Low-Temperature Effects on the Growth and Phytochemical Properties of Wheat Sprouts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wheat Sprouts
2.3. Cell Viability
2.4. Quantification of Phenolics and Flavonoids in Wheat Sprouts
2.5. Radical Scavenging Activity
2.6. Statistical Analysis
3. Results and Discussion
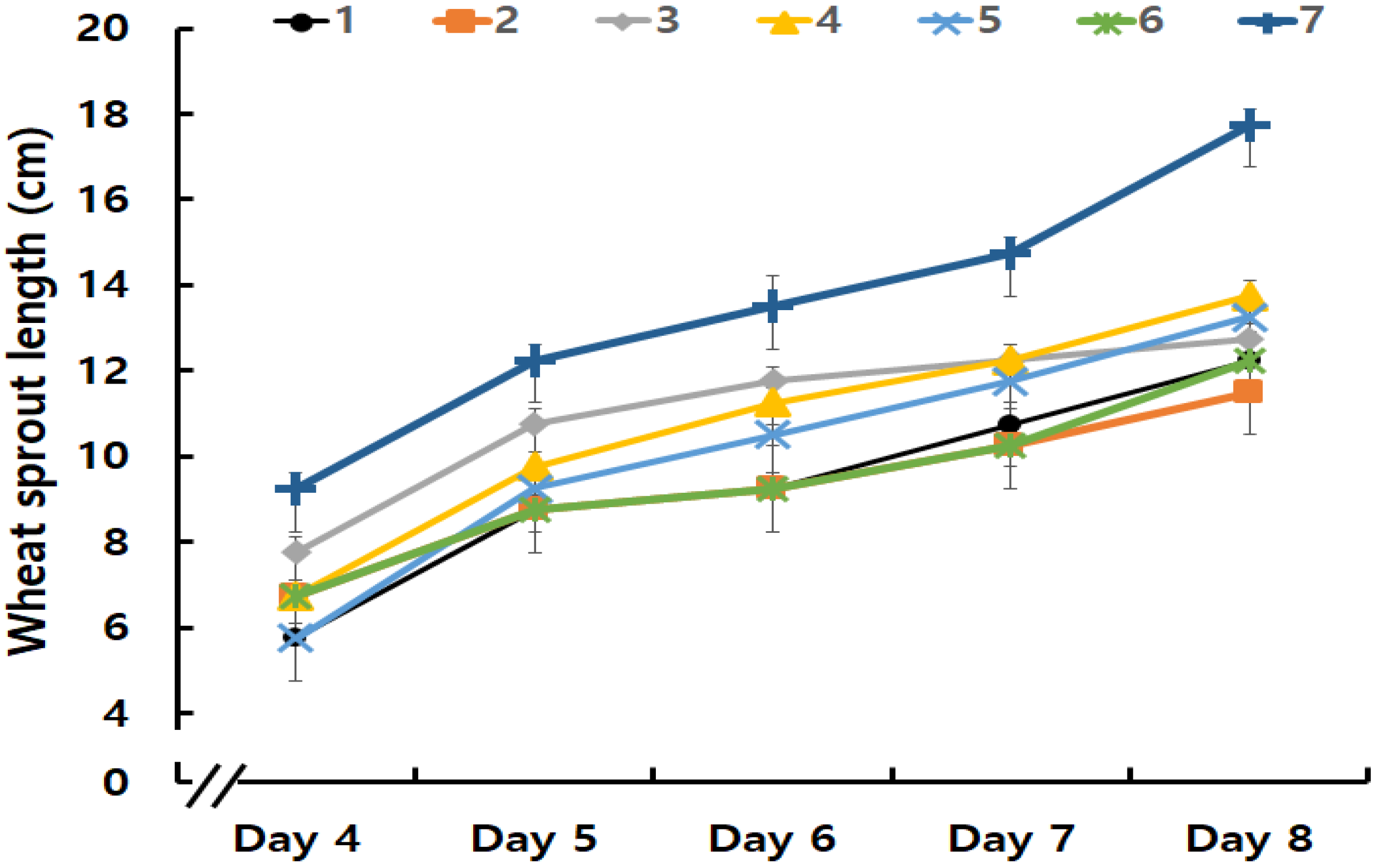
