Investigating the Impact of Ultrasound, Microwave, and High-Pressure Processing of Milk on the Volatile Compounds and Sensory Properties of Cheddar Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procurement of Raw Material
2.2. Pre-Treatments
2.3. Manufacturing of Cheddar Cheese
2.4. Determination of Volatile Compounds in Milk and Cheese by GC-MS
2.4.1. Oven
2.4.2. Front Inlet
2.4.3. Column
2.4.4. Front Injector
2.5. Sensory Evaluation of Cheddar Cheese
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Processing Techniques on Volatile Compounds of Milk
3.1.1. Alcohols
3.1.2. Aldehydes
3.1.3. Ketones
3.1.4. Hydrocarbons
3.1.5. Acids
3.1.6. Esters
3.1.7. Furan
3.2. Effect of Processing Techniques on Volatile Compounds of Cheddar Cheese
3.2.1. Alcohols
3.2.2. Aldehydes
3.2.3. Ketones
3.2.4. Hydrocarbons
3.2.5. Alkenes
3.2.6. Esters
3.2.7. Acids
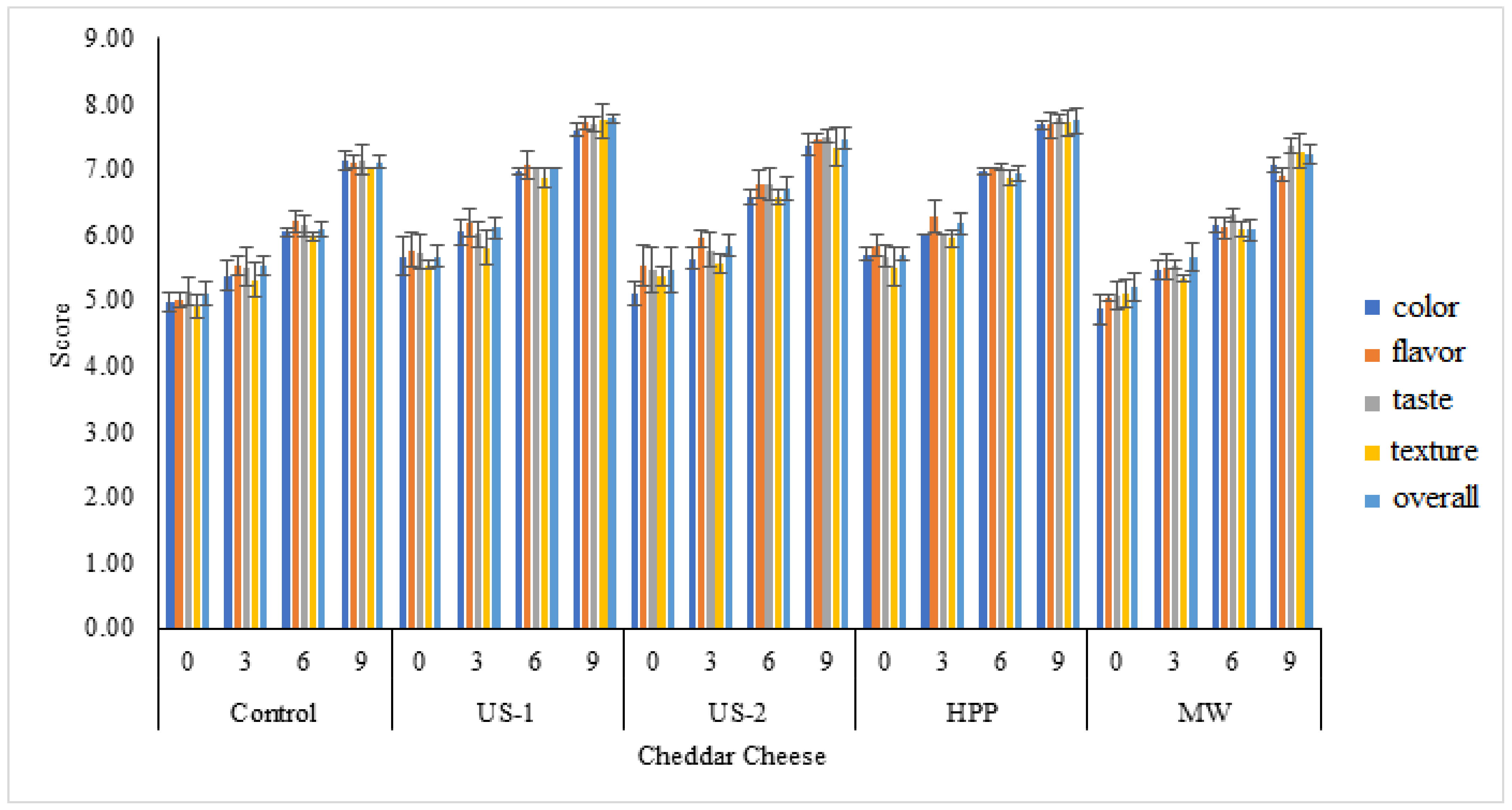
3.3. Effect of Processing Techniques on Sensory Quality of Cheddar Cheese
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, P.N.; Arora, S. Comprehensive Statistical Methods. Chapter 19; S. Chand Publishing: New Delhi, India, 2007; pp. 19–37. [Google Scholar]
- Food and Agricultural Organization of the United Nations. Dairy Production and Products. Available online: http://www.fao.org/agriculture/dairygateway/milk-and-milk-products/en/#.VuL1rLnSnrd (accessed on 20 December 2021).
