First Report on Purpureocillium lilacinum Infection of Indoor-Cultivated Morel Primordia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of the Contaminating Fungus
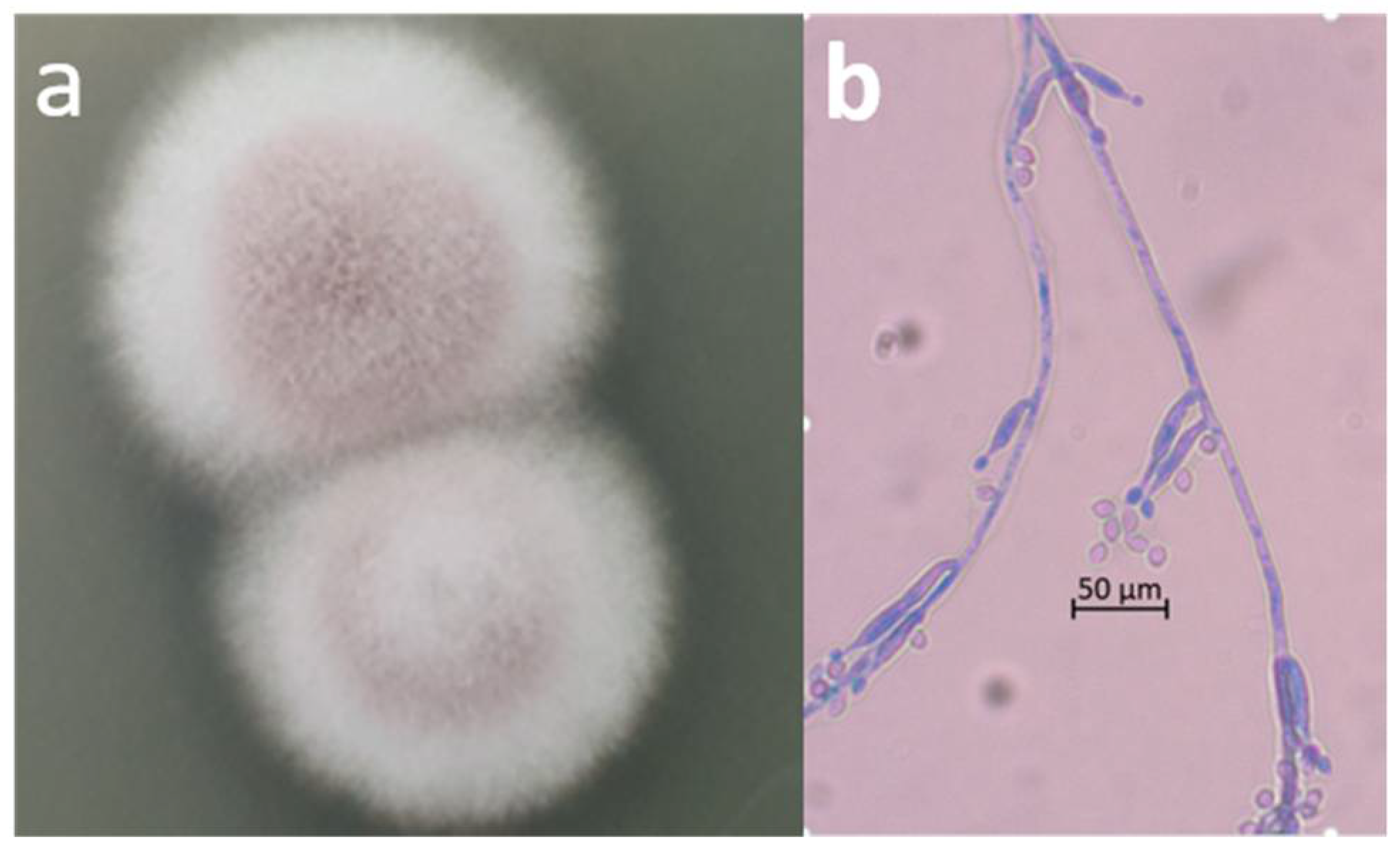
2.2. Microscopic Observation and Molecular Identification of the Contaminating Fungus
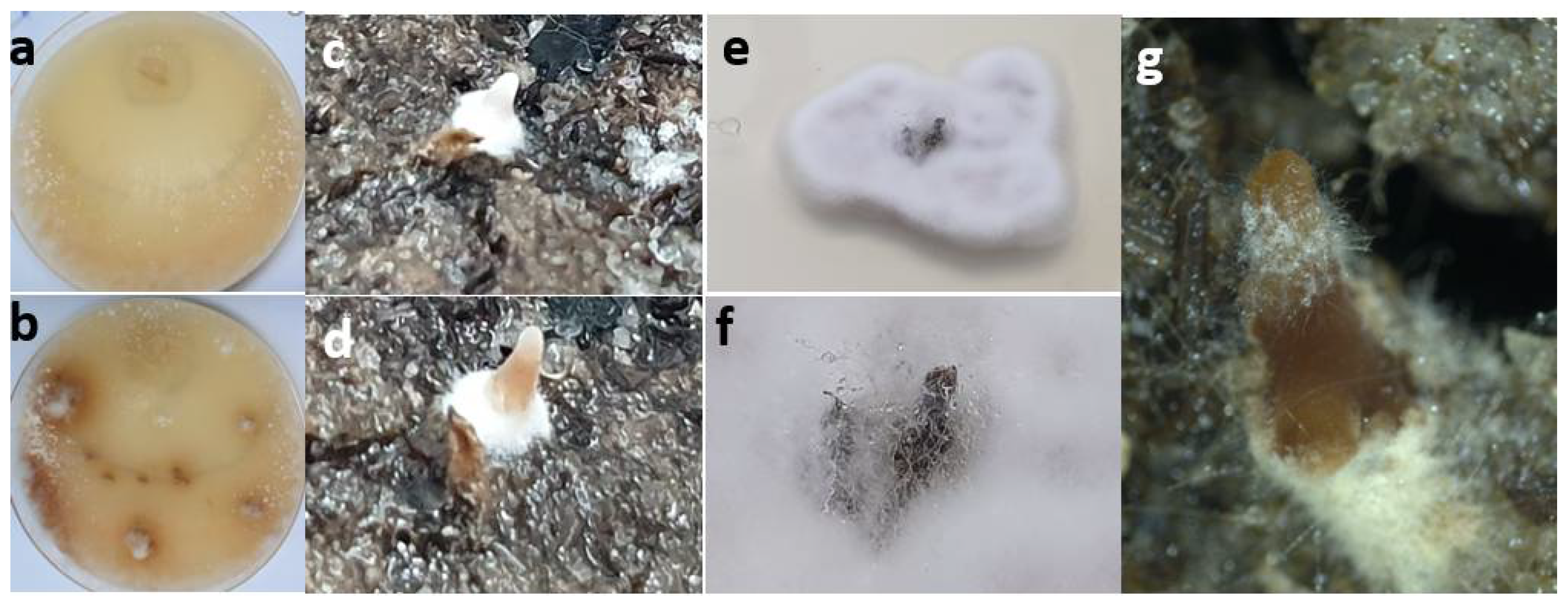
2.3. Pathogenicity Test (Koch’s Postulates)
3. Results and Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tietel, Z.; Masaphy, S. True morels (Morchella)—nutritional and phytochemical composition, health benefits and flavor: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1888–1901. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Zhang, W.M.; Wu, S.L.; Shi, J.; Zhang, G.L. Preparing of new health food Morchella capsule. Edible Fungi China 2001, 20, 38–40. [Google Scholar]
- Ower, R. Notes on the development of the morel ascocarp: Morchella esculenta. Mycologia 1982, 74, 142–144. [Google Scholar] [CrossRef]
- Ower, R.; Mills, G.L.; Malachowski, J.A. Cultivation of Morchella. U.S. Patent 4,594,809, 17 June 1986. [Google Scholar]
- Masaphy, S. Biotechnology of morel mushrooms: Successful fruiting body formation and development in a soilless system. Biotechnol. Lett. 2010, 32, 1523–1527. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial cultivation of true morels: Current state, issues and perspectives. Crit. Rev. Biotechnol. 2018, 38, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Sambyal, K.; Singh, R.V. A comprehensive review on Morchella importuna: Cultivation aspects, phytochemistry, and other significant applications. Folia Microbiol. 2021, 66, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.H. History, current status and prospects of morel cultivation. Edible Med. Mushrooms 2016, 24, 140–144. (In Chinese) [Google Scholar]
- Longley, R.; Benucci, G.M.N.; Mills, G.; Bonito, G. Fungal and bacterial community dynamics in substrates during the cultivation of morels (Morchella rufobrunnea) indoors. FEMS Microbiol. Lett. 2019, 366, fnz215. [Google Scholar] [CrossRef]
