Developmental and Physiological Responses of Pisum sativum L. after Short- and Long-Time Cadmium Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
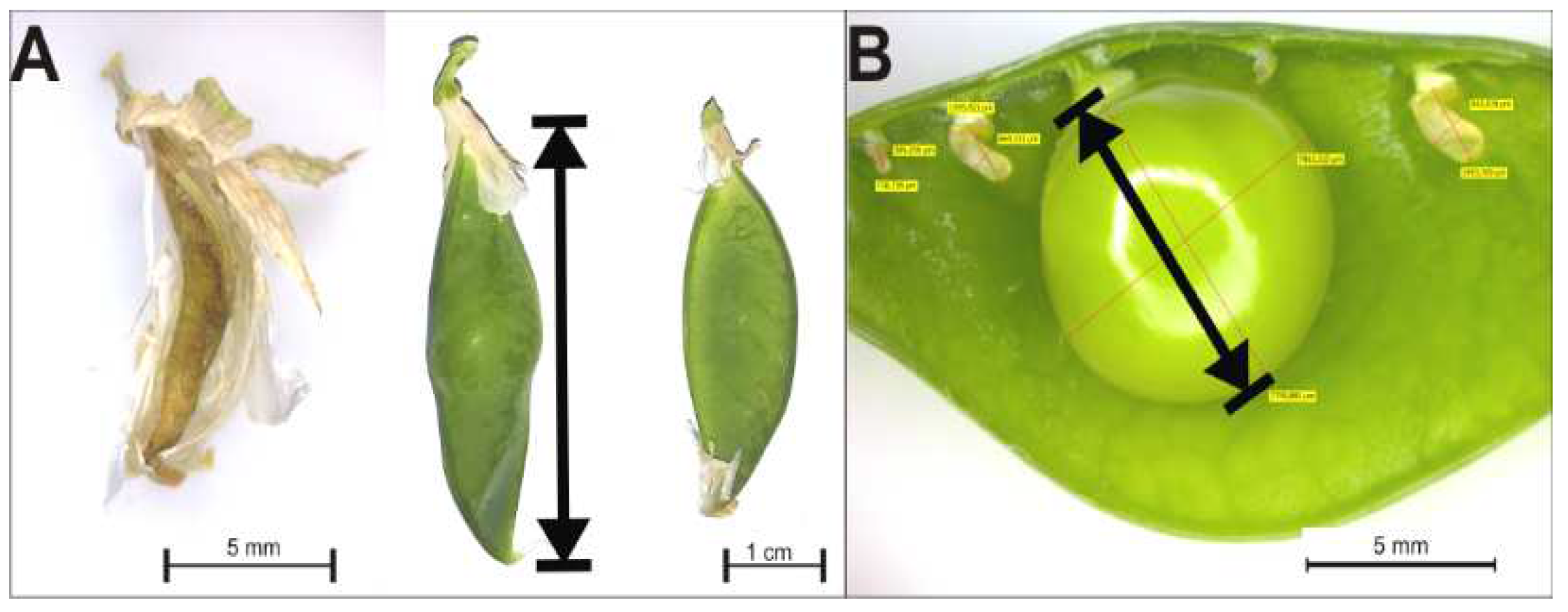
2.2. Analysis of the Development of P. sativum L
2.3. Water Content (WC) on a Dry Weight Basis Measurement
2.4. Measurement of Gas Exchange
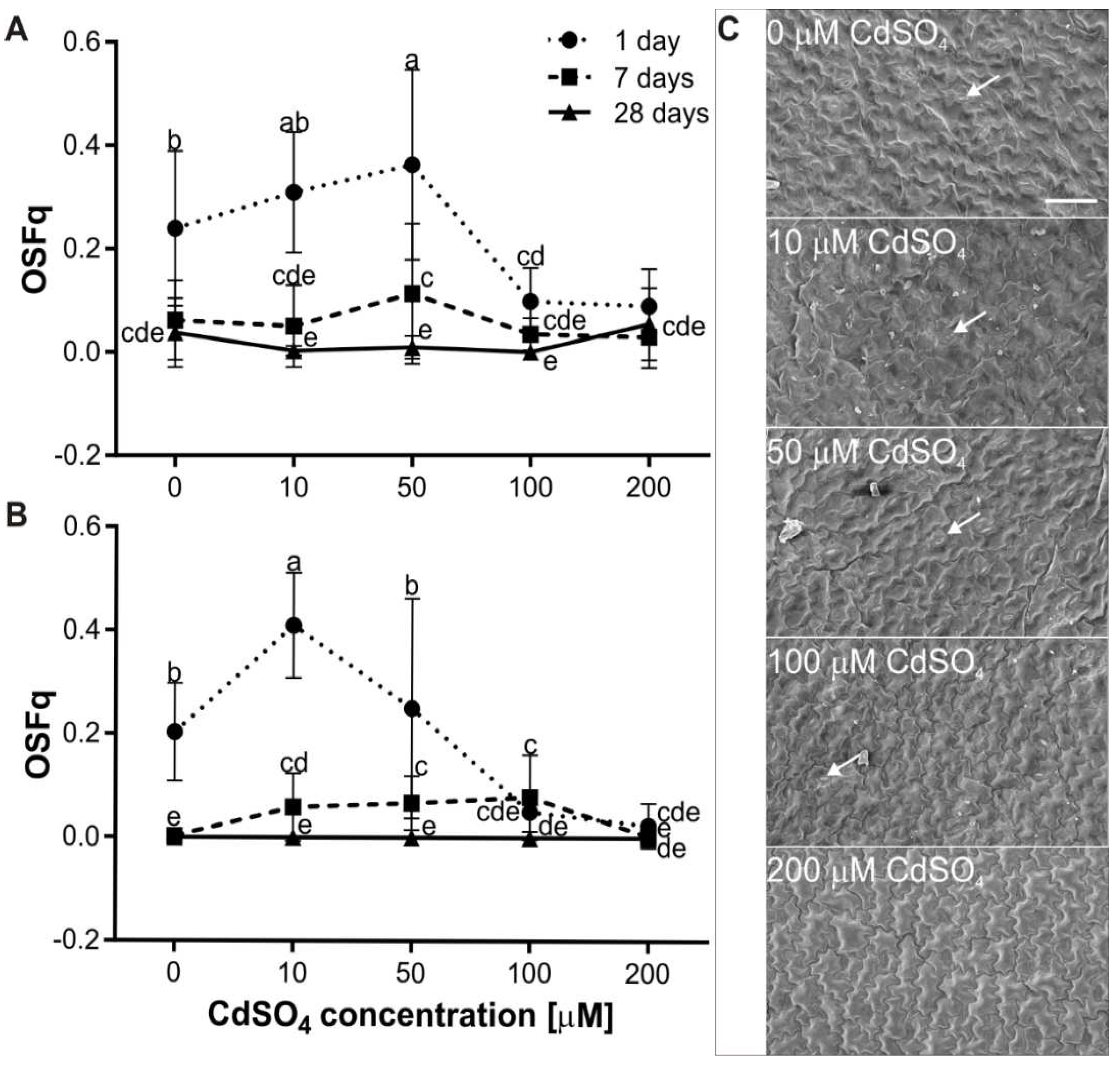
2.5. Measurement of Chlorophyll Fluorescence Ratio
2.6. Analysis of the Stomatal Opening
2.7. Determination of Macronutrients and Micronutrients Concentration
2.8. Statistical Analysis
3. Results
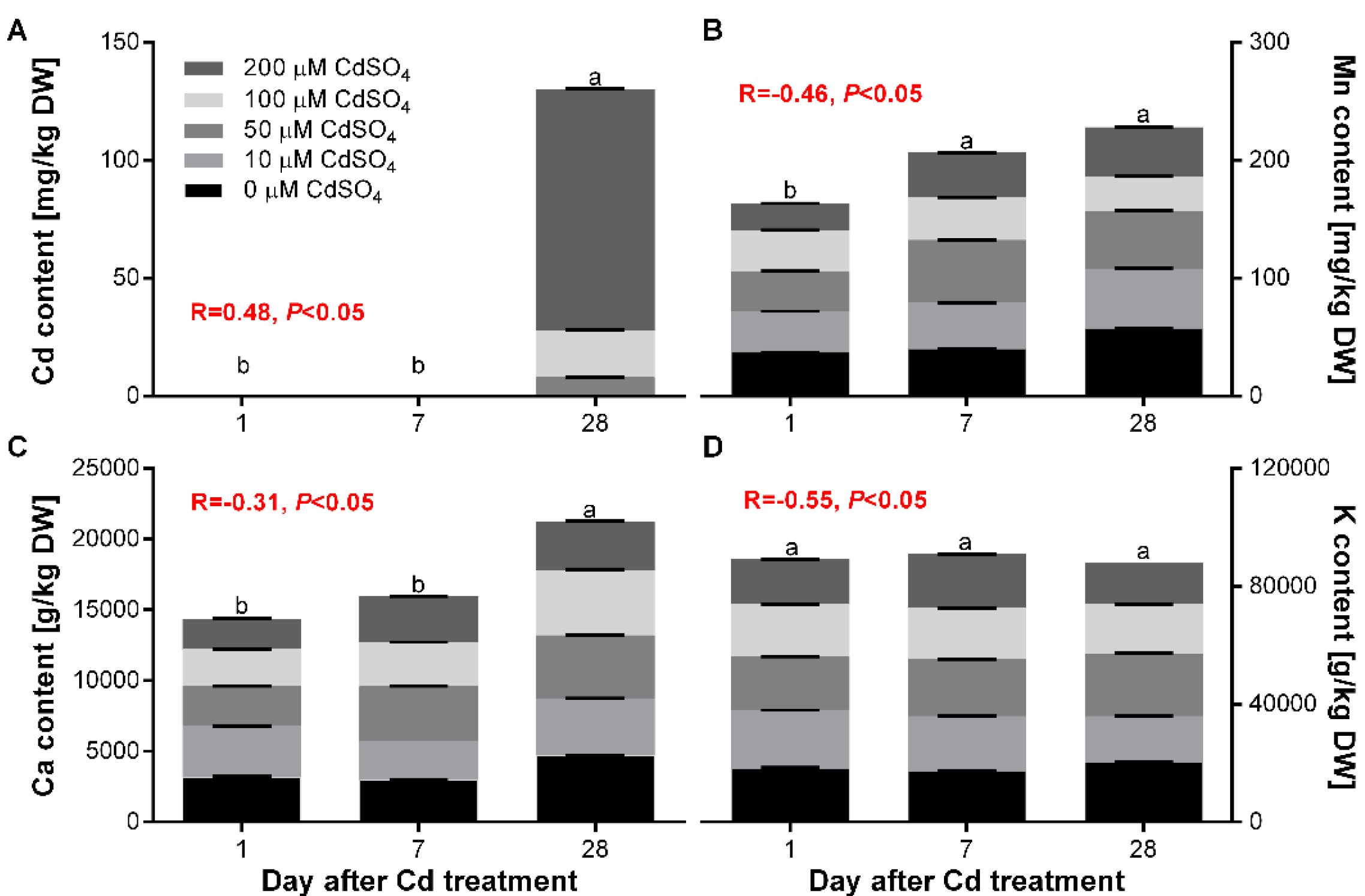
3.1. Macroelements and Microelements Content Analysis
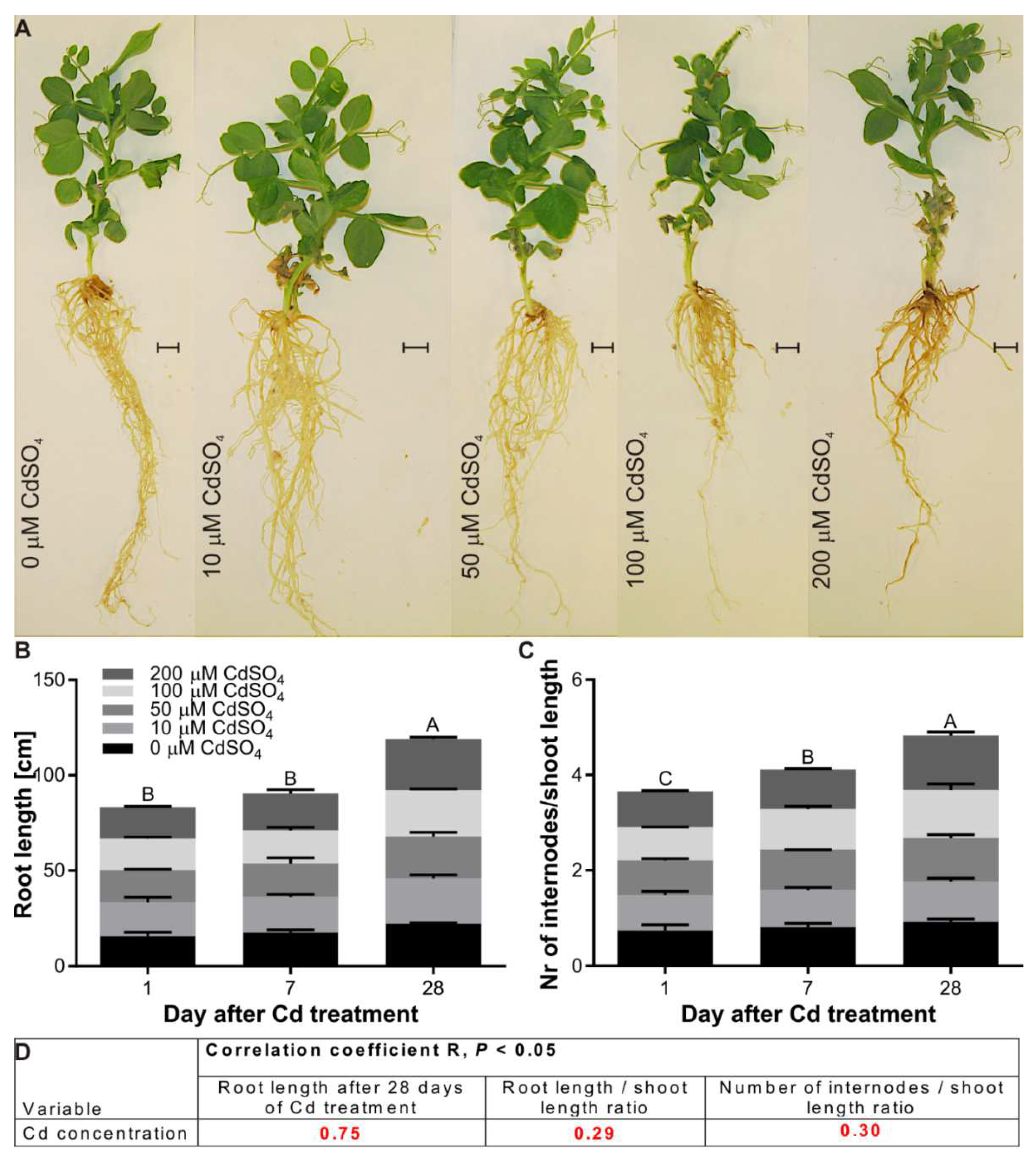
3.2. Effect of Cd on the Growth of P. sativum L
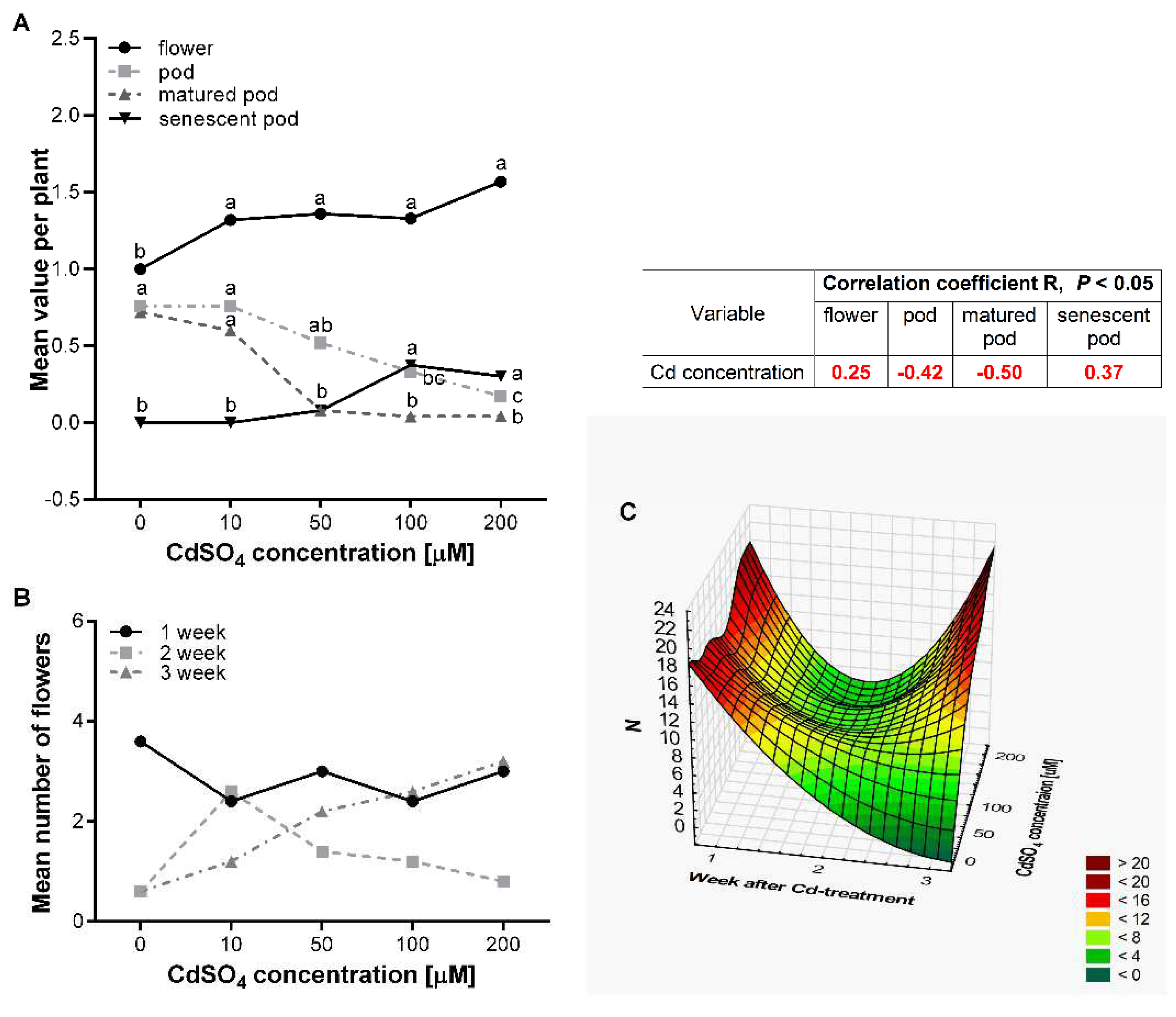
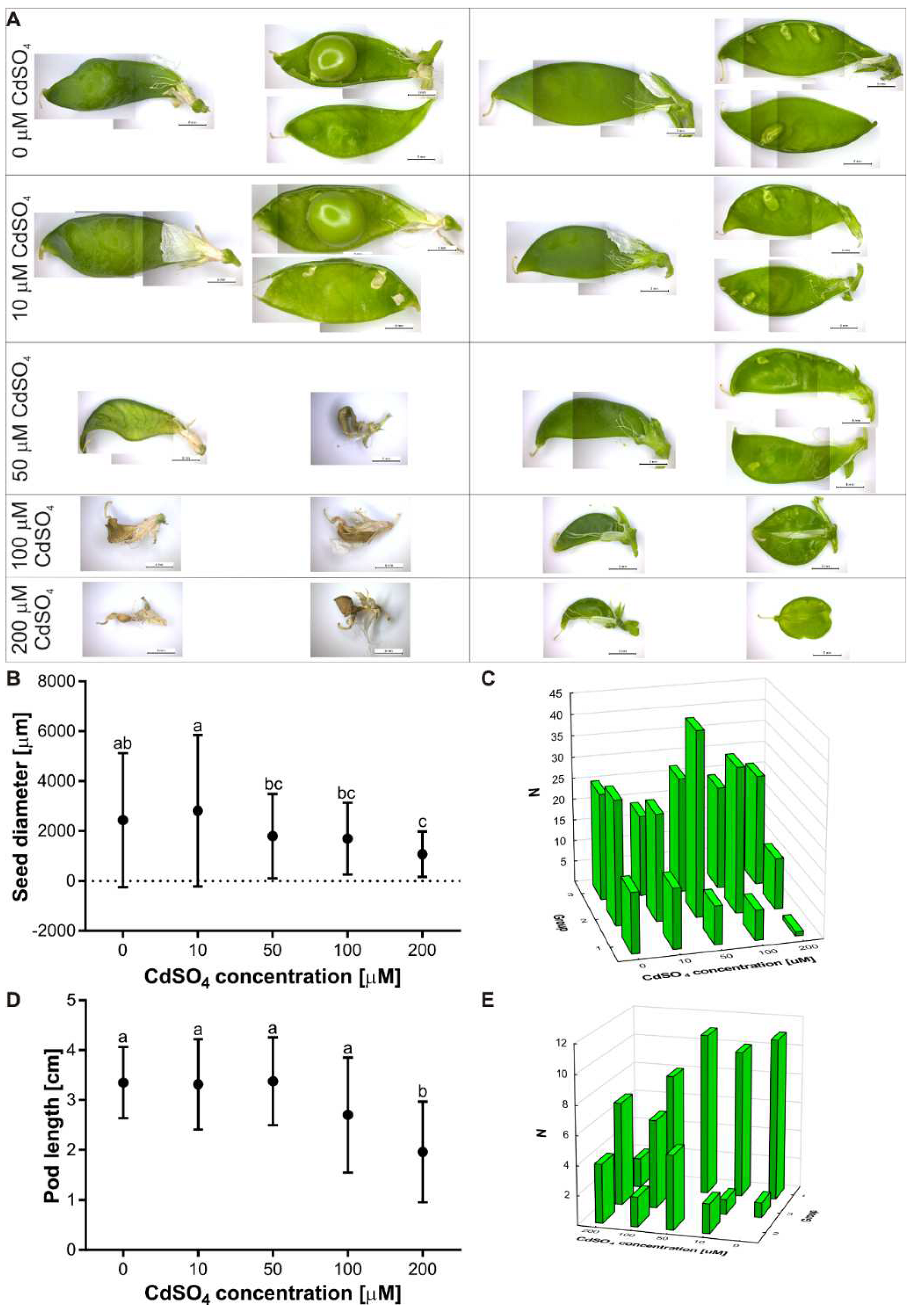
3.3. Effect of Cd on the Flowering and Seed Production of P. sativum L
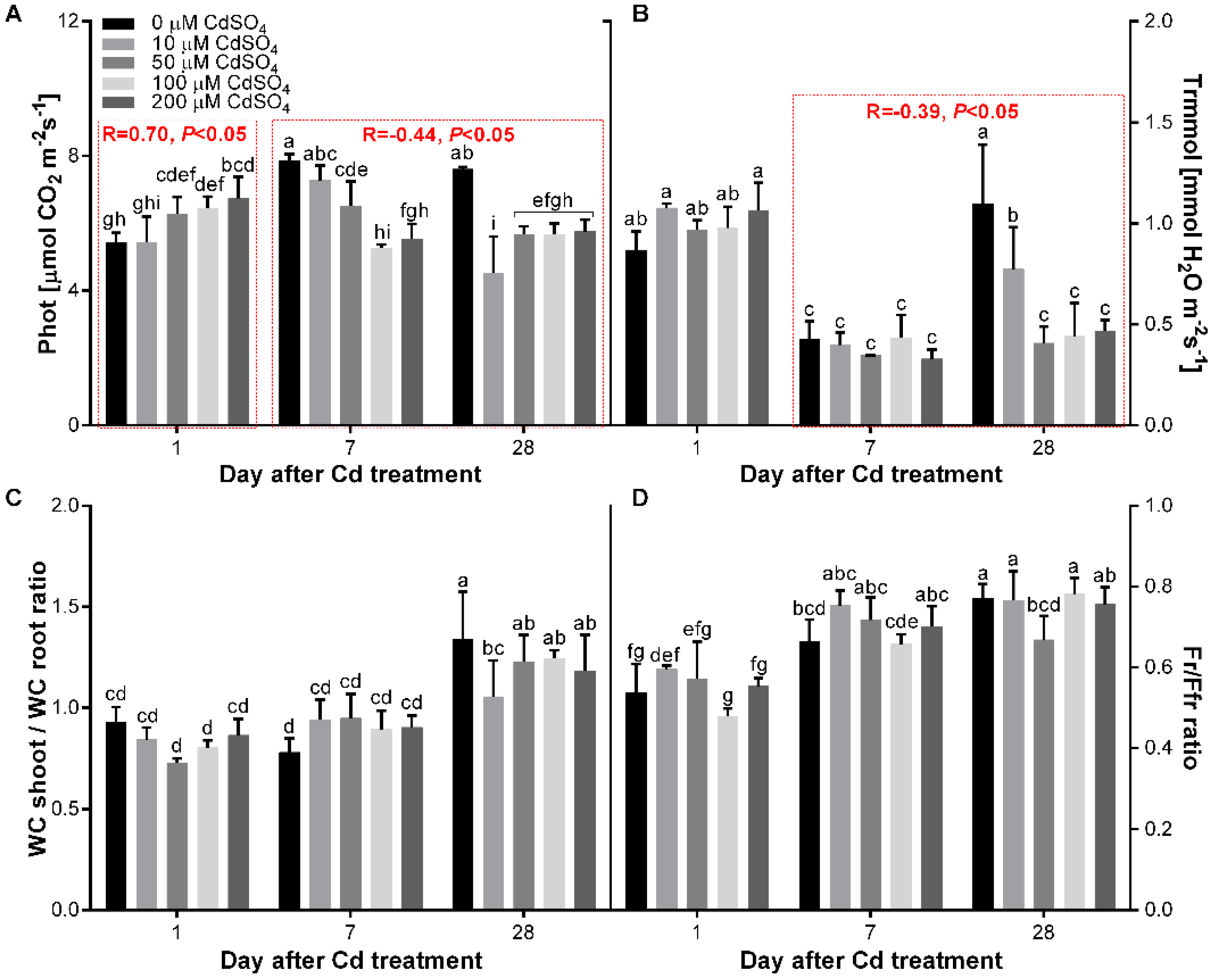
3.4. Effect of Cd on the Gas Exchange and Water Content of P. sativum L
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Sanità Toppi, L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; ISBN 0849315751. [Google Scholar]
- Gusmão Lima, A.I.; Pereira, S.I.A.; de Almeida Figueira, E.M.; Caldeira, G.C.N.; Caldeira, H.D.Q. Cadmium Uptake in PEA Plants under Environmentally-Relevant Exposures: The Risk of Food-Chain Transfer. J. Plant Nutr. 2006, 29, 2165–2177. [Google Scholar] [CrossRef]