- Rozenberg, S.; Body, J.-J.; Bruyere, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelaer, J.-P.; Gielen, E.; Goemaere, S.; Kaufman, J.-M. Effects of Dairy Products Consumption on Health: Benefits and Beliefs—A Commentary from the Belgian Bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif. Tissue Int. 2016, 98, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, Z.F.; Bhat, H. Milk and Dairy Products as Functional Foods: A Review. Int. J. Dairy Sci. 2011, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Raza, N.; Kim, K.-H. Quantification Techniques for Important Environmental Contaminants in Milk and Dairy Products. TrAC Trends Anal. Chem. 2018, 98, 79–94. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. (Eds.) Biochemistry of Cheese Ripening BT—Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2017; pp. 391–442. ISBN 978-1-4899-7681-9. [Google Scholar]
- McSweeney, P.L.H. Biochemistry of Cheese Ripening: Introduction and Overview. Cheese Chem. Phys. Microbiol. 2004, 1, 347–360. [Google Scholar]
- Niimi, J.; Eddy, A.I.; Overington, A.R.; Silcock, P.; Bremer, P.J.; Delahunty, C.M. Sensory Interactions between Cheese Aroma and Taste. J. Sens. Stud. 2015, 30, 247–257. [Google Scholar] [CrossRef]
- Zehentbauer, G.; Reineccius, G.A. Determination of Key Aroma Components of Cheddar Cheese Using Dynamic Headspace Dilution Assay. Flavour Fragr. J. 2002, 17, 300–305. [Google Scholar] [CrossRef]
- Urbach, G. The Flavour of Milk and Dairy Products: II. Cheese: Contribution of Volatile Compounds. Int. J. Dairy Technol. 1997, 50, 79–89. [Google Scholar] [CrossRef]
- Ganesan, B.; Weimer, B.C. Amino Acid Catabolism and Its Relationship to Cheese Flavor Outcomes. In Cheese; Elsevier: Amsterdam, The Netherlands, 2017; pp. 483–516. [Google Scholar]
- Barron, L.J.R.; Redondo, Y.; Flanagan, C.E.; Pérez-Elortondo, F.J.; Albisu, M.; Nájera, A.I.; de Renobales, M.; Fernández-García, E. Comparison of the Volatile Composition and Sensory Characteristics of Spanish PDO Cheeses Manufactured from Ewes’ Raw Milk and Animal Rennet. Int. Dairy J. 2005, 15, 371–382. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical Pathways for the Production of Flavour Compounds in Cheeses during Ripening: A Review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Drake, M.A.; Delahunty, C.M. Sensory Character of Cheese and Its Evaluation. In Cheese; Elsevier: Amsterdam, The Netherlands, 2017; pp. 517–545. [Google Scholar]
- Kilcawley, K.N. Cheese Flavour BT—Fundamentals of Cheese Science; Fox, P.F., Guinee, T.P., Cogan, T.M., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2017; pp. 443–474. ISBN 978-1-4899-7681-9. [Google Scholar]
- Meshram, B.D.; Vyahaware, A.N.; Wasnik, P.G.; Agrawal, A.K.; Sandey, K.K. Microwave Processing of Milk: A Review. In Processing Technologies for Milk and Milk Products; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 219–251. [Google Scholar]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave Food Processing—A Review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Costa, N.R.; Cappato, L.P.; Ferreira, M.V.S.; Pires, R.P.S.; Moraes, J.; Esmerino, E.A.; Silva, R.; Neto, R.P.C.; Tavares, M.I.B.; Freitas, M.Q. Ohmic Heating: A Potential Technology for Sweet Whey Processing. Food Res. Int. 2018, 106, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Amaral, G.V.; Silva, E.K.; Cavalcanti, R.N.; Cappato, L.P.; Guimaraes, J.T.; Alvarenga, V.O.; Esmerino, E.A.; Portela, J.B.; Sant’Ana, A.S.; Freitas, M.Q. Dairy Processing Using Supercritical Carbon Dioxide Technology: Theoretical Fundamentals, Quality and Safety Aspects. Trends Food Sci. Technol. 2017, 64, 94–101. [Google Scholar] [CrossRef]