- Guo, M.P.; Chen, K.; Wang, G.Z.; Bian, Y.B. First report of stipe rot disease on Morchella importuna caused by Fusarium incarnatum–F. equiseti species complex in China. Plant Dis. 2016, 100, 2530. [Google Scholar] [CrossRef]
- He, P.; Li, C.; Cai, Y.; Zhang, Y.; Bian, Y.; Liu, W. First report of pileus rot disease on cultivated Morchella importuna caused by Diploöspora longispora in China. J. Gen. Plant Pathol. 2018, 84, 65–69. [Google Scholar] [CrossRef]
- He, X.L.; Peng, W.H.; Miao, R.Y.; Tang, J.; Chen, Y.; Liu, L.X.; Wang, D.; Gan, B.C. White mold on cultivated morels caused by Paecilomyces penicillatus. FEMS Microbiol. Lett. 2017, 364, fnx037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Peng, J.; Sun, L.; Bonito, G.; Guo, Y.; Li, Y.; Fu, Y. Genome sequencing of Paecilomyces penicillatus provides insights into its phylogenetic placement and mycoparasitism mechanisms on morel mushrooms. Pathogens 2020, 9, 834. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.F.; Cong, Q.Q.; Wang, Q.W.; Tang, L.N.; Li, X.M.; Yu, Q.W.; Cui, X.; An, X.R.; Yu, C.X.; Kong, F.H.; et al. First report of Cladobotryum protrusum causing cobweb disease on cultivated Morchella importuna. Plant Dis. 2020, 104, 977. [Google Scholar] [CrossRef]
- Liu, W.; Cai, Y.; He, P.; Ma, X.; Bian, Y. Occurrence and control of pests and diseases in field cultivation of Morchella mushrooms. Acta Edulis Fungi 2019, 26, 128–134. [Google Scholar]
- Albaum, S.; Masaphy, S. Comparison of rose bengal-chloramphenicol and modified aureomycin-rose bengal-glucose-peptone agar as media for the enumeration of molds and yeasts in water by membrane filtration techniques. J. Microbiol. Methods 2019, 76, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Luangsa-Ard, J.; Houbraken, J.; van Doorn, T.; Hong, S.B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef]
- Volk, T.J.; Leonard, T.J. Cytology of the life-cycle of Morchella. Mycol Res. 1990, 94, 399–406. [Google Scholar] [CrossRef]
