 8.1 |
| Albendazole | 69.2 | 2.0 | 2.4 | 72.5 | 5.8 | 6.7 | 69.8 | 4.0 | 6.1 | 0.5 | 0.2 | 9.9 | 19.3 |
| Albendazole sulfone | 71.2 | 3.1 | 3.9 | 75.3 | 3.9 | 4.6 | 77.6 | 4.9 | 7.8 | 0.5 | 0.2 | 5.3 | 10.0 |
| Alclomethasone dipropionate | 73.0 | 3.7 | 4.1 | 73.5 | 3.6 | 4.8 | 73.0 | 3.8 | 4.3 | 0.5 | 0.2 | 18.7 | 36.9 |
| Amantadine | 76.2 | 2.0 | 2.5 | 75.6 | 3.9 | 5.3 | 75.7 | 3.8 | 4.0 | 0.5 | 0.2 | 14.7 | 28.9 |
| Amcinonide | 79.5 | 6.3 | 7.6 | 81.7 | 4.8 | 6.9 | 73.8 | 3.4 | 3.7 | 0.5 | 0.2 | 15.6 | 30.7 |
| Ampicillin | 58.4 | 8.5 | 11.1 | 53.8 | 10.2 | 11.2 | 53.3 | 7.6 | 9.3 | 5.0 | 2.0 | 12.9 | 20.9 |
| Antipyrine | 77.5 | 1.5 | 1.9 | 78.6 | 1.3 | 1.3 | 83.1 | 1.6 | 2.0 | 0.5 | 0.2 | 13.5 | 26.5 |
| Azaperol | 76.4 | 2.3 | 3.5 | 77.8 | 1.2 | 1.9 | 78.6 | 1.7 | 2.3 | 0.5 | 0.2 | 4.1 | 7.7 |
| Azaperone | 76.1 | 3.1 | 3.2 | 76.8 | 2.1 | 3.0 | 79.5 | 2.3 | 3.0 | 0.5 | 0.2 | 14.0 | 27.5 |
| Bambuterol | 86.8 | 1.9 | 2.2 | 84.4 | 1.9 | 2.7 | 87.2 | 1.4 | 1.8 | 0.5 | 0.2 | 8.1 | 15.6 |
| Beclomethasone | 73.4 | 4.5 | 6.1 | 73.9 | 3.5 | 4.0 | 69.2 | 3.3 | 6.2 | 1.0 | 0.5 | 14.7 | 28.3 |
| Beclomethasone dipropionate | 82.7 | 7.4 | 9.0 | 83.8 | 2.4 | 4.3 | 84.3 | 3.5 | 3.5 | 0.5 | 0.2 | 25.6 | 50.7 |
| Benzimidazole | 76.7 | 3.5 | 4.3 | 80.3 | 3.5 | 4.6 | 80.5 | 1.2 | 1.7 | 2.0 | 1.0 | 7.4 | 12.7 |
| Betamethasone | 76.8 | 3.4 | 3.8 | 75.7 | 3.7 | 3.9 | 77.1 | 2.6 | 2.7 | 0.5 | 0.2 | 18.7 | 36.8 |
| Betamethasone valerate | 86.2 | 5.7 | 7.4 | 71.8 | 6.6 | 9.7 | 76.1 | 4.4 | 4.9 | 0.5 | 0.2 | 4.4 | 8.3 |
| Betamethasone dipropionate | 85.3 | 2.8 | 3.1 | 81.5 | 4.0 | 4.4 | 80.7 | 3.1 | 4.0 | 0.5 | 0.2 | 16.2 | 31.9 |
| Bromchlorbuterol | 77.1 | 1.8 | 2.1 | 80.6 | 1.5 | 2.1 | 84.4 | 1.8 | 2.5 | 0.5 | 0.2 | 19.4 | 38.2 |
| Brompheniramine | 55.3 | 3.6 | 4.2 | 58.0 | 4.2 | 4.4 | 57.4 | 3.1 | 3.6 | 0.5 | 0.2 | 8.1 | 15.6 |
| Budesonide | 84.2 | 5.2 | 6.0 | 73.4 | 5.7 | 8.2 | 71.3 | 1.4 | 3.8 | 0.5 | 0.2 | 10.1 | 19.7 |
| Cambendazole | 65.7 | 2.6 | 3.1 | 68.1 | 2.7 | 2.8 | 67.3 | 5.0 | 7.5 | 0.5 | 0.2 | 5.9 | 11.3 |
| Carazolol | 86.3 | 1.7 | 1.8 | 87.8 | 2.7 | 3.3 | 86.0 | 1.1 | 1.6 | 0.5 | 0.2 | 3.3 | 6.1 |
| Carbadox | 104.2 | 4.0 | 5.9 | 107.8 | 4.2 | 6.0 | 108.1 | 2.5 | 3.1 | 1.0 | 0.5 | 12.5 | 24.1 |
| Carbamazepine | 76.1 | 1.1 | 1.3 | 74.6 | 2.6 | 2.8 | 81.1 | 1.3 | 1.8 | 0.5 | 0.2 | 4.9 | 9.3 |
| Carbofuran | 74.0 | 1.8 | 2.4 | 71.3 | 3.0 | 4.7 | 77.6 | 1.3 | 1.9 | 0.5 | 0.2 | 14.7 | 28.8 |
| Cefapirin | 104.4 | 7.0 | 7.4 | 91.3 | 11.9 | 12.4 | 110.7 | 3.3 | 4.7 | 2.0 | 1.0 | 14.6 | 27.2 |
| Cefotaxime | 101.6 | 6.7 | 8.1 | 101.4 | 7.1 | 9.0 | 105.4 | 1.9 | 3.1 | 5.0 | 2.0 | 15.9 | 26.8 |
| Ceftiofur | 72.8 | 5.2 | 5.7 | 76.6 | 6.7 | 7.2 | 83.4 | 10.0 | 12.4 | 2.0 | 1.0 | 8.7 | 15.3 |
| Chlordimeform | 70.9 | 4.5 | 4.9 | 75.3 | 3.4 | 4.4 | 80.5 | 3.7 | 5.5 | 2.0 | 1.0 | 7.7 | 13.4 |
| Chlormadinone acetate | 77.3 | 6.8 | 7.4 | 74.2 | 4.7 | 5.9 | 76.9 | 1.6 | 3.9 | 0.5 | 0.2 | 18.0 | 35.5 |
| Chloroprocaine | 72.1 | 2.0 | 2.0 | 71.6 | 2.3 | 2.8 | 72.4 | 1.3 | 1.4 | 0.5 | 0.2 | 13.9 | 27.4 |