- Kasahara, I.; Carrasco, V.; Aguilar, L. Inactivation of Escherichia Coli in Goat Milk Using Pulsed Ultraviolet Light. J. Food Eng. 2015, 152, 43–49. [Google Scholar] [CrossRef]
- McAuley, C.M.; Singh, T.K.; Haro-Maza, J.F.; Williams, R.; Buckow, R. Microbiological and Physicochemical Stability of Raw, Pasteurised or Pulsed Electric Field-Treated Milk. Innov. Food Sci. Emerg. Technol. 2016, 38, 365–373. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Silva, E.K.; Alvarenga, V.O.; Costa, A.L.R.; Cunha, R.L.; Sant’Ana, A.S.; Freitas, M.Q.; Meireles, M.A.A.; Cruz, A.G. Physicochemical Changes and Microbial Inactivation after High-Intensity Ultrasound Processing of Prebiotic Whey Beverage Applying Different Ultrasonic Power Levels. Ultrason. Sonochem. 2018, 44, 251–260. [Google Scholar] [CrossRef]
- De Toledo Guimarães, J.; Silva, E.K.; de Freitas, M.Q.; de Almeida Meireles, M.A.; da Cruz, A.G. Non-Thermal Emerging Technologies and Their Effects on the Functional Properties of Dairy Products. Curr. Opin. Food Sci. 2018, 22, 62–66. [Google Scholar] [CrossRef]
- Monteiro, S.H.M.C.; Silva, E.K.; Alvarenga, V.O.; Moraes, J.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Sant’Ana, A.S.; Meireles, M.A.A.; Cruz, A.G. Effects of Ultrasound Energy Density on the Non-Thermal Pasteurization of Chocolate Milk Beverage. Ultrason. Sonochem. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S. Cold Plasma Processing of Milk and Dairy Products. Trends Food Sci. Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Miller, B.M.; Sauer, A.; Moraru, C.I. Inactivation of Escherichia Coli in Milk and Concentrated Milk Using Pulsed-Light Treatment. J. Dairy Sci. 2012, 95, 5597–5603. [Google Scholar] [CrossRef] [Green Version]
- Ashokkumar, M.; Mason, T.J. Sonochemistry. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Vercet, A.; Oria, R.; Marquina, P.; Crelier, S.; Lopez-Buesa, P. Rheological Properties of Yoghurt Made with Milk Submitted to Manothermosonication. J. Agric. Food Chem. 2002, 50, 6165–6171. [Google Scholar] [CrossRef]
- Yasui, K. Influence of Ultrasonic Frequency on Multibubble Sonoluminescence. J. Acoust. Soc. Am. 2002, 112, 1405–1413. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Kentish, S.; Ashokkumar, M. Influence of Ultrasound on Chemically Induced Gelation of Micellar Casein Systems. J. Dairy Res. 2013, 80, 138–143. [Google Scholar] [CrossRef]
- Leong, T.S.H.; Walter, V.; Gamlath, C.J.; Yang, M.; Martin, G.J.O.; Ashokkumar, M. Functionalised Dairy Streams: Tailoring Protein Functionality Using Sonication and Heating. Ultrason. Sonochem. 2018, 48, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, A.J.; Capellas, M.; Buffa, M.; Royo, C.; Gervilla, R.; Felipe, X.; Sendra, E.; Saldo, J.; Ferragut, V.; Guamis, B. Application of High Pressure Treatment for Cheese Production. Food Res. Int. 2000, 33, 311–316. [Google Scholar] [CrossRef]
- O’Reilly, C.E.; O’Connor, P.M.; Murphy, P.M.; Kelly, A.L.; Beresford, T.P. The Effect of Exposure to Pressure of 50 MPa on Cheddar Cheese Ripening. Innov. Food Sci. Emerg. Technol. 2000, 1, 109–117. [Google Scholar] [CrossRef]
- Calzada, J.; del Olmo, A.; Picon, A.; Nuñez, M. Effect of High-Pressure-Processing on Lipolysis and Volatile Compounds of Brie Cheese during Ripening and Refrigerated Storage. Int. Dairy J. 2014, 39, 232–239. [Google Scholar] [CrossRef]
- Kudra, T.; Voort, F.; Raghavan, G.; Ramaswamy, H. Heating Characteristics of Milk Constituents in a Microwave Pasteurization System. J. Food Sci. 1991, 56, 931–934. [Google Scholar] [CrossRef]
- Salazar-González, C.; Martín-González, S.; Fernanda, M.; López-Malo, A.; Sosa-Morales, M.E. Recent Studies Related to Microwave Processing of Fluid Foods. Food Bioprocess Technol. 2012, 5, 31–46. [Google Scholar] [CrossRef]