3.1. Wheat Sprout Characteristics Differed among Varieties
3.1.1. Wheat Sprout Growth Rates
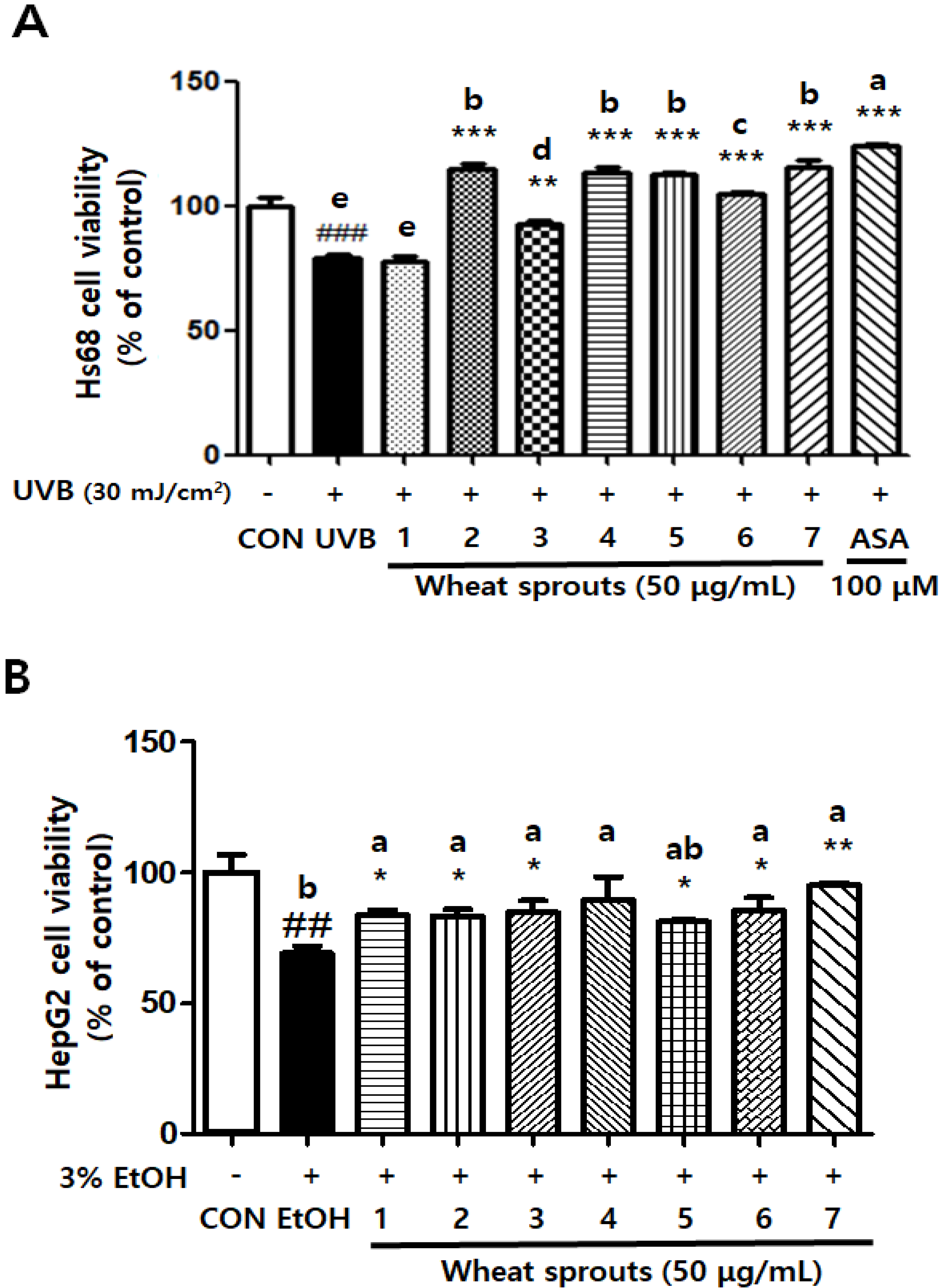
3.1.2. Wheat Sprouts Protect Cells against Oxidative Stress
3.2. Effects of Low-Temperature Treatment on Purple Wheat Sprout Properties
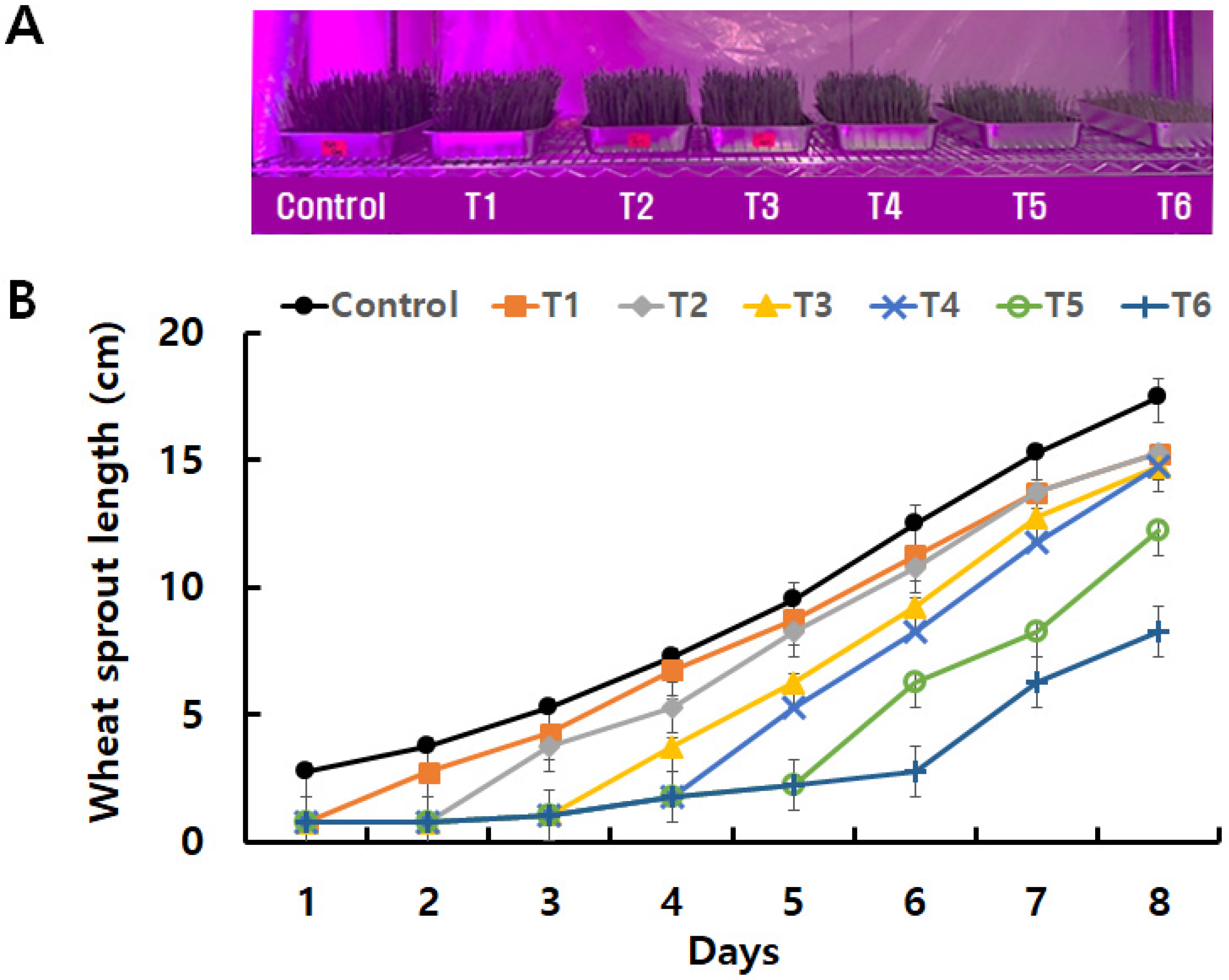
3.2.1. The Growth Rate of Purple Wheat Sprouts Depends on Duration of Low-Temperature Exposure