- Simpson, G.G.; Dean, C. Arabidopsis, the Rosetta Stone of Flowering Time? Science 2002, 296, 285–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szőllősi, R.; Varga, I.S.; Erdei, L.; Mihalik, E. Cadmium-induced oxidative stress and antioxidative mechanisms in germinating Indian mustard (Brassica juncea L.) seeds. Ecotoxicol. Environ. Saf. 2009, 72, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Możdżeń, K.; Zagata, P.; Migdałek, G.; Rut, G.; Rzepka, A. Effect of cadmium nitrate on morphological parameters of Lupinus luteus L. and L. angustifolius L. Mod. Phytomorphology 2015, 7, 75–79. [Google Scholar] [CrossRef]
- Liu, S.; Yang, C.; Xie, W.; Xia, C.; Fan, P. The Effects of Cadmium on Germination and Seedling Growth of Suaeda salsa. Procedia Environ. Sci. 2012, 16, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Kastori, R.; Maksimovic, I.; Putnik-Delic, M.; Momcilovic, V.; Rajic, M. Effect of cadmium on germination and growth of wheat. Zb. Matice Srp. Za Prir. Nauk. 2019, 136, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Almuwayhi, M.A. Effect of cadmium on the molecular and morpho-physiological traits of Pisum sativum L. Biotechnol. Biotechnol. Equip. 2021, 35, 1374–1384. [Google Scholar] [CrossRef]
- Keunen, E.; Truyens, S.; Bruckers, L.; Remans, T.; Vangronsveld, J.; Cuypers, A. Survival of Cd-exposed Arabidopsis thaliana: Are these plants reproductively challenged? Plant Physiol. Biochem. 2011, 49, 1084–1091. [Google Scholar] [CrossRef]
- Maistri, S.; DalCorso, G.; Vicentini, V.; Furini, A. Cadmium affects the expression of ELF4, a circadian clock gene in Arabidopsis. Environ. Exp. Bot. 2011, 72, 115–122. [Google Scholar] [CrossRef]