- Iuliana, C.; Rodica, C.; Sorina, R.; Oana, M. Impact of Microwaves on the Physico-Chemical Characteristics of Cow Milk. Rom. Rep. Phys. 2015, 67, 423–430. [Google Scholar]
- Bai, Y.; Saren, G.; Huo, W. Response Surface Methodology (RSM) in Evaluation of the Vitamin C Concentrations in Microwave Treated Milk. J. Food Sci. Technol. 2015, 52, 4647–4651. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Alcalá, L.M.; Alonso, L.; Fontecha, J. Stability of Fatty Acid Composition after Thermal, High Pressure, and Microwave Processing of Cow Milk as Affected by Polyunsaturated Fatty Acid Concentration. J. Dairy Sci. 2014, 97, 7307–7315. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.; Shah, N.P. Probiotic Cheddar Cheese: Influence of Ripening Temperatures on Survival of Probiotic Microorganisms, Cheese Composition and Organic Acid Profiles. LWT Food Sci. Technol. 2009, 42, 1260–1268. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Gamlath, C.J.; Martin, G.J.O.; Hemar, Y.; Ashokkumar, M. Effect of Sonication, Microwaves and High-Pressure Processing on ACE-Inhibitory Activity and Antioxidant Potential of Cheddar Cheese during Ripening. Ultrason. Sonochem. 2020, 67, 105140. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Martínez, F.J.; Carrapiso, A.I.; Contador, R.; Ramírez, M.R. Volatile Compounds and Sensory Changes after High Pressure Processing of Mature “Torta Del Casar”(Raw Ewe’s Milk Cheese) during Refrigerated Storage. Innov. Food Sci. Emerg. Technol. 2019, 52, 34–41. [Google Scholar] [CrossRef]
- Molimard, P.; Spinnler, H.E. Review: Compounds Involved in the Flavor of Surface Mold-Ripened Cheeses: Origins and Properties. J. Dairy Sci. 1996, 79, 169–184. [Google Scholar] [CrossRef]
- Vazquez-Landaverde, P.A.; Torres, J.A.; Qian, M.C. Effect of High-Pressure−Moderate-Temperature Processing on the Volatile Profile of Milk. J. Agric. Food Chem. 2006, 54, 9184–9192. [Google Scholar] [CrossRef]
- Valero, E.; Villamiel, M.; Sanz, J.; Martínez-Castro, I. Chemical and Sensorial Changes in Milk Pasteurised by Microwave and Conventional Systems during Cold Storage. Food Chem. 2000, 70, 77–81. [Google Scholar] [CrossRef]
- Chouliara, E.; Georgogianni, K.G.; Kanellopoulou, N.; Kontominas, M.G. Effect of Ultrasonication on Microbiological, Chemical and Sensory Properties of Raw, Thermized and Pasteurized Milk. Int. Dairy J. 2010, 20, 307–313. [Google Scholar] [CrossRef]
- Karatapanis, A.E.; Badeka, A.V.; Riganakos, K.A.; Savvaidis, I.N.; Kontominas, M.G. Changes in Flavour Volatiles of Whole Pasteurized Milk as Affected by Packaging Material and Storage Time. Int. Dairy J. 2006, 16, 750–761. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Silva, E.K.; Ranadheera, C.S.; Moraes, J.; Raices, R.S.L.; Silva, M.C.; Ferreira, M.S.; Freitas, M.Q.; Meireles, M.A.A.; Cruz, A.G. Effect of High-Intensity Ultrasound on the Nutritional Profile and Volatile Compounds of a Prebiotic Soursop Whey Beverage. Ultrason. Sonochem. 2019, 55, 157–164. [Google Scholar] [CrossRef]
- Juliano, P.; Torkamani, A.E.; Leong, T.; Kolb, V.; Watkins, P.; Ajlouni, S.; Singh, T.K. Lipid Oxidation Volatiles Absent in Milk after Selected Ultrasound Processing. Ultrason. Sonochem. 2014, 21, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Grewell, D.; Annandarajah, C.; Benner, L.; Clark, S. Quality Characteristics and Plasmin Activity of Thermosonicated Skim Milk and Cream. J. Dairy Sci. 2015, 98, 6678–6691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchesini, G.; Fasolato, L.; Novelli, E.; Balzan, S.; Contiero, B.; Montemurro, F.; Andrighetto, I.; Segato, S. Ultrasonic Inactivation of Microorganisms: A Compromise between Lethal Capacity and Sensory Quality of Milk. Innov. Food Sci. Emerg. Technol. 2015, 29, 215–221. [Google Scholar] [CrossRef]