- Masaphy, S. External ultrastructure of fruit body initiation in Morchella. Myco Res. 2005, 109, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Antioxidant compounds for the inhibition of enzymatic browning by polyphenol oxidases in the fruiting body extract of the edible mushroom Hericium erinaceus. Foods 2020, 9, 951. [Google Scholar] [CrossRef] [PubMed]
- Soler-Rivas, C.; Jolivet, S.; Arpin, N.; Olivier, J.M.; Wichers, H.J. Biochemical and physiological aspects of brown blotch disease of Agaricus bisporus. FEMS Microbiol. Rev. 1999, 23, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A. Paecilomyces and some allied hyphomycetes. Stud. Mycol. 1974, 6, 1–119. [Google Scholar]
- Lopez, D.C.; Zhu-Salzman, K.; Ek-Ramos, M.J.; Sword, G.A. The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. PLoS ONE 2014, 9, e103891. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Pandey, R.K.; Goswami, B.K. Bio-control activity of Purpureocillium lilacinum strains in managing root-knot disease of tomato caused by Meloidogyne incognita. Biocontr. Sci. Technol. 2013, 23, 1469–1489. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Z.; Lin, R.; Li, E.; Mao, Z.; Ling, J.; Yang, Y.; Yin, W.-B.; Xie, B. Biosynthesis of antibiotic leucinostatins in bio-control fungus Purpureocillium lilacinum and their inhibition on Phytophthora revealed by genome mining. PLoS Pathog. 2016, 12, e1005685. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Xu, P.; Xiao, H.; Zhang, Z.; Zhao, J.; Wang, G.; Xiao, X.; Xiao, Y. Efficacy of Purpureocillium lilacinus on gray mold of post-harvest tomato fruits. Chin. J. Biol. Control 2017, 33, 525–530. [Google Scholar]
- Lan, X.; Zhang, J.; Zong, Z.; Ma, Q.; Wang, Y. Evaluation of the biocontrol potential of Purpureocillium lilacinum QLP12 against Verticillium dahliae in eggplant. BioMed Res. Int. 2017, 2017, 4101357. [Google Scholar] [CrossRef] [Green Version]