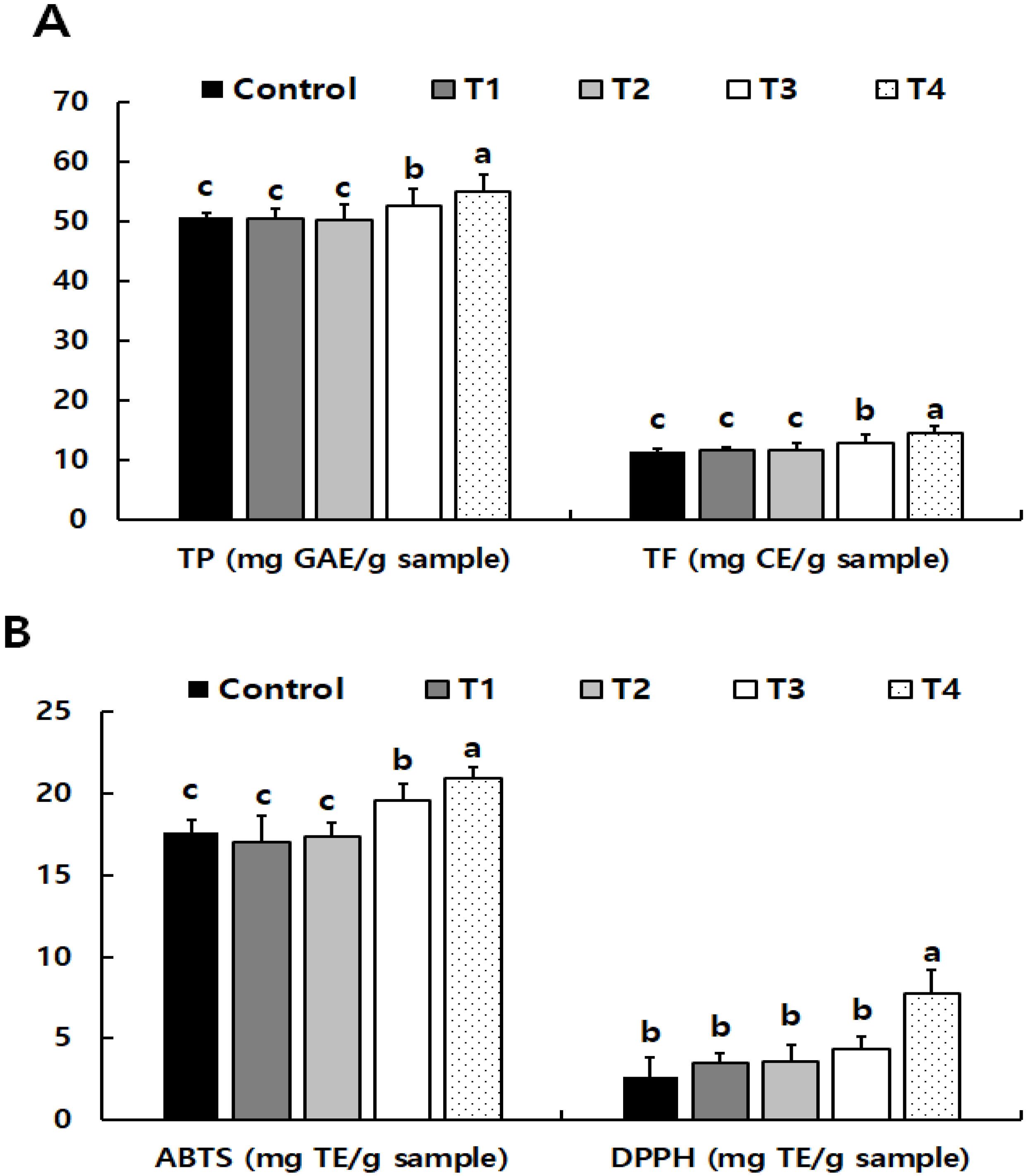
3.2.2. Phytochemical Properties of Low-Temperature-Treated Purple Wheat Sprouts
4. Conclusions
5. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincai, L. Cold Stress in Wheat: Plant Acclimation Responses and Management Strategies. Front. Plant Sci. 2021, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Moon, Y.; Kweon, M. Effect of Purple-Colored Wheat Bran Addition on Quality and Antioxidant Property of Bread and Optimization of Bread-Making Conditions. Appl. Sci. 2021, 11, 4034. [Google Scholar] [CrossRef]
- Donley, A. South Korea Wheat Imports Forecast Higher. Available online: world-grain.com/articles/16078-south-korea-wheat-imports-forecast-higher (accessed on 16 March 2022).