| Chlorpheniramine | 58.3 | 2.6 | 3.2 | 53.9 | 4.5 | 5.8 | 55.0 | 1.5 | 2.8 | 0.5 | 0.2 | 7.9 | 15.2 |
| Chlorpromazine | 75.8 | 2.0 | 3.6 | 74.8 | 2.7 | 3.4 | 74.1 | 2.4 | 3.4 | 0.5 | 0.2 | 3.1 | 5.7 |
| Cinchocaine | 80.5 | 2.3 | 2.4 | 81.8 | 2.5 | 3.8 | 88.3 | 2.0 | 2.2 | 0.5 | 0.2 | 10.1 | 19.7 |
| Cinoxacin | 61.3 | 3.1 | 3.5 | 59.7 | 2.6 | 2.9 | 60.1 | 2.7 | 3.0 | 0.5 | 0.2 | 11.0 | 21.5 |
| Ciprofloxacin | 74.5 | 1.8 | 2.1 | 75.6 | 1.6 | 2.3 | 78.4 | 1.6 | 4.3 | 2.0 | 1.0 | 4.5 | 7.0 |
| Clenbuterol | 84.1 | 2.0 | 2.5 | 83.0 | 1.7 | 2.4 | 84.7 | 1.3 | 1.6 | 0.5 | 0.2 | 6.4 | 12.3 |
| Clenbuterol hydroxymethyl | 76.9 | 2.0 | 2.2 | 82.0 | 1.5 | 2.8 | 81.4 | 1.1 | 1.2 | 0.5 | 0.2 | 6.7 | 12.9 |
| Clencyclohexerol | 73.7 | 1.7 | 2.1 | 80.7 | 1.8 | 2.3 | 83.5 | 1.6 | 1.9 | 0.5 | 0.2 | 10.4 | 20.3 |
| Clenhexerol | 80.8 | 1.7 | 2.2 | 81.3 | 2.0 | 2.3 | 80.2 | 1.6 | 2.2 | 0.5 | 0.2 | 6.7 | 12.8 |
| Clenisopenterol | 76.8 | 2.6 | 3.2 | 77.9 | 2.5 | 3.3 | 76.7 | 1.8 | 2.0 | 0.5 | 0.2 | 5.2 | 9.8 |
| Clenpenterol | 78.9 | 3.9 | 4.6 | 83.1 | 1.1 | 2.6 | 83.3 | 1.2 | 1.9 | 0.5 | 0.2 | 8.6 | 16.6 |
| Clenproperol | 75.5 | 1.9 | 2.1 | 77.8 | 2.0 | 2.5 | 81.1 | 1.7 | 1.9 | 0.5 | 0.2 | 7.3 | 14.1 |
| Clindamycin | 72.1 | 4.1 | 4.9 | 71.2 | 3.5 | 4.3 | 73.2 | 1.1 | 1.2 | 0.5 | 0.2 | 7.8 | 15.1 |
| Clobetasol 17-propionate | 79.1 | 4.8 | 5.5 | 83.6 | 3.3 | 3.9 | 77.2 | 3.9 | 5.2 | 0.5 | 0.2 | 19.1 | 37.6 |
| Clobetasone 17-butyrate | 83.4 | 5.0 | 5.6 | 80.6 | 2.7 | 3.2 | 76.0 | 4.6 | 5.5 | 0.5 | 0.2 | 23.1 | 45.7 |
| Clopidol | 72.5 | 1.1 | 2.7 | 73.5 | 1.2 | 2.7 | 78.8 | 1.8 | 1.9 | 0.5 | 0.2 | 9.6 | 18.8 |
| Clorprenaline | 71.7 | 2.7 | 2.7 | 71.6 | 2.1 | 3.0 | 72.1 | 2.4 | 2.4 | 0.5 | 0.2 | 7.5 | 14.5 |
| Cortisone | 66.3 | 8.6 | 9.0 | 64.5 | 3.2 | 4.4 | 64.4 | 2.1 | 2.3 | 0.5 | 0.2 | 6.1 | 11.8 |
| Coumaphos | 96.1 | 1.6 | 1.7 | 91.2 | 4.5 | 6.9 | 83.9 | 4.3 | 5.8 | 0.5 | 0.2 | 7.0 | 13.5 |
| Cyproheptadine | 77.6 | 1.7 | 2.4 | 77.6 | 2.9 | 3.0 | 78.4 | 2.9 | 3.9 | 0.5 | 0.2 | 10.7 | 21.0 |
| Danofloxacin | 76.2 | 2.8 | 2.9 | 77.9 | 2.5 | 2.7 | 76.3 | 1.6 | 1.7 | 0.5 | 0.2 | 4.1 | 7.7 |
| Dapsone | 61.9 | 1.6 | 1.7 | 66.6 | 2.0 | 2.1 | 63.1 | 1.8 | 2.2 | 0.5 | 0.2 | 8.5 | 16.5 |
| Deflazacort | 82.9 | 3.9 | 4.8 | 79.0 | 2.8 | 3.6 | 80.7 | 4.7 | 5.3 | 0.5 | 0.2 | 25.1 | 49.7 |
| Demeclocycline | 58.2 | 5.8 | 6.1 | 56.9 | 9.7 | 10.3 | 67.3 | 7.3 | 9.1 | 5.0 | 2.0 | 18.6 | 32.2 |
| Desloratadine | 74.7 | 4.3 | 6.9 | 70.7 | 7.2 | 9.4 | 74.3 | 4.0 | 5.7 | 2.0 | 1.0 | 7.1 | 12.3 |
| Desoxycarbadox | 51.3 | 3.3 | 4.1 | 52.3 | 3.0 | 4.6 | 55.0 | 3.7 | 4.9 | 0.5 | 0.2 | 5.4 | 10.2 |
| Dexamethasone | 77.8 | 5.4 | 5.7 | 72.3 | 4.6 | 6.7 | 74.3 | 3.3 | 3.9 | 0.5 | 0.2 | 18.0 | 35.4 |
| Dichlorvos | 59.9 | 4.1 | 4.7 | 60.9 | 5.9 | 7.6 | 64.6 | 1.6 | 1.6 | 5.0 | 2.0 | 10.4 | 15.7 |
| Diflorasone diacetate | 71.5 | 5.3 | 6.3 | 77.0 | 6.0 | 8.5 | 80.2 | 4.4 | 5.5 | 0.5 | 0.2 | 6.7 | 13.0 |
| Difloxacin | 62.4 | 3.9 | 4.0 | 66.6 | 3.6 | 3.7 | 69.9 | 4.9 | 7.1 | 0.5 | 0.2 | 16.5 | 32.5 |
| Dimetridazole | 71.4 | 2.8 | 3.2 | 71.4 | 3.0 | 4.4 | 74.1 | 2.2 | 2.5 | 0.5 | 0.2 | 27.3 | 54.1 |