- Wu, H.; Hulbert, G.J.; Mount, J.R. Effects of Ultrasound on Milk Homogenization and Fermentation with Yogurt Starter. Innov. Food Sci. Emerg. Technol. 2000, 1, 211–218. [Google Scholar] [CrossRef]
- Pereda, J.; Jaramillo, D.P.; Quevedo, J.M.; Ferragut, V.; Guamis, B.; Trujillo, A.J. Characterization of Volatile Compounds in Ultra-High-Pressure Homogenized Milk. Int. Dairy J. 2008, 18, 826–834. [Google Scholar] [CrossRef]
- Yao, Y. Enhancement of Mass Transfer by Ultrasound: Application to Adsorbent Regeneration and Food Drying/Dehydration. Ultrason. Sonochem. 2016, 31, 512–531. [Google Scholar] [CrossRef]
- Ardö, Y.; McSweeney, P.L.H.; Magboul, A.A.A.; Upadhyay, V.K.; Fox, P.F. Chapter 18—Biochemistry of Cheese Ripening: Proteolysis; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W.B.T.-C., Fourth, E., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 445–482. ISBN 978-0-12-417012-4. [Google Scholar]
- Juan, B.; Barron, L.J.R.; Ferragut, V.; Trujillo, A.J. Effects of High Pressure Treatment on Volatile Profile During Ripening of Ewe Milk Cheese. J. Dairy Sci. 2007, 90, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Ávila, M.; Gómez-Torres, N.; Delgado, D.; Gaya, P.; Garde, S. Effect of High-Pressure Treatments on Proteolysis, Volatile Compounds, Texture, Colour, and Sensory Characteristics of Semi-Hard Raw Ewe Milk Cheese. Food Res. Int. 2017, 100, 595–602. [Google Scholar] [CrossRef]
- Curioni, P.M.G.; Bosset, J.O. Key Odorants in Various Cheese Types as Determined by Gas Chromatography-Olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Pico, J.; Bernal, J.; Gómez, M. Wheat bread aroma compounds in crumb and crust: A review. Food Res. Int. 2015, 75, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Collins, Y.F.; McSweeney, P.L.H.; Wilkinson, M.G. Lipolysis and Free Fatty Acid Catabolism in Cheese: A Review of Current Knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Holland, R.; Crow, V.L. Esters and Their Biosynthesis in Fermented Dairy Products: A Review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- Ortigosa, M.; Torre, P.; Izco, J.M. Effect of Pasteurization of Ewe’s Milk and Use of a Native Starter Culture on the Volatile Components and Sensory Characteristics of Roncal Cheese. J. Dairy Sci. 2001, 84, 1320–1330. [Google Scholar] [CrossRef]
- Corrëa Lelles Nogueira, M.; Lubachevsky, G.; Rankin, S.A. A Study of the Volatile Composition of Minas Cheese. LWT-Food Sci. Technol. 2005, 38, 555–563. [Google Scholar] [CrossRef]
- Moio, L.; Addeo, F. Grana Padano Cheese Aroma. J. Dairy Res. 1998, 65, 317–333. [Google Scholar] [CrossRef]
- Frank, D.C.; Owen, C.M.; Patterson, J. Solid Phase Microextraction (SPME) Combined with Gas-Chromatography and Olfactometry-Mass Spectrometry for Characterization of Cheese Aroma Compounds. LWT-Food Sci. Technol. 2004, 37, 139–154. [Google Scholar] [CrossRef]
- Chen, G.; Song, H.; Ma, C. Aroma-Active Compounds of Beijing Roast Duck. Flavour Fragr. J. 2009, 24, 186–191. [Google Scholar] [CrossRef]
- Hickey, C.D.; O’Sullivan, M.G.; Davis, J.; Scholz, D.; Kilcawley, K.N.; Wilkinson, M.G.; Sheehan, J.J. The Effect of Buttermilk or Buttermilk Powder Addition on Functionality, Textural, Sensory and Volatile Characteristics of Cheddar-Style Cheese. Food Res. Int. 2018, 103, 468–477. [Google Scholar] [CrossRef] [Green Version]
- Bertuzzi, A.S.; McSweeney, P.L.H.; Rea, M.C.; Kilcawley, K.N. Detection of Volatile Compounds of Cheese and Their Contribution to the Flavor Profile of Surface-Ripened Cheese. Compr. Rev. Food Sci. Food Saf. 2018, 17, 371–390. [Google Scholar] [CrossRef] [Green Version]