- Moon, Y.; Seo, Y.; Kim, K.H.; Kweon, M. Identification of Significant Formula and Processing Factors for Bread Formulated with the Blends of Korean Domestic Wheat Flour and Purple Wheat Bran Using a Factorial Design. Korean J. Food Cook. Sci. 2021, 37, 144–152. [Google Scholar] [CrossRef]
- Shin, Y.J.; Jegal, J.M.; Lee, M.H. Quality and Antioxidant Properties of White Bread with Gynura procumbens Powder. Culin. Sci. Hosp. Res. 2019, 25, 1–11. [Google Scholar] [CrossRef]
- Grausgruber, H.; Atzgersdorfer, K.; Bohmdorfer, S. Purple and blue wheat-health-promoting grains with increased antioixdant activity. Cereal Foods World 2018, 63, 217–220. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, S.; Sharma, N.; Chunduri, V.; Kapoor, P.; Kaur, S.; Goyal, A.; Garg, M. Influence of Biofortified Colored Wheats (Purple, Blue, Black) on Physicochemical, Antioxidant and Sensory Characteristics of Chapatti (Indian Flatbread). Molecules 2020, 25, 5071. [Google Scholar] [CrossRef]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and microgreens: Trends, opportunities, and horizons for novel research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Stagnari, F.; Galieni, A.; D’Egidio, S.; Falcinelli, B.; Pagnani, G.; Pace, R.; Pisante, M.; Benincasa, P. Effects of sprouting and salt stress on polyphenol composition and antiradical activity of einkorn, emmer and durum wheat. Ital. J. Agron. 2017, 12, 293–301. [Google Scholar] [CrossRef]
- Sytar, O.; Bosko, P.; Zivcak, M.; Brestic, M.; Smetanska, I. Bioactive Phytochemicals and Antioxidant Properties of the Grains and Sprouts of Colored Wheat Genotypes. Molecules 2018, 23, 2282. [Google Scholar] [CrossRef]
- Ham, H.; Choi, I.D.; Park, H.Y.; Yoon, S.D.; Oh, S.G.; Kim, W.H.; Woo, K.S. Phenolic Compounds and Radical Scavenging Activity of the Korean Wheat (Triticum aestivum L.) according to Germination Times. Korean J. Food Nutr. 2015, 28, 737–744. [Google Scholar] [CrossRef]
- Yi, B.; Kasai, H.; Lee, H.S.; Kang, Y.; Park, J.Y.; Yang, M. Inhibition by wheat sprout (Triticum aestivum) juice of bisphenol A-induced oxidative stress in young women. Mutat. Res. 2011, 724, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cang, J.; Lu, Q.; Fan, B.; Xu, Q.; Li, W.; Wang, X. ABA enhanced cold tolerance of wheat ‘dn1’ via increasing ROS scavenging system. Plant Signal. Behav. 2020, 15, 1780403. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Seo, H.Y.; Seo, W.D.; Lee, M.J.; Ham, H. Evaluation of biological activities of wheat sprouts with different extraction solvents. Korean J. Food Nutr. 2019, 32, 636–642. [Google Scholar] [CrossRef]
- Hong, S.; Heo, H.; Lee, H.; Lee, M.; Lee, J.; Park, J.H. Protective Effects of the Methanol Extract from Calyx of Diospyros kaki on Alcohol-Induced Liver Injury. J. Korean Soc. Food Sci. Nutr. 2021, 50, 339–346. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, J.; Chang, B.; Wang, B.F.; Zhang, D.; Wang, B.Y. Effects of ethanol on the expression of caveolin-1 in HepG2 cells. Mol. Med. Rep. 2015, 11, 4409–4413. [Google Scholar] [CrossRef][Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Kim, M.; Nam, D.G.; Ju, W.T.; Choe, J.S.; Choi, A.J. Response Surface Methodology for Optimization of Process Parameters and Antioxidant Properties of Mulberry (Morus alba L.) Leaves by Extrusion. Molecules 2020, 25, 5231. [Google Scholar] [CrossRef]
- Choi, S.; Hinkle, A.F. Rice Production Stays Steady Despite Government’s Rice Reduction Program; GAIN Report Number: KS1913; USDA Foreign Agricultural Service: Washington, DC, USA, 2019; pp. 1–36. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Grain%20and%20Feed%20Annual_Seoul_Korea%20-%20Republic%20of_4-1-2019.pdf (accessed on 1 April 2022).