| Diphenhydramine | 83.6 | 1.0 | 1.7 | 83.1 | 2.6 | 2.9 | 82.2 | 1.3 | 1.6 | 0.5 | 0.2 | 4.4 | 8.3 |
| Doxepin | 76.8 | 5.3 | 6.5 | 80.2 | 3.8 | 4.4 | 79.9 | 7.5 | 11.1 | 2.0 | 0.2 | 10.2 | 18.4 |
| Econazole | 67.8 | 4.9 | 6.0 | 69.9 | 3.9 | 4.2 | 69.2 | 5.2 | 8.1 | 0.5 | 0.2 | 12.1 | 23.6 |
| Enoxacin | 73.5 | 1.6 | 1.6 | 73.2 | 3.7 | 5.6 | 72.7 | 1.4 | 1.9 | 5.0 | 2.0 | 9.0 | 13.1 |
| Enrofloxacin | 64.8 | 1.5 | 1.7 | 66.1 | 2.2 | 2.8 | 69.2 | 4.6 | 7.0 | 0.5 | 0.2 | 16.3 | 32.1 |
| Epitestosterone | 65.1 | 3.1 | 3.6 | 64.5 | 2.6 | 2.6 | 66.2 | 3.9 | 4.3 | 0.5 | 0.2 | 12.6 | 24.7 |
| Eprinomectin | 71.6 | 6.1 | 7.5 | 86.6 | 11.8 | 12.5 | 74.2 | 6.2 | 8.1 | 2.0 | 1.0 | 10.7 | 19.5 |
| Febantel | 85.7 | 1.3 | 1.7 | 83.5 | 3.7 | 5.1 | 76.3 | 4.2 | 5.7 | 0.5 | 0.2 | 4.5 | 8.5 |
| Fenbendazole | 56.4 | 2.9 | 4.2 | 58.4 | 4.3 | 6.3 | 59.2 | 3.6 | 4.0 | 0.5 | 0.2 | 8.3 | 16.0 |
| Fenoterol | 59.6 | 1.6 | 1.8 | 58.7 | 2.6 | 2.9 | 64.1 | 2.9 | 3.2 | 0.5 | 0.2 | 4.0 | 7.5 |
| Fenthion sulfoxide | 74.0 | 2.0 | 3.5 | 78.6 | 5.4 | 8.2 | 72.6 | 2.2 | 2.5 | 0.5 | 0.2 | 10.4 | 20.2 |
| Fleroxacin | 71.1 | 2.0 | 2.2 | 73.1 | 2.0 | 2.3 | 71.3 | 1.3 | 1.6 | 0.5 | 0.2 | 5.1 | 9.7 |
| Florfenicol | 82.9 | 7.8 | 10.4 | 81.3 | 14.2 | 15.0 | 87.5 | 10.0 | 10.1 | 5.0 | 2.0 | 20.2 | 35.4 |
| Flubendazole | 85.7 | 2.0 | 2.6 | 89.3 | 4.7 | 5.7 | 84.6 | 5.5 | 6.8 | 2.0 | 0.2 | 403.6 | 407.2 |
| Fluconazole | 64.9 | 3.9 | 4.7 | 69.3 | 6.1 | 6.7 | 68.7 | 6.2 | 7.1 | 2.0 | 0.2 | 3.0 | 4.0 |
| Fludrocortisone 21-acetate | 75.6 | 9.0 | 9.3 | 72.7 | 10.3 | 10.6 | 72.0 | 7.3 | 8.6 | 5.0 | 2.0 | 16.2 | 27.3 |
| Fludroxycortide | 70.4 | 8.7 | 10.1 | 71.0 | 4.9 | 6.1 | 70.2 | 2.5 | 3.0 | 2.0 | 1.0 | 16.9 | 31.8 |
| Flumequine | 62.6 | 2.8 | 3.3 | 63.2 | 2.3 | 2.4 | 63.0 | 2.5 | 3.3 | 0.5 | 0.2 | 4.9 | 9.2 |
| Flumethasone | 68.7 | 10.5 | 12.7 | 75.1 | 6.2 | 9.6 | 73.4 | 4.9 | 5.9 | 0.5 | 0.2 | 14.2 | 27.9 |
| Flumethasone pivalate | 76.7 | 4.9 | 5.7 | 86.2 | 4.3 | 5.2 | 81.6 | 4.0 | 4.5 | 0.5 | 0.2 | 9.4 | 18.3 |
| Flunixin | 71.0 | 1.3 | 1.4 | 74.5 | 3.2 | 4.0 | 75.8 | 4.4 | 5.4 | 2.0 | 1.0 | 3.6 | 5.2 |
| Fluocinolone acetonide | 66.6 | 5.4 | 6.8 | 73.1 | 4.2 | 5.7 | 68.0 | 6.7 | 8.5 | 1.0 | 0.5 | 8.8 | 16.6 |
| Fluoromethalone | 73.3 | 2.6 | 4.8 | 71.7 | 8.4 | 12.2 | 73.9 | 4.6 | 7.8 | 2.0 | 1.0 | 5.1 | 8.1 |
| Fluoxetine | 86.9 | 2.5 | 3.0 | 86.6 | 2.1 | 2.9 | 89.6 | 1.5 | 2.0 | 0.5 | 0.2 | 9.5 | 18.5 |
| Fluphenazine | 78.6 | 4.4 | 4.9 | 80.9 | 2.4 | 3.0 | 80.5 | 3.1 | 4.0 | 0.5 | 0.2 | 11.2 | 21.8 |
| Fluticasone propionate | 87.0 | 3.7 | 4.9 | 79.4 | 2.4 | 3.5 | 78.1 | 5.2 | 5.7 | 0.5 | 0.2 | 19.4 | 38.3 |
| Formoterol | 75.7 | 1.1 | 1.1 | 79.2 | 3.0 | 3.2 | 78.4 | 1.1 | 1.1 | 0.5 | 0.2 | 9.3 | 18.2 |
| Gatifloxacin | 56.0 | 3.5 | 4.3 | 53.6 | 3.2 | 3.6 | 56.6 | 2.4 | 3.2 | 0.5 | 0.2 | 4.5 | 8.6 |
| Gemifioxacin | 53.4 | 4.7 | 7.8 | 56.1 | 3.8 | 4.0 | 60.4 | 2.9 | 4.7 | 2.0 | 1.0 | 6.0 | 10.0 |
| Griseofulvin | 81.6 | 2.8 | 3.0 | 76.3 | 2.2 | 2.4 | 79.1 | 2.2 | 2.5 | 0.5 | 0.2 | 13.9 | 27.3 |