- Voigt, D.D.; Donaghy, J.A.; Patterson, M.F.; Stephan, S.; Kelly, A.L. Manufacture of Cheddar Cheese from High-Pressure-Treated Whole Milk. Innov. Food Sci. Emerg. Technol. 2010, 11, 574–579. [Google Scholar] [CrossRef]
- Voigt, D.D.; Chevalier, F.; Donaghy, J.A.; Patterson, M.F.; Qian, M.C.; Kelly, A.L. Effect of High-Pressure Treatment of Milk for Cheese Manufacture on Proteolysis, Lipolysis, Texture and Functionality of Cheddar Cheese during Ripening. Innov. Food Sci. Emerg. Technol. 2012, 13, 23–30. [Google Scholar] [CrossRef]
- Jeličić, I.; Božanić, R.; Brnčić, M.; Tripalo, B. Influence and Comparison of Thermal, Ultrasonic and Thermo-Sonic Treatments on Microbiological Quality and Sensory Properties of Rennet Cheese Whey. Mljekarstvo Časopis Za Unaprjeđenje Proizvodnje I Prerade Mlijeka 2012, 62, 165–178. [Google Scholar]
- Clare, D.A.; Bang, W.S.; Cartwright, G.; Drake, M.A.; Coronel, P.; Simunovic, J. Comparison of Sensory, Microbiological, and Biochemical Parameters of Microwave Versus Indirect UHT Fluid Skim Milk During Storage. J. Dairy Sci. 2005, 88, 4172–4182. [Google Scholar] [CrossRef] [Green Version]
- Villamiel, M.; Corzo, N.; Martínez-Castro, I.; Olano, A. Chemical Changes during Microwave Treatment of Milk. Food Chem. 1996, 56, 385–388. [Google Scholar] [CrossRef]
| Volatile Compounds Alcohols | CAS No. | RT (Min) | Control (% Area) | HPP (% Area) | US (% Area) | MW (% Area) |
|---|---|---|---|---|---|---|
| Pentanol | 001320-98-5 | 1.5763 | 1.18 ± 0.002 a | 1.15 ± 0.003 b | 1.15 ± 0.002 b | 1.15 ± 0.004 b |
| Hexanol | 000111-27-3 | 1.6246 | 0.81 ± 0.01 b | 0.76 ± 0.03 b | 1.02 ± 0.02 a | 0.79 ± 0.02 b |
| 2-methyl-3-Buten-2-Ol | 024509-88-4 | 1.7833 | ND | ND | 1.22 ± 0.05 a | ND |
| Octanol | 000589-98-0 | 1.804 | 2.97 ± 0.04 b | 2.89 ± 0.02 c | 3.144 ± 0.06 a | ND |
| 2,6-Dimethyl-2-Heptanol | 013254-34-7 | 2.1486 | 0.558 ± 0.001 a | ND | ND | 0.609 ± 0.002 a |
| 3-Methyl-2-Buten-1-Ol | 000556-82-1 | 2.2522 | ND | 1.375 ± 0.01 a | 1.387 ± 0.01 a | ND |
| Isopentyl alcohol | 000123-51-3 | 3.6518 | 2.479 ± 0.01 a | ND | ND | ND |
| 3-Methyl-1-Butanol | 000123-51-3 | 3.6794 | ND | ND | ND | 2.454 ± 0.03 a |
| Aldehydes | ||||||
| Heptanal | 000111-71-7 | 1.4936 | ND | 1.594 ± 0.004 b | 1.615 ± 0.002 a | ND |
| Ketones | ||||||
| Pentadecanone | 002345-28-0 | 1.6177 | 1.693 ± 0.02 a | 1.643 ± 0.01 ab | 1.670 ± 0.05 a | 1.596 ± 0.02 b |
| 2-Heptanone | 000110-43-0 | 1.6591 | 0.901 ± 0.003 d | 2.837 ± 0.03 b | 3.1718 ± 0.04 a | 1.289 ± 0.001 c |
| Octanone | 000111-13-7 | 1.7282 | 11.37 ± 0.11 c | 11.69 ± 0.18 b | 12.88 ± 0.13 a | 11.723 ± 0.12 b |
| 2-Butanonoe | 000078-93-3 | 2.0935 | 2.094 ± 0.005 b | 2.127 ± 0.006 b | 2.388 ± 0.02 a | 2.132 ± 0.02 b |
| Acetone | 000067-64-1 | 1.766 | 10.260 ± 0.034 c | 10.74 ± 0.01 b | 10.83 ± 0.05 a | 10.80 ± 0.07 ab |
| 2,3-pentadione | 000600-14-6 | 2.0452 | 1.1753 ± 0.002 a | ND | ND | ND |
| 3-octanone | 000106-68-3 | 2.0591 | ND | ND | 2.1237 ± a | ND |
| Hydrocarbons | ||||||
| Trichloromethane | 000067-66-3 | 2.2107 | 0.631 ± 0.001 c | 0.748 ± 0.00 b | 0.828 ± 0.002 a | 0.759 ± 0.004 ab |
| 4-Methyl-Heptane | 000589-53-7 | 3.4175 | ND | ND | 0.5914 ± 0.00 a | ND |
| 3-Ethyl-Pentane | 000617-78-7 | 3.4244 | 0.484 ± 0.001 c | 0.466 ± 0.00 c | 0.701 ± 0.001 a | 0.577 ± 0.003 b |
| 2,3,3-Trimethyl-Pentane | 000560-21-4 | 3.5139 | 1.507 ± 0.002 a | ND | ND | 1.3684 ± 0.003 b |
| Hexadecane | 000544-76-3 | 3.541 | ND | ND | ND | 1.390± a |
| Decamethyl-cyclopentasiloxane | 0541-02-06 | 9.5956 | 16.23 ± 0.05 c | 16.15 ± 0.06 c | 18.54 ± 0.05 b | 21.72 ± 0.08 a |
| 1-Hexene | 000592-41-6 | 1.9695 | ND | 1.022 ± 0.00 b | 1.562 ± 0.005 a | ND |