- Wang, Y.; Zong, K.; Jiang, L.; Sun, J.; Ren, Y.; Sun, Z.; Wen, C.; Chen, X.; Cao, S. Characterization of an Arabidopsis cadmium-resistant mutant cdr3-1D reveals a link between heavy metal resistance as well as seed development and flowering. Planta 2011, 233, 697–706. [Google Scholar] [CrossRef]
- Wang, W.Y.; Xu, J.; Liu, X.J.; Yu, Y.; Ge, Q. Cadmium induces early flowering in Arabidopsis. Biol. Plant. 2012, 56, 117–120. [Google Scholar] [CrossRef]
- Eid, R.A.; Mazher, A.A.M.; Khalifa, R.K.M.; Shaaban, S.H.A. Effect of cadmium concentrations on the vegetative growth, flowering and chemical constituents of Tagets erecta L. plant. Int. J. PharmTech Res. 2016, 9, 18–24. [Google Scholar]
- Sandalio, L.M.; Dalurzo, H.C.; Gómez, M.; Romero-Puertas, M.C.; Del Río, L.A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001, 52, 2115–2126. [Google Scholar] [CrossRef]
- Nada, E.; Ferjani, B.A.; Ali, R.; Bechir, B.R.; Imed, M.; Makki, B. Cadmium-induced growth inhibition and alteration of biochemical parameters in almond seedlings grown in solution culture. Acta Physiol. Plant 2007, 29, 57–62. [Google Scholar] [CrossRef]
- Liu, L.; Sun, H.; Chen, J.; Zhang, Y.; Li, D.; Li, C. Effects of cadmium (Cd) on growth, chlorophyll content, and photosynthesis of cotton seedlings (Gossypium hirsutum L.). Plant Omics 2014, 7, 284–290. [Google Scholar]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavasseur, A.; Forestier, C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef]