| Halcinonide | 73.3 | 9.8 | 10.5 | 70.8 | 4.7 | 6.2 | 70.1 | 3.7 | 4.7 | 0.5 | 0.2 | 9.4 | 18.2 |
| Halofuginone | 64.9 | 3.9 | 4.6 | 64.6 | 3.7 | 4.0 | 69.0 | 1.2 | 1.4 | 0.5 | 0.2 | 10.8 | 21.2 |
| Haloperidol | 77.7 | 1.2 | 1.2 | 79.5 | 2.6 | 2.7 | 76.8 | 1.5 | 2.2 | 0.5 | 0.2 | 6.9 | 13.4 |
| 2-Hydroxymethyl-1-methyl-5-nitroimidazole (HMMNI) | 70.0 | 1.9 | 2.3 | 72.8 | 4.0 | 5.1 | 79.6 | 2.0 | 3.7 | 2.0 | 0.2 | 3.0 | 4.1 |
| Hydrocortisone | 72.2 | 4.5 | 5.3 | 71.3 | 3.3 | 3.7 | 71.1 | 3.3 | 3.7 | 2.0 | 1.0 | 7.3 | 12.6 |
| Hydroxy-ipronidazole | 72.6 | 2.5 | 3.5 | 71.5 | 2.2 | 3.4 | 72.2 | 4.8 | 7.4 | 2.0 | 0.2 | 4.9 | 7.7 |
| Hydroxyzine | 75.3 | 3.2 | 4.5 | 75.0 | 2.5 | 2.9 | 75.6 | 1.7 | 2.4 | 0.5 | 0.2 | 9.2 | 18.0 |
| Imipramine | 77.4 | 2.7 | 3.0 | 80.0 | 2.1 | 3.8 | 80.9 | 2.1 | 2.6 | 0.5 | 0.2 | 5.9 | 11.2 |
| Indoprofen | 54.5 | 2.0 | 2.5 | 58.3 | 8.7 | 10.7 | 57.0 | 2.9 | 3.2 | 0.5 | 0.2 | 10.8 | 21.2 |
| Ipronidazole | 73.9 | 2.7 | 2.8 | 77.9 | 2.7 | 3.8 | 78.4 | 1.2 | 1.6 | 0.5 | 0.2 | 10.4 | 20.4 |
| Isoxsuprine | 84.4 | 1.2 | 1.3 | 86.5 | 1.8 | 2.8 | 87.3 | 1.5 | 1.5 | 0.5 | 0.2 | 7.6 | 14.8 |
| Ivermectin | 80.2 | 9.4 | 10.3 | 88.3 | 5.3 | 6.7 | 85.9 | 2.1 | 2.4 | 0.5 | 0.2 | 22.9 | 45.3 |
| Ketoconazole | 56.4 | 2.4 | 3.1 | 55.0 | 5.6 | 6.7 | 58.0 | 4.4 | 6.5 | 2.0 | 0.2 | 4.2 | 6.4 |
| Ketoprofen | 65.0 | 6.6 | 7.4 | 65.1 | 2.5 | 3.1 | 65.0 | 3.8 | 4.1 | 0.5 | 0.2 | 9.3 | 18.1 |
| Ketotifen | 83.3 | 2.2 | 2.4 | 81.2 | 2.7 | 3.0 | 81.6 | 1.1 | 1.8 | 0.5 | 0.2 | 8.4 | 16.3 |
| Labetalol | 72.6 | 2.0 | 4.0 | 72.2 | 3.2 | 3.3 | 79.1 | 2.1 | 3.1 | 0.5 | 0.2 | 7.8 | 15.1 |
| Levamisole | 78.4 | 2.8 | 2.9 | 82.9 | 1.7 | 2.6 | 84.4 | 1.9 | 2.3 | 0.5 | 0.2 | 5.7 | 11.0 |
| Lidocaine/Diocaine | 77.0 | 1.7 | 1.8 | 78.6 | 1.8 | 2.6 | 81.0 | 1.7 | 2.1 | 0.5 | 0.2 | 5.4 | 10.3 |
| Lincomycin | 71.3 | 1.8 | 2.4 | 72.5 | 6.4 | 8.4 | 81.3 | 10.6 | 13.1 | 0.5 | 0.2 | 55.1 | 60.2 |
| Invisible malachite green (LMG) | 69.8 | 1.8 | 2.1 | 67.5 | 4.5 | 5.9 | 69.1 | 5.6 | 7.8 | 2.0 | 0.2 | 4.3 | 6.7 |
| Lomefloxacin | 72.0 | 2.9 | 3.1 | 74.8 | 3.2 | 4.1 | 72.4 | 1.6 | 1.6 | 0.5 | 0.2 | 5.3 | 10.0 |
| Loratadine | 68.7 | 1.5 | 2.2 | 66.2 | 1.4 | 3.0 | 64.5 | 4.5 | 4.9 | 0.5 | 0.2 | 10.4 | 20.3 |
| Lornoxicam | 55.4 | 4.3 | 4.5 | 58.9 | 7.0 | 7.3 | 57.0 | 4.0 | 5.3 | 2.0 | 0.2 | 5.9 | 9.8 |
| Maduramycin | 82.9 | 11.9 | 11.9 | 92.0 | 4.7 | 7.1 | 86.9 | 3.3 | 3.5 | 0.5 | 0.2 | 21.9 | 43.4 |
| Marbofloxacin | 63.3 | 2.5 | 3.8 | 64.6 | 4.0 | 5.7 | 70.6 | 3.6 | 5.1 | 0.5 | 0.2 | 11.1 | 21.8 |
| Mebendazole | 64.7 | 2.8 | 3.1 | 68.1 | 5.1 | 6.3 | 67.1 | 5.1 | 7.7 | 2.0 | 0.2 | 5.0 | 7.9 |
| Mebendazole-amine (HMEB) | 61.3 | 1.7 | 2.4 | 64.8 | 5.2 | 7.1 | 62.1 | 4.0 | 6.0 | 2.0 | 0.2 | 4.4 | 6.8 |
| Mefenamic acid | 54.5 | 1.5 | 1.7 | 57.6 | 5.5 | 8.2 | 54.8 | 3.9 | 4.7 | 1.0 | 0.5 | 9.9 | 18.8 |
| Megestrol acetate | 74.4 | 3.2 | 3.6 | 77.6 | 2.0 | 2.9 | 77.3 | 5.8 | 6.8 | 0.5 | 0.2 | 20.6 | 40.6 |
| Melengestrol acetate | 81.2 | 3.2 | 3.7 | 75.4 | 2.3 | 2.8 | 73.3 | 4.9 | 5.2 | 0.5 | 0.2 | 15.0 | 29.6 |