| 2-Methyl-1-pentene | 000763-29-1 | 2.0039 | 0.8512 ± 0.00 c | 1.053 ± 0.003 b | 1.85 ± 0.003 a | 0.615 ± 0.004 d |
| 1,2,3,6,7,8-Hexahydro-Pyrene | 001732-13-4 | 4.4518 | ND | ND | 5.563 ± 0.01 a | ND |
| 2,4-Dimethyl-1-Heptene | 019549-87-2 | 4.7689 | ND | 0.884 ± 0.000 | 1.229 ± 0.000 a | ND |
| Furan | ||||||
| Tetrahydrofuran | 000109-99-9 | 2.2383 | 2.1603 ± 0.002 a | ND | ND | ND |
| Acids | ||||||
| Butanoic Acid | 000107-92-6 | 4.1759 | 1.731 ± 0.02 a | 1.718 ± 0.01 a | 0.685 ± 0.01 b | ND |
| Valeric Acid | 000105-43-1 | 7.2789 | ND | ND | 1.039 ± 0.002 a | ND |
| Heptanoic Acid | 000111-14-8 | 7.3201 | ND | ND | ND | 1.894 ± 0.001 |
| Hexanoic Acid | 000142-62-1 | 7.3133 | 3.14 ± 0.02 b | 3.21 ± 0.002 b | 3.416 ± 0.005 a | 2.74 ± 0.003 c |
| Octanoic Acid | 0124-07-02 | 10.2783 | ND | ND | 0.153 ± 0.00 a | ND |
| Benzene | ||||||
| Benzene | 001014-60-4 | 11.409 | ND | ND | 0.423 ± 0.00 a | ND |
| Esters | ||||||
| Ally Butanoate | 002051-78-7 | 2.2865 | 2.0 ± 0.001 b | ND | ND | 2.38 ± 0.001 a |
| Amyl Isobutyrate | 002445-72-9 | 3.5209 | ND | 0.907 ± 0.002 b | 1.035 ± 0.004 | ND |
| Methyl-Pentanoate | 000624-24-8 | 7.3684 | 2.223 ± 0.001 b | 2.260 ± 0.001 | 3.868 ± 0.009 a | ND |
| Category | Volatile Compounds | CAS | RT | Control | Us-2 | Us-1 | HPP | MW |
|---|---|---|---|---|---|---|---|---|
| Alcohols | 2,3-Butanediol | 000513-85-9 | 1.3283 | 0.63 ± 0.00 d | 4.54 ± 0.006 b | 1.8 ± 0.001 c | 5.58 ± 0.003 a | 1.58 ± 0.003 c |
| 2-Octanol | 000123-96-6 | 1.5902 | ND | 1.45 ± 0.00 b | 2.56 ± 0.001 a | 1.84 ± 0.002 b | ND | |
| Ethanethiol | 1975-08-01 | 1.804 | ND | 1.29 ± 0.002 a | ND | ND | ND | |
| Ethanol | 000064-17-5 | 1.3007 | 0.45 ± 0.00 d | ND | 3.62 ± 0.002 a | 2.95 ± 0.005 b | 2.03 ± 0.004 c | |
| Melaleucol | 000000-00-0 | 6.541 | ND | 1.36 ± 0.002 b | 1.06 ± 0.003 c | 1.59 ± 0.003 a | 0.98 ± 0.001 d | |
| Aldehydes | Hexanal | 000066-25-1 | 0.58 ± 0.00 a | ND | 0.17 ± 0.00 | 0.215 ± 0.00 | 0.51 ± 0.00 | |
| Pentanal | 000110-62-3 | 2.79 | ND | 1.93 ± 0.00 a | 1.88 ± 0.001 b | ND | ND | |
| 2-Methyl Undecanal | 000110-41-8 | 2.8038 | ND | ND | ND | 1.82 ± 0.001 a | ND | |
| Ketones | 2-Heptanone | 000110-43-0 | 1.304 | 0.64 ± 0.002 c | 0.85 ± 0.002 c | ND | 4.64 ± 0.001 a | 3.06 ± 0.001 b |
| 2-Decanone | 000693-54-9 | 1.5764 | ND | ND | 1.94 ± 0.002 b | 2.67 ± 0.003 a | 1.05 ± 0.002 c | |
| 2-Octanone | 000111-13-7 | 1.7281 | ND | 7.1 ± 0.002 a | ND | 6.0 ± 0.002 b | ND | |
| 2-Pentadecanone | 002345-28-0 | 1.7558 | 2.25 ± 0.002 a | ND | ND | ND | ND | |
| 2,3-Butanedione | 0431-03-08 | 2.0108 | ND | ND | ND | 2.56 ± 0.003 b | 1.28 ± 0.001 b | |
| 2,3-Pentadione | 000600-14-6 | 2.066 | ND | 3.48 ± 0.04 a | 3.09 ± 0.001 b | ND | ND | |
| 5-Methyl-3-Heptanone | 000541-85-5 | 2.0868 | 2.35 ± 0.002 b | ND | ND | 2.92 ± 0.003 a | ND | |
| 3-Hydroxybutnone | 000513-86-0 | 2.9487 | 1.49 ± 0.001 d | 3.63 ± 0.04 c | 7.17 ± 0.01 b | ND | 16.01 ± 0.02 a | |
| 2-pentanone | 000107-87-9 | 2.6934 | ND | 1.7 ± 0.001 a | 0.89 ± 0.003 b | 0.88 ± 0.00 b | 0.41 ± 0.00 c | |
| Acetone | 000067-64-1 | 1.7075 | ND | 3.66 ± 0.002 b | 3.78 ± 0.001 a | 3.47 ± 0.001 c | ND | |
| Alkanes | n-Hexane | 000110-54-3 | 2.0522 | ND | 6.20 ± 0.001 a | ND | ND | ND |
| Trichloromethane | 000067-66-3 | 2.2454 | 1.31 ± 0.001 a | ND | ND | ND | ND | |
| Cyclotrisiloxane, hexamethyl- | 0541-05-09 | 4.3278 | 6.57 ± 0.06 b | 6.1 ± 0.002 d | 6.2± c | 8.36± a | 3.59± e | |
| Octamethyl- Cyclotetrasiloxane | 000556-67-2 | 7.1341 | 8.99 ± 0.11 a | 7.31 ± 0.13 c | 8.12 ± 0.08 b | ND | 6.92 ± 0.08 d | |
| Tricosane | 000638-67-5 | 7.3547 | ND | ND | ND | 2.0 ± 0.002 a | ND | |
| Eicosane | 000112-95-8 | 8.0856 | ND | 2.58 ± 0.00 a | 2.42± b | ND | ND | |