- Shi, G.; Liu, C.; Cai, Q.; Liu, Q.; Hou, C. Cadmium accumulation and tolerance of two safflower cultivars in relation to photosynthesis and antioxidative enzymes. Bull. Environ. Contam. Toxicol. 2010, 85, 256–263. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Głowacka, K.; Źróbek-Sokolnik, A.; Okorski, A.; Najdzion, J. The Effect of Cadmium on the Activity of Stress-Related Enzymes and the Ultrastructure of Pea Roots. Plants 2019, 8, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Chong, J.; Li, C.; Kim, C.; Shi, D.; Wang, D. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 2007, 294, 263–276. [Google Scholar] [CrossRef]
- Gunderson, C.A.; Sholtis, J.D.; Wullschleger, S.D.; Tissue, D.T.; Hanson, P.J.; Norby, R.J. Environmental and stomatal control of photosynthetic enhancement in the canopy of a sweetgum (Liquidambar styraciflua L.) plantation during 3 years of CO2 enrichment. Plant Cell Environ. 2002, 25, 379–393. [Google Scholar] [CrossRef]
- Pfündel, E.; Neubohn, B. Assessing photosystem I and II distribution in leaves from C4 plants using confocal laser scanning microscopy. Plant Cell Environ. 1999, 22, 1569–1577. [Google Scholar] [CrossRef]
- Gouveia-Neto, S.A.; Silva-Jr, E.A.; Cunha, P.C.; Oliveira-Filho, R.A.; Silva, L.H.M.; Da Costa, E.B.; Câmara, T.J.R.; Willadino, L.G. Abiotic Stress Diagnosis via Laser Induced Chlorophyll Fluorescence Analysis in Plants for Biofuel: 1. In Biofuel Production; Bernardes, M.A.d.S., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- White, R.G. Using Chlorophyll Fluorescence to Rapidly Discriminate C3 from C4 Photosynthesis in Plants. 2012. Available online: http://www.emc2012.org.uk/documents/Abstracts/Abstracts/EMC2012_1121.pdf (accessed on 8 June 2018).