| Melitracene | 82.6 | 1.5 | 2.9 | 77.5 | 3.4 | 3.6 | 77.2 | 2.8 | 3.7 | 0.5 | 0.2 | 11.2 | 22.0 |
| Meloxicam | 68.5 | 2.8 | 3.1 | 68.2 | 4.6 | 7.0 | 69.4 | 3.0 | 5.5 | 2.0 | 0.2 | 5.7 | 9.3 |
| Metaproterenol | 71.4 | 7.2 | 8.9 | 74.3 | 2.1 | 3.7 | 81.0 | 2.3 | 2.7 | 0.5 | 0.2 | 17.8 | 35.2 |
| Methylprednisolone | 74.6 | 3.8 | 4.6 | 76.6 | 4.3 | 5.1 | 75.9 | 3.1 | 4.0 | 0.5 | 0.2 | 25.6 | 50.7 |
| Methylprednisolone 21-acetate | 94.7 | 5.1 | 5.3 | 93.7 | 6.0 | 6.6 | 84.5 | 5.4 | 7.1 | 0.5 | 0.2 | 6.2 | 11.8 |
| Methyltestosterone | 71.3 | 4.0 | 4.2 | 74.8 | 4.9 | 7.7 | 78.9 | 6.2 | 7.1 | 0.5 | 0.2 | 13.4 | 26.2 |
| Metronidazole | 64.9 | 2.2 | 2.8 | 66.2 | 2.1 | 2.7 | 65.1 | 6.0 | 9.3 | 0.5 | 0.2 | 16.4 | 32.4 |
| Miconazole | 62.9 | 1.1 | 1.2 | 64.8 | 2.8 | 4.2 | 64.4 | 4.1 | 6.3 | 0.5 | 0.2 | 7.5 | 14.5 |
| Hydroxy metronidazole (MNZOH) | 55.2 | 4.2 | 4.7 | 58.1 | 2.5 | 2.7 | 61.2 | 5.7 | 8.8 | 2.0 | 1.0 | 6.1 | 10.3 |
| Mometasone furoate | 116.1 | 6.9 | 8.2 | 114.7 | 1.8 | 1.9 | 107.7 | 6.7 | 7.1 | 0.5 | 0.2 | 20.3 | 40.1 |
| Monensin | 69.3 | 1.7 | 1.8 | 69.7 | 5.1 | 7.7 | 70.3 | 1.8 | 1.8 | 0.5 | 0.2 | 2.7 | 5.0 |
| Moxifloxacin | 61.9 | 1.9 | 4.3 | 54.9 | 2.9 | 3.4 | 53.6 | 1.2 | 1.8 | 2.0 | 0.5 | 13.5 | 25.1 |
| Nabumetone | 93.3 | 2.9 | 3.8 | 88.5 | 3.4 | 3.7 | 83.4 | 5.4 | 6.3 | 0.5 | 0.2 | 9.0 | 17.6 |
| N-Acetyl dapson | 63.8 | 8.9 | 10.7 | 63.0 | 3.5 | 4.8 | 69.7 | 4.9 | 5.4 | 0.5 | 0.2 | 12.4 | 24.2 |
| Nadifloxacin | 58.2 | 3.4 | 3.9 | 53.2 | 6.2 | 7.2 | 52.9 | 4.0 | 5.4 | 0.5 | 0.2 | 17.0 | 33.4 |
| Nafcillin | 77.3 | 7.2 | 9.4 | 82.5 | 13.1 | 13.5 | 86.3 | 9.9 | 11.2 | 5.0 | 2.0 | 11.3 | 17.5 |
| Naftifine | 59.7 | 6.7 | 9.1 | 61.8 | 11.4 | 13.1 | 66.0 | 5.6 | 7.1 | 2.0 | 1.0 | 13.5 | 25.1 |
| Nalidixic acid | 65.7 | 1.3 | 2.4 | 64.1 | 3.0 | 3.0 | 66.1 | 1.6 | 3.2 | 0.5 | 0.2 | 14.7 | 28.9 |
| Nandrolone | 78.8 | 3.6 | 3.8 | 75.6 | 4.0 | 4.5 | 76.6 | 4.8 | 5.8 | 0.5 | 0.2 | 11.8 | 23.2 |
| Naproxen | 58.1 | 5.7 | 6.5 | 54.0 | 4.1 | 5.2 | 60.8 | 4.7 | 5.2 | 5.0 | 2.0 | 9.2 | 13.4 |
| Nequinate | 55.9 | 2.8 | 3.1 | 56.4 | 4.3 | 6.3 | 56.6 | 3.8 | 4.1 | 0.5 | 0.2 | 21.3 | 42.1 |
| Nigericin | 75.8 | 1.8 | 2.1 | 72.6 | 2.2 | 3.0 | 74.5 | 1.8 | 2.3 | 0.5 | 0.2 | 7.2 | 13.9 |
| Nimorazole | 78.7 | 9.2 | 10.2 | 80.6 | 10.1 | 11.7 | 78.4 | 7.2 | 8.2 | 2.0 | 0.2 | 13.4 | 24.9 |
| Norfloxacin | 74.0 | 1.6 | 2.2 | 72.2 | 1.6 | 1.7 | 71.8 | 5.2 | 7.4 | 0.5 | 0.2 | 10.8 | 21.2 |
| Ofloxacin | 72.7 | 1.4 | 1.7 | 76.2 | 2.4 | 2.6 | 73.1 | 5.1 | 7.7 | 0.5 | 0.2 | 15.4 | 30.3 |
| Oleandomycin | 83.6 | 1.2 | 1.8 | 86.5 | 4.1 | 5.4 | 81.9 | 1.1 | 1.2 | 0.5 | 0.2 | 13.7 | 26.9 |
| Orbifloxacin | 76.9 | 3.0 | 3.1 | 71.1 | 3.8 | 4.4 | 75.1 | 3.1 | 4.1 | 0.5 | 0.2 | 7.1 | 13.8 |
| Oxaprozin | 56.2 | 2.7 | 3.3 | 55.4 | 3.1 | 3.1 | 53.2 | 3.9 | 4.8 | 0.5 | 0.2 | 18.6 | 36.7 |
| Oxfendazole | 83.8 | 6.4 | 7.2 | 90.1 | 9.0 | 9.6 | 84.4 | 7.1 | 8.6 | 0.5 | 0.2 | 11.9 | 23.3 |
| Oxibendazole | 62.6 | 1.6 | 2.5 | 64.2 | 3.4 | 4.0 | 64.1 | 5.1 | 5.7 | 0.5 | 0.2 | 4.3 | 8.1 |
| Oxolinic acid | 71.0 | 2.0 | 3.7 | 75.5 | 6.8 | 8.8 | 74.3 | 11.5 | 14.2 | 2.0 | 1.0 | 2.4 | 2.8 |