- Elsherbiny, E.A.; Taher, M.A.; Elsebai, M.F. Activity of Purpureocillium lilacinum filtrates on biochemical characteristics of Sclerotinia sclerotiorum and induction of defense responses in common bean. Eur. J. Plant Pathol. 2019, 155, 39–52. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Taher, M.A.; Abd El-Aziz, M.H.; Mohamed, S.Y. Action mechanisms and biocontrol of Purpureocillium lilacinum against green mould caused by Penicillium digitatum in orange fruit. J. Appl. Microbiol. 2021, 131, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.S. Role of Purpureocillium lilacinum cultural filtrate in controlling onion white rot. J. Plant Protect. Pathol. 2020, 11, 175–184. [Google Scholar] [CrossRef]
- Prasad, P.; Varshney, D.; Adholeya, A. Whole genome annotation and comparative genomic analyses of bio-control fungus Purpureocillium lilacinum. BMC Genom. 2015, 16, 1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.K.; Arthurs, S. Recent advances in the biological control of citrus nematodes: A review. Biol. Control 2021, 157, 104593. [Google Scholar] [CrossRef]

| NCBI Accession No. | Homology (%) | From |
|---|---|---|
| HQ842812 | 100 | Nematoda |
| HQ842815 | 100 | Other |
| HQ842816 | 100 | Human |
| HQ842817 | 100 | Entomogenous |
| HQ842819 | 100 | Nematoda |
| HQ842820 | 100 | Miscellaneous |
| HQ842821 | 100 | Other |
| HQ842824 | 100 | Human |
| HQ842825 | 100 | Entomogenous |
| HQ842828 | 100 | Human |
| HQ842838 | 100 | Environmental |
| HQ842841 | 97.51 | Environmental |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masaphy, S. First Report on Purpureocillium lilacinum Infection of Indoor-Cultivated Morel Primordia. Agriculture 2022, 12, 695. https://doi.org/10.3390/agriculture12050695
Masaphy S. First Report on Purpureocillium lilacinum Infection of Indoor-Cultivated Morel Primordia. Agriculture. 2022; 12(5):695. https://doi.org/10.3390/agriculture12050695
Chicago/Turabian StyleMasaphy, Segula. 2022. "First Report on Purpureocillium lilacinum Infection of Indoor-Cultivated Morel Primordia" Agriculture 12, no. 5: 695. https://doi.org/10.3390/agriculture12050695
APA StyleMasaphy, S. (2022). First Report on Purpureocillium lilacinum Infection of Indoor-Cultivated Morel Primordia. Agriculture, 12(5), 695. https://doi.org/10.3390/agriculture12050695