- Lichtenthaler, U.; Rinderle, H.K. The Chlorophyll Fluorescence Ratio F690/F735 as a Possible Stress Indicator. In Applications of Chlorophyll Fluorescence in Photosynthesis Research, Stress Physiology, Hydrobiology and Remote Sensing; Lichtenthaler, H.K., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 1988; pp. 189–196. [Google Scholar]
- Hédiji, H.; Djebali, W.; Belkadhi, A.; Cabasson, C.; Moing, A.; Rolin, D.; Brouquisse, R.; Gallusci, P.; Chaïbi, W. Impact of long-term cadmium exposure on mineral content of Solanum lycopersicum plants: Consequences on fruit production. S. Afr. J. Bot. 2015, 97, 176–181. [Google Scholar] [CrossRef]
- Carvalho Bertoli, A.; Gabriel Cannata, M.; Carvalho, R.; Ribeiro Bastos, A.R.; Puggina Freitas, M.; dos Santos Augusto, A. Lycopersicon esculentum submitted to Cd-stressful conditions in nutrition solution: Nutrient contents and translocation. Ecotoxicol. Environ. Saf. 2012, 86, 176–181. [Google Scholar] [CrossRef]
- Gonçalves, J.F.; Antes, F.G.; Maldaner, J.; Pereira, L.B.; Tabaldi, L.A.; Rauber, R.; Rossato, L.V.; Bisognin, D.A.; Dressler, V.L.; de Moraes Flores, É.M.; et al. Cadmium and mineral nutrient accumulation in potato plantlets grown under cadmium stress in two different experimental culture conditions. Plant Physiol. Biochem. 2009, 47, 814–821. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Zheng, L.; Wang, Y.; Luo, L.; Ma, M.; Zhang, C.; Han, Y.; Beeckman, T.; Xu, G.; et al. Cadmium stress suppresses lateral root formation by interfering with the root clock. Plant Cell Environ. 2019, 42, 3182–3196. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Xuan, W.; Huang, X.-Y.; Mao, C.; Zhao, F.-J. Cadmium Inhibits Lateral Root Emergence in Rice by Disrupting OsPIN-Mediated Auxin Distribution and the Protective Effect of OsHMA3. Plant Cell Physiol. 2021, 62, 166–177. [Google Scholar] [CrossRef]
- Djebali, W.; Hédiji, H.; Abbes, Z.; Barhoumi, Z.; Yaakoubi, H.; Zoghlami, L.B.; Chaïbi, W. Aspects on growth and anatomy of internodes and leaves of cadmium-treated Solanum lycopersicum L. plants. J. Biol. Res. 2010, 13, 75–84. [Google Scholar]
- Mayonde, S.; Cron, G.V.; Glennon, K.L.; Byrne, M.J. Effects of cadmium toxicity on the physiology and growth of a halophytic plant, Tamarix usneoides (E. Mey. ex Bunge). Int. J. Phytoremediat. 2021, 23, 130–138. [Google Scholar] [CrossRef]
- Elobeid, M.; Göbel, C.; Feussner, I.; Polle, A. Cadmium interferes with auxin physiology and lignification in poplar. J. Exp. Bot. 2012, 63, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Nowak, J. Effects of cadmium and lead concentrations and arbuscular mycorrhiza on growth, flowering and heavy metal accumulation in scarlet sage (Salvia splendens Sello ‘torreador’). Acta Agrobot. 2012, 60, 79–83. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Shi, Y.; Zhao, M.Q.; Chi, G.Y. Effects of cadmium on growth and photosynthetic activities in pakchoi and mustard. Bot. Stud. 2011, 52, 41–46. [Google Scholar]