| Oxytetracycline (OTC) | 77.3 | 4.7 | 5.1 | 75.6 | 1.8 | 2.4 | 71.0 | 2.9 | 4.6 | 5.0 | 2.0 | 202.7 | 205.5 |
| Paracetamol | 85.3 | 2.1 | 2.4 | 88.1 | 2.2 | 2.3 | 81.7 | 1.1 | 1.4 | 0.5 | 0.2 | 5.5 | 10.6 |
| Pefloxacin | 72.2 | 2.4 | 2.5 | 73.7 | 5.9 | 7.2 | 79.5 | 5.2 | 7.0 | 0.5 | 0.2 | 16.9 | 33.2 |
| Penbutolol | 87.6 | 1.4 | 2.8 | 79.8 | 4.2 | 6.2 | 77.3 | 2.1 | 2.4 | 0.5 | 0.2 | 10.4 | 20.2 |
| Phenacetin | 79.8 | 1.5 | 1.8 | 78.5 | 2.3 | 2.5 | 80.3 | 1.9 | 2.0 | 2.0 | 0.2 | 2.5 | 3.0 |
| Phenylbutazone | 79.0 | 6.1 | 9.0 | 74.1 | 8.2 | 9.7 | 86.6 | 5.8 | 6.2 | 0.5 | 0.2 | 8.7 | 16.8 |
| Phenylethanolamine A | 88.2 | 1.6 | 1.9 | 87.5 | 2.0 | 2.6 | 94.5 | 2.1 | 2.2 | 0.5 | 0.2 | 1.9 | 3.3 |
| Pipemidic acid | 75.3 | 4.1 | 4.8 | 76.3 | 2.4 | 3.6 | 72.1 | 2.7 | 3.4 | 2.0 | 1.0 | 6.3 | 10.5 |
| Pirbuterol acetate | 52.7 | 3.3 | 3.8 | 52.5 | 4.9 | 5.2 | 68.6 | 5.1 | 7.7 | 5.0 | 0.2 | 8.8 | 12.6 |
| Piroxicam | 64.5 | 5.6 | 8.3 | 65.7 | 4.6 | 5.3 | 66.9 | 4.6 | 7.5 | 2.0 | 0.2 | 10.7 | 19.4 |
| Prednicarbate | 77.9 | 4.0 | 4.4 | 74.4 | 4.8 | 5.1 | 73.5 | 3.9 | 4.3 | 0.5 | 0.2 | 9.8 | 19.1 |
| Prednisolone | 62.0 | 1.8 | 2.0 | 57.1 | 3.4 | 4.0 | 59.1 | 4.8 | 5.2 | 0.5 | 0.2 | 15.2 | 30.0 |
| Prednisone | 74.2 | 5.8 | 5.9 | 76.6 | 4.8 | 5.5 | 78.9 | 1.8 | 3.8 | 2.0 | 0.5 | 7.0 | 12.0 |
| Procainamide | 58.1 | 1.6 | 2.5 | 53.1 | 6.9 | 10.3 | 63.2 | 1.7 | 1.8 | 0.5 | 0.2 | 7.2 | 13.9 |
| Procaine/Novocaine | 60.2 | 2.4 | 4.6 | 58.4 | 9.5 | 11.6 | 55.1 | 2.2 | 4.7 | 2.0 | 1.0 | 4.4 | 6.7 |
| Procaterol | 61.5 | 3.2 | 3.8 | 64.6 | 4.5 | 5.4 | 71.8 | 1.7 | 3.7 | 2.0 | 0.2 | 5.7 | 9.5 |
| Progesterone | 92.4 | 1.1 | 1.7 | 87.8 | 3.7 | 5.3 | 81.6 | 6.2 | 7.5 | 2.0 | 0.5 | 22.6 | 43.3 |
| Promethazine | 60.9 | 4.9 | 6.2 | 61.7 | 4.3 | 5.3 | 63.0 | 1.0 | 2.8 | 2.0 | 0.2 | 8.1 | 14.2 |
| Propetamphos | 58.9 | 10.5 | 11.0 | 63.2 | 9.0 | 10.9 | 52.7 | 9.2 | 9.6 | 5.0 | 0.2 | 14.7 | 24.5 |
| Propionylpromazine | 78.1 | 1.2 | 1.2 | 79.3 | 3.7 | 4.0 | 79.7 | 2.0 | 3.4 | 0.5 | 0.2 | 8.5 | 16.4 |
| Propranolol | 80.2 | 2.1 | 2.5 | 73.8 | 5.0 | 6.9 | 88.6 | 3.8 | 5.5 | 2.0 | 1.0 | 12.6 | 23.2 |
| Propyl thiouracil | 64.3 | 6.5 | 7.1 | 64.6 | 7.4 | 8.3 | 67.6 | 5.0 | 5.3 | 5.0 | 1.0 | 12.0 | 19.0 |
| Ractopamine | 76.1 | 2.8 | 3.3 | 79.9 | 2.9 | 3.5 | 82.2 | 1.1 | 1.4 | 0.5 | 0.2 | 22.0 | 43.5 |
| Ritodrine | 83.7 | 3.5 | 3.6 | 81.5 | 3.8 | 5.1 | 71.8 | 3.7 | 4.5 | 0.5 | 0.2 | 19.0 | 37.5 |
| Robenidine | 54.8 | 2.9 | 4.5 | 57.8 | 4.7 | 5.9 | 54.0 | 2.0 | 5.6 | 5.0 | 0.2 | 9.1 | 13.1 |
| Ronidazole | 75.9 | 1.5 | 2.4 | 73.0 | 3.8 | 4.3 | 72.8 | 1.9 | 3.4 | 2.0 | 0.2 | 3.9 | 5.7 |
| Salbutamol | 78.1 | 4.4 | 5.3 | 72.2 | 3.2 | 4.1 | 76.7 | 1.1 | 2.6 | 0.5 | 0.2 | 3.6 | 6.6 |
| Salmeterol | 70.9 | 4.4 | 5.2 | 71.2 | 4.2 | 5.6 | 73.2 | 3.0 | 3.0 | 0.5 | 0.2 | 9.8 | 19.1 |
| Sarafloxacin | 76.2 | 1.0 | 1.1 | 72.8 | 4.0 | 5.8 | 74.4 | 3.9 | 4.0 | 2.0 | 0.5 | 3.8 | 5.6 |
| Secnidazole | 66.3 | 3.6 | 3.7 | 69.3 | 2.2 | 2.9 | 67.0 | 1.8 | 5.1 | 0.5 | 0.2 | 3.6 | 6.7 |
| Sineptina | 73.3 | 3.2 | 3.9 | 79.2 | 6.7 | 7.7 | 85.3 | 4.8 | 5.5 | 2.0 | 1.0 | 6.6 | 11.3 |