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M.; Mildaziene, V. Impact of seed color and storage time on the radish seed germination and sprout growth in plasma agriculture. Sci. Rep. 2021, 11, 2539. [Google Scholar] [CrossRef]
- Calderon Flores, P.; Yoon, J.S.; Kim, D.Y.; Seo, Y.W. Effect of chilling acclimation on germination and seedlings response to cold in different seed coat colored wheat (Triticum aestivum L.). BMC Plant Biol. 2021, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.G.; Kim, K.H.; Sin, D.J.; Kim, K.M.; Kim, Y.J.; Ko, J.M. Screening of wheat varieties for wheat sprout and analysis of nutritional compositions. In Proceedings of the Korean Society of Crop Science, Seoul, Korea, 19 April 2018. [Google Scholar]
- Bhatt, A.; Gairola, S.; El-Keblawy, A.A. Seed colour affects light and temperature requirements during germination in two Lotus species (Fabaceae) of the Arabian subtropical deserts. Rev. Biol. Trop. 2016, 64, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Atis, I.; Atak, M.; Can, E.; Mavi, K. Seed Coat Color Effects on Seed Quality and Salt Tolerance of Red Clover (Trifolium pratense). Int. J. Agric. Biol. 2011, 13, 363–368. [Google Scholar]
- Kim, K.H.; Kim, K.M.; Shin, D.J.; Park, H.H.; Kang, C.S. A New Variety of Wheat (KCTC 1859 1P) and Food Composition for Anti-oxidative Activity Comprising Thereof. 10-2035666, 17 October 2019. [Google Scholar]
- Bonfili, L.; Amici, M.; Cecarini, V.; Cuccioloni, M.; Tacconi, R.; Angeletti, M.; Fioretti, E.; Keller, J.N.; Eleuteri, A.M. Wheat sprout extract-induced apoptosis in human cancer cells by proteasomes modulation. Biochimie 2009, 91, 1131–1144. [Google Scholar] [CrossRef]
- Gutierrez-Ruiz, M.C.; Quiroz, S.C.; Souza, V.; Bucio, L.; Hernandez, E.; Olivares, I.P.; Llorente, L.; Vargas-Vorackova, F.; Kershenobich, D. Cytokines, growth factors, and oxidative stress in HepG2 cells treated with ethanol, acetaldehyde, and LPS. Toxicology 1999, 134, 197–207. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Wu, P.Y.; Chen, C.W.; Lin, P.; Wen, K.C.; Lin, C.Y.; Chiang, H.M. N-(4-bromophenethyl) Caffeamide Protects Skin from UVB-Induced Inflammation Through MAPK/IL-6/NF-kappaB-Dependent Signaling in Human Skin Fibroblasts and Hairless Mouse Skin. Molecules 2017, 22, 1639. [Google Scholar] [CrossRef]
- Nagappan, A.; Jung, D.Y.; Kim, J.H.; Lee, H.; Jung, M.H. Gomisin N Alleviates Ethanol-Induced Liver Injury through Ameliorating Lipid Metabolism and Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 2601. [Google Scholar] [CrossRef]
- Idowu, A.T.; Olatunde, O.O.; Adekoya, A.E.; Idowu, S. Germination: An alternative source to promote phytonutrients in edible seeds. Food Qual. Saf. 2019, 4, 129–133. [Google Scholar] [CrossRef]
- Samec, D.; Ljubej, V.; Redovnikovic, I.R.; Fistanic, S.; Salopek-Sondi, B. Low Temperatures Affect the Physiological Status and Phytochemical Content of Flat Leaf Kale (Brassica oleracea var. acephala) Sprouts. Foods 2022, 11, 264. [Google Scholar] [CrossRef]
- Zabihi-e-Mahmoodabad, R.; Jamaati-e-Somarin, S.; Khayatnezhad, M.; Gholamin, R. Effect of Cold Stress on Germination and Growth of Wheat Cultivars. Adv. Environ. Biol. 2011, 5, 94–97. Available online: https://www.researchgate.net/publication/228883778 (accessed on 1 April 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Park, J.; Kim, K.-M.; Kim, Y.; Kang, C.-S.; Son, J.; Ko, J.; Kim, K.-H. Low-Temperature Effects on the Growth and Phytochemical Properties of Wheat Sprouts. Agriculture 2022, 12, 745. https://doi.org/10.3390/agriculture12060745
Kim M, Park J, Kim K-M, Kim Y, Kang C-S, Son J, Ko J, Kim K-H. Low-Temperature Effects on the Growth and Phytochemical Properties of Wheat Sprouts. Agriculture. 2022; 12(6):745. https://doi.org/10.3390/agriculture12060745
Chicago/Turabian StyleKim, Mina, Jinhee Park, Kyeong-Min Kim, Yurim Kim, Chon-Sik Kang, Jiyoung Son, Jongmin Ko, and Kyeong-Hoon Kim. 2022. "Low-Temperature Effects on the Growth and Phytochemical Properties of Wheat Sprouts" Agriculture 12, no. 6: 745. https://doi.org/10.3390/agriculture12060745
APA StyleKim, M., Park, J., Kim, K.-M., Kim, Y., Kang, C.-S., Son, J., Ko, J., & Kim, K.-H. (2022). Low-Temperature Effects on the Growth and Phytochemical Properties of Wheat Sprouts. Agriculture, 12(6), 745. https://doi.org/10.3390/agriculture12060745