- Wada, K.C.; Takeno, K. Stress-Induced Flowering. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 331–345. ISBN 978-1-4614-0633-4. [Google Scholar]
- Takeno, K. Stress-induced flowering: The third category of flowering response. J. Exp. Bot. 2016, 67, 4925–4934. [Google Scholar] [CrossRef] [Green Version]
- Maurya, R.; Prasad, S.M.; Gopal, R. LIF technique offers the potential for the detection of cadmium-induced alteration in photosynthetic activities of Zea Mays L. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 29–35. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Deng, G.; Li, M.; Li, H.; Yin, L.; Li, W. Exposure to cadmium causes declines in growth and photosynthesis in the endangered aquatic fern (Ceratopteris pteridoides). Aquat. Bot. 2014, 112, 23–32. [Google Scholar] [CrossRef]
- Chugh, L.K.; Sawhney, S.K. Photosynthetic activities of Pisum sativum seedlings grown in presence of cadmium. Plant Physiol. Biochem. 1999, 37, 297–303. [Google Scholar] [CrossRef]
- Datta, J.K.; Ghosh, D.; Banerjee, A.; Mondal, N.K. Studies on the impact of cadmium on growth, yield attributes, yield and biochemistry of mung bean (Vigna radiata L. Wilczek) under natural field condition, burdwan, west bengal. Sci. Agric. 2016, 14, 202. [Google Scholar] [CrossRef]
| A | |||||
|---|---|---|---|---|---|
| Variable | Correlation Coefficient R, p < 0.05 | ||||
| Photosynthesis Rate after 28 Days of Cd Treatment | |||||