| Sotalol | 73.4 | 1.6 | 2.8 | 73.4 | 3.3 | 3.3 | 77.3 | 2.2 | 2.6 | 0.5 | 0.2 | 21.8 | 43.1 |
| Sparfloxacin | 85.4 | 2.4 | 2.5 | 74.1 | 4.4 | 5.0 | 81.6 | 2.2 | 2.2 | 0.5 | 0.2 | 3.7 | 6.9 |
| Sulfabenzamide | 73.4 | 2.9 | 3.2 | 77.2 | 3.2 | 4.5 | 73.9 | 3.5 | 4.9 | 0.5 | 0.2 | 3.7 | 6.9 |
| Sulfachloropyridazine | 57.4 | 4.7 | 6.0 | 62.1 | 6.8 | 7.4 | 61.0 | 3.1 | 5.5 | 2.0 | 0.2 | 6.4 | 10.8 |
| Sulfadiazine | 61.7 | 2.8 | 2.9 | 65.4 | 3.8 | 4.5 | 63.4 | 1.1 | 5.5 | 0.5 | 0.2 | 8.5 | 16.4 |
| Sulfadimethoxine | 66.6 | 1.7 | 2.0 | 67.9 | 4.6 | 5.9 | 66.3 | 1.4 | 4.3 | 0.5 | 0.2 | 5.2 | 9.9 |
| Sulfadimidine | 64.3 | 2.7 | 3.6 | 67.1 | 3.8 | 4.0 | 66.3 | 3.4 | 3.4 | 0.5 | 0.2 | 11.2 | 21.9 |
| Sulfadoxine | 67.5 | 2.8 | 3.4 | 69.2 | 3.9 | 4.3 | 69.1 | 5.6 | 6.4 | 0.5 | 0.2 | 6.8 | 13.1 |
| Sulfamerazine | 60.4 | 3.0 | 3.0 | 63.5 | 3.0 | 3.0 | 64.9 | 5.4 | 5.8 | 0.5 | 0.2 | 6.8 | 13.1 |
| Sulfameter | 60.9 | 2.8 | 3.9 | 62.7 | 3.0 | 3.0 | 62.4 | 5.7 | 7.5 | 0.5 | 0.2 | 11.5 | 22.6 |
| Sulfamethizole | 57.2 | 4.9 | 6.0 | 61.9 | 4.7 | 5.5 | 62.8 | 3.3 | 5.1 | 2.0 | 0.2 | 7.6 | 13.2 |
| Sulfamethoxazole | 57.2 | 5.3 | 7.4 | 61.3 | 2.3 | 3.0 | 59.9 | 5.7 | 7.1 | 2.0 | 0.2 | 8.9 | 15.8 |
| Sulfamethoxypyridazine | 64.8 | 3.8 | 4.1 | 69.6 | 2.7 | 3.7 | 69.3 | 4.2 | 5.7 | 0.5 | 0.2 | 10.8 | 21.2 |
| Sulfamonomethoxine | 63.0 | 2.8 | 3.3 | 64.4 | 4.8 | 5.2 | 64.1 | 6.0 | 7.8 | 2.0 | 0.2 | 4.9 | 7.7 |
| Sulfamoxol | 62.3 | 5.3 | 7.1 | 65.1 | 2.1 | 3.2 | 62.5 | 6.8 | 7.4 | 0.5 | 0.2 | 14.1 | 27.7 |
| Sulfanilamide | 73.1 | 2.4 | 2.9 | 83.2 | 5.4 | 6.8 | 84.1 | 1.3 | 1.9 | 0.5 | 0.2 | 8.1 | 15.6 |
| Sulfanitran | 70.3 | 4.6 | 5.0 | 75.1 | 8.6 | 11.4 | 77.2 | 6.6 | 9.7 | 5.0 | 1.0 | 14.6 | 24.2 |
| Sulfaphenazole | 68.8 | 2.6 | 3.8 | 70.4 | 2.3 | 2.8 | 70.2 | 2.2 | 5.0 | 0.5 | 0.2 | 10.1 | 19.7 |
| Sulfapyrazole | 71.2 | 1.7 | 2.2 | 71.3 | 3.7 | 4.4 | 71.6 | 5.3 | 5.3 | 0.5 | 0.2 | 10.7 | 20.9 |
| Sulfapyridine | 61.4 | 2.8 | 3.6 | 63.5 | 3.5 | 4.0 | 63.8 | 5.5 | 7.4 | 0.5 | 0.2 | 9.1 | 17.6 |
| Sulfaquinoxaline | 56.7 | 6.3 | 6.8 | 63.2 | 6.1 | 7.6 | 60.9 | 5.6 | 5.9 | 0.5 | 0.2 | 13.0 | 25.5 |
| Sulfathiazole | 53.0 | 3.7 | 4.6 | 56.1 | 3.5 | 3.9 | 56.9 | 5.6 | 7.4 | 5.0 | 0.2 | 8.0 | 10.9 |
| Sulfisomidine | 60.9 | 2.8 | 3.3 | 63.5 | 2.5 | 2.6 | 67.5 | 5.0 | 5.6 | 2.0 | 0.5 | 4.8 | 7.7 |
| Sulindac | 70.5 | 4.0 | 4.7 | 72.9 | 4.1 | 5.6 | 73.8 | 1.9 | 2.7 | 2.0 | 0.5 | 16.9 | 31.7 |
| Sulphacetamide | 117.8 | 3.9 | 4.0 | 118.3 | 2.8 | 3.1 | 106.5 | 1.3 | 1.3 | 0.5 | 0.2 | 3.1 | 5.8 |
| Sulpiride | 72.5 | 1.6 | 3.2 | 77.8 | 4.4 | 6.6 | 83.4 | 5.3 | 6.0 | 0.5 | 0.2 | 9.0 | 17.4 |
| Tenoxicam | 62.4 | 2.9 | 3.4 | 61.6 | 3.7 | 4.9 | 65.1 | 4.2 | 5.1 | 2.0 | 0.2 | 5.5 | 8.9 |
| Terbutaline | 85.2 | 3.2 | 3.3 | 82.4 | 9.0 | 12.1 | 72.7 | 3.8 | 4.2 | 0.5 | 0.2 | 18.0 | 35.5 |
| Terfenadine | 84.8 | 1.0 | 3.5 | 84.6 | 3.8 | 5.3 | 83.6 | 2.4 | 3.4 | 0.5 | 0.2 | 6.7 | 13.0 |
| Testosterone | 62.9 | 1.1 | 2.3 | 63.3 | 3.4 | 3.7 | 58.0 | 4.5 | 5.3 | 0.5 | 0.2 | 8.1 | 15.7 |