| Octadecane | 000593-45-3 | 8.0924 | ND | ND | ND | 2.1 ± 0.002 a | ND | |
| 2,6,10,14-tetramethyl-Hexadecane | 000638-36-8 | 8.0925 | ND | 1.71± a | ND | 0.54± b | ND | |
| Nonadecane | 000629-92-5 | 8.4028 | ND | 0.68 ± 0.001 a | 0.66 ± 0.00 b | ND | 0.64 ± 0.00 c | |
| Hexamethyl- Cyclotrisiloxane | 0541-05-09 | 8.6373 | 0.63 ± 0.00 d | 5.12 ± 0.005 c | 7.22 ± 0.007 b | 8.37 ± 0.03 a | 5.26 ± 0.02 c | |
| Heptadecane | 000629-78-7 | 8.7613 | ND | 0.33 ± 0.00 a | ND | ND | ND | |
| Hexadecane | 000544-76-3 | 8.7614 | 0.37± a | ND | ND | ND | ND | |
| Decamethyl-Cyclopentasiloxane | 0541-02-06 | 9.6027 | 1.32 ± 0.00 e | 17.84 ± 0.12 a | 11.94 ± 0.15 b | 10.82 ± 0.11 c | 9.47 ± 0.09 c | |
| Esters | Ethyl Acetate | 000141-78-6 | 2.1281 | ND | 6.74 ± 0.01 a | 6.7 ± 0.001 b | ND | ND |
| Amyl Acetate | 000628-63-7 | 2.2039 | ND | 6.55 ± 0.003 a | ND | ND | ND | |
| Ethyl Butanoate | 000105-54-4 | 2.983 | ND | ND | ND | 2.68 ± 0.001 a | ND | |
| Pentyl Isobutanoate | 002445-72-9 | 3.7416 | ND | 0.14 ± 0.00 b | 0.57 ± 0.00 a | ND | ND | |
| Furan | Tetrahydrofuran | 000109-99-9 | 2.2798 | 0.94 ± 0.0 a | ND | ND | ND | ND |
| Acids | Acetic Acid | 000064-19-7 | 3.3072 | 5.09 ± 0.001 b | ND | ND | 7.61 ± 0.01 a | ND |
| Nonanoic Acid | 000112-05-0 | 4.2243 | ND | 4.89 ± 0.003 a | 3.98 ± 0.005 b | ND | ND | |
| Butanoic acid | 000107-92-6 | 4.2863 | 1.08 ± 0.00 e | 8.13 ± 0.08 a | 3.59 ± 0.03 c | 6.97 ± 0.008 b | 2.08 ± 0.006 d | |
| Isovaleric Acid | 000503-74-2 | 4.4657 | 1.07 ± 0.00 b | ND | ND | ND | 0.47 ± 0.002 a | |
| 3-Methyl-Valereic Acid | 000105-43-1 | 4.5484 | 1.06 ± 0.003 a | ND | ND | ND | ND | |
| Hexanoic acid | 000142-62-1 | 7.2857 | ND | 1.8 ± 0.001 b | ND | 2.55 ± 0.001 a | ND | |
| Heptanoic acid | 000111-14-8 | 7.2858 | ND | 2.83 ± 0.002 a | 2.8 ± 0.001 b | ND | ND | |
| Dodecanoic acid | 0143-07-07 | 7.2996 | ND | 4.88 ± 0.001 a | 2.12 ± 0.001 b | ND | ND | |
| Pentanoic acid | 000109-52-4 | 7.3824 | 2.12± a | ND | ND | ND | ND | |
| Benzene | 1-methyl-3-1-methyethyl-Benzene | 000535-77-3 | 7.9753 | ND | 0.68 ± 0.00 a | 0.5 ± 0.00 b | ND | ND |
| Alkenes | Toluene | 000108-88-3 | 3.6795 | ND | 1.9 ± 0.001 b | 1.43 ± 0.002 c | 0.94 ± 0.002 d | 2.42 ± 0.002 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munir, M.; Nadeem, M.; Ali, B.; Sultan, M.; Kanwal, R.; Al-Jumayi, H.A.; Algarni, E.H.A.; Alnofeai, M.B.; Mahmoud, S.F. Investigating the Impact of Ultrasound, Microwave, and High-Pressure Processing of Milk on the Volatile Compounds and Sensory Properties of Cheddar Cheese. Agriculture 2022, 12, 577. https://doi.org/10.3390/agriculture12050577
Munir M, Nadeem M, Ali B, Sultan M, Kanwal R, Al-Jumayi HA, Algarni EHA, Alnofeai MB, Mahmoud SF. Investigating the Impact of Ultrasound, Microwave, and High-Pressure Processing of Milk on the Volatile Compounds and Sensory Properties of Cheddar Cheese. Agriculture. 2022; 12(5):577. https://doi.org/10.3390/agriculture12050577
Chicago/Turabian StyleMunir, Masooma, Muhammad Nadeem, Barkat Ali, Muhammad Sultan, Rabia Kanwal, Huda Abdalrahman Al-Jumayi, Eman Hassan Ahmed Algarni, Maged B. Alnofeai, and Samy F. Mahmoud. 2022. "Investigating the Impact of Ultrasound, Microwave, and High-Pressure Processing of Milk on the Volatile Compounds and Sensory Properties of Cheddar Cheese" Agriculture 12, no. 5: 577. https://doi.org/10.3390/agriculture12050577
APA StyleMunir, M., Nadeem, M., Ali, B., Sultan, M., Kanwal, R., Al-Jumayi, H. A., Algarni, E. H. A., Alnofeai, M. B., & Mahmoud, S. F. (2022). Investigating the Impact of Ultrasound, Microwave, and High-Pressure Processing of Milk on the Volatile Compounds and Sensory Properties of Cheddar Cheese. Agriculture, 12(5), 577. https://doi.org/10.3390/agriculture12050577