| WC of the shoot/WC of the root ratio after 28 days of Cd treatment | 0.93 | ||||
| B | |||||
| Variable | Correlation Coefficient R, p < 0.05 | ||||
| Fr/Ffr Ratio | F690/F735 Ratio | Transpiration Rate | WC of the Shoot/WC of the Root Ratio | OSFq Abaxial | |
| Fr/Ffr ratio | - | 0.99 | −0.54 | 0.68 | ns |
| F690/F735 ratio | 0.99 | - | ns | 0.72 | ns |
| OSFq adaxial | −0.61 | −0.54 | 0.55 | ns | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głowacka, K.; Olszewski, J.; Sowiński, P.; Kalisz, B.; Najdzion, J. Developmental and Physiological Responses of Pisum sativum L. after Short- and Long-Time Cadmium Exposure. Agriculture 2022, 12, 637. https://doi.org/10.3390/agriculture12050637
Głowacka K, Olszewski J, Sowiński P, Kalisz B, Najdzion J. Developmental and Physiological Responses of Pisum sativum L. after Short- and Long-Time Cadmium Exposure. Agriculture. 2022; 12(5):637. https://doi.org/10.3390/agriculture12050637
Chicago/Turabian StyleGłowacka, Katarzyna, Jacek Olszewski, Paweł Sowiński, Barbara Kalisz, and Janusz Najdzion. 2022. "Developmental and Physiological Responses of Pisum sativum L. after Short- and Long-Time Cadmium Exposure" Agriculture 12, no. 5: 637. https://doi.org/10.3390/agriculture12050637
APA StyleGłowacka, K., Olszewski, J., Sowiński, P., Kalisz, B., & Najdzion, J. (2022). Developmental and Physiological Responses of Pisum sativum L. after Short- and Long-Time Cadmium Exposure. Agriculture, 12(5), 637. https://doi.org/10.3390/agriculture12050637