| Tetracaine | 55.4 | 4.7 | 5.8 | 53.4 | 7.0 | 10.9 | 53.8 | 2.8 | 3.4 | 0.5 | 0.2 | 4.3 | 8.1 |
| Thiabendazole | 59.9 | 1.1 | 1.3 | 62.2 | 2.9 | 2.9 | 65.0 | 5.5 | 5.7 | 2.0 | 0.2 | 3.1 | 4.2 |
| Tilmicosin | 95.6 | 6.9 | 7.1 | 81.1 | 7.5 | 8.4 | 81.7 | 2.5 | 2.5 | 2.0 | 0.2 | 12.8 | 23.7 |
| Tinidazole | 63.2 | 2.3 | 2.5 | 65.5 | 3.6 | 3.6 | 66.6 | 4.7 | 5.0 | 0.5 | 0.2 | 5.6 | 10.7 |
| Tolfenamic acid | 75.7 | 3.8 | 4.2 | 76.9 | 5.1 | 6.5 | 72.2 | 3.1 | 4.0 | 2.0 | 1.0 | 7.3 | 12.5 |
| Tolmetin | 75.4 | 6.9 | 7.1 | 76.0 | 7.6 | 9.6 | 76.5 | 3.5 | 4.8 | 2.0 | 0.2 | 10.4 | 18.7 |
| Toltrazuril | 74.9 | 3.4 | 4.2 | 71.5 | 1.9 | 2.0 | 71.7 | 9.9 | 12.8 | 5.0 | 2.0 | 7.2 | 9.3 |
| Toltrazuril sulfone | 88.6 | 4.3 | 5.1 | 80.0 | 1.9 | 2.5 | 91.2 | 6.5 | 8.7 | 5.0 | 2.0 | 12.8 | 20.7 |
| Toltrazuril-sulfoxide | 73.7 | 5.0 | 5.7 | 76.7 | 3.8 | 5.2 | 77.1 | 6.0 | 6.8 | 2.0 | 1.0 | 10.0 | 18.0 |
| Tosufloxacin | 55.2 | 5.0 | 5.4 | 62.2 | 11.7 | 14.0 | 66.8 | 8.3 | 11.0 | 5.0 | 0.2 | 15.9 | 26.8 |
| Triamcinolone acetonide | 81.5 | 3.2 | 6.7 | 81.0 | 3.3 | 3.5 | 81.8 | 2.5 | 4.3 | 0.5 | 0.2 | 7.8 | 15.1 |
| Triclabendazole | 54.6 | 3.0 | 3.9 | 57.2 | 2.6 | 2.7 | 57.4 | 4.6 | 5.9 | 5.0 | 0.2 | 7.3 | 9.6 |
| Trimethoprim | 74.1 | 1.1 | 1.2 | 80.7 | 2.9 | 2.9 | 81.7 | 1.1 | 1.4 | 0.5 | 0.2 | 11.2 | 21.8 |
| Tulobuterol | 80.7 | 1.8 | 1.8 | 81.2 | 2.5 | 2.7 | 82.1 | 1.6 | 2.0 | 0.5 | 0.2 | 4.7 | 9.0 |
| Tylosin | 74.2 | 3.3 | 4.6 | 78.7 | 2.6 | 3.2 | 81.3 | 1.2 | 1.9 | 0.5 | 0.2 | 206.4 | 212.8 |
| Valnemulin | 66.9 | 6.1 | 7.3 | 56.3 | 4.8 | 6.0 | 60.8 | 6.4 | 7.9 | 5.0 | 2.0 | 10.1 | 15.1 |
| Virginiamycin M1 | 62.1 | 3.9 | 4.2 | 68.9 | 3.3 | 3.8 | 66.1 | 1.8 | 3.0 | 0.5 | 0.2 | 11.7 | 22.9 |
| Xylazine | 76.1 | 1.5 | 1.7 | 77.3 | 2.9 | 3.5 | 78.1 | 1.2 | 1.4 | 0.5 | 0.2 | 9.2 | 17.9 |
| Zolpidem | 77.2 | 1.0 | 1.1 | 79.3 | 2.4 | 2.5 | 78.2 | 1.9 | 1.9 | 0.5 | 0.2 | 3.9 | 7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jia, Q.; Liao, G.; Qian, Y.; Qiu, J. Multi-Residue Determination of 244 Chemical Contaminants in Chicken Eggs by Liquid Chromatography-Tandem Mass Spectrometry after Effective Lipid Clean-Up. Agriculture 2022, 12, 869. https://doi.org/10.3390/agriculture12060869
Zhang L, Jia Q, Liao G, Qian Y, Qiu J. Multi-Residue Determination of 244 Chemical Contaminants in Chicken Eggs by Liquid Chromatography-Tandem Mass Spectrometry after Effective Lipid Clean-Up. Agriculture. 2022; 12(6):869. https://doi.org/10.3390/agriculture12060869
Chicago/Turabian StyleZhang, Lin, Qi Jia, Guangqin Liao, Yongzhong Qian, and Jing Qiu. 2022. "Multi-Residue Determination of 244 Chemical Contaminants in Chicken Eggs by Liquid Chromatography-Tandem Mass Spectrometry after Effective Lipid Clean-Up" Agriculture 12, no. 6: 869. https://doi.org/10.3390/agriculture12060869
APA StyleZhang, L., Jia, Q., Liao, G., Qian, Y., & Qiu, J. (2022). Multi-Residue Determination of 244 Chemical Contaminants in Chicken Eggs by Liquid Chromatography-Tandem Mass Spectrometry after Effective Lipid Clean-Up. Agriculture, 12(6), 869. https://doi.org/10.3390/agriculture12060869






