The Changes in GABA, GAD and DAO Activities, and Microbial Safety of Soaking- and High Voltage Electric Field-Treated Adzuki Bean Sprouts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Materials
2.2. Soaking (S) Treatment
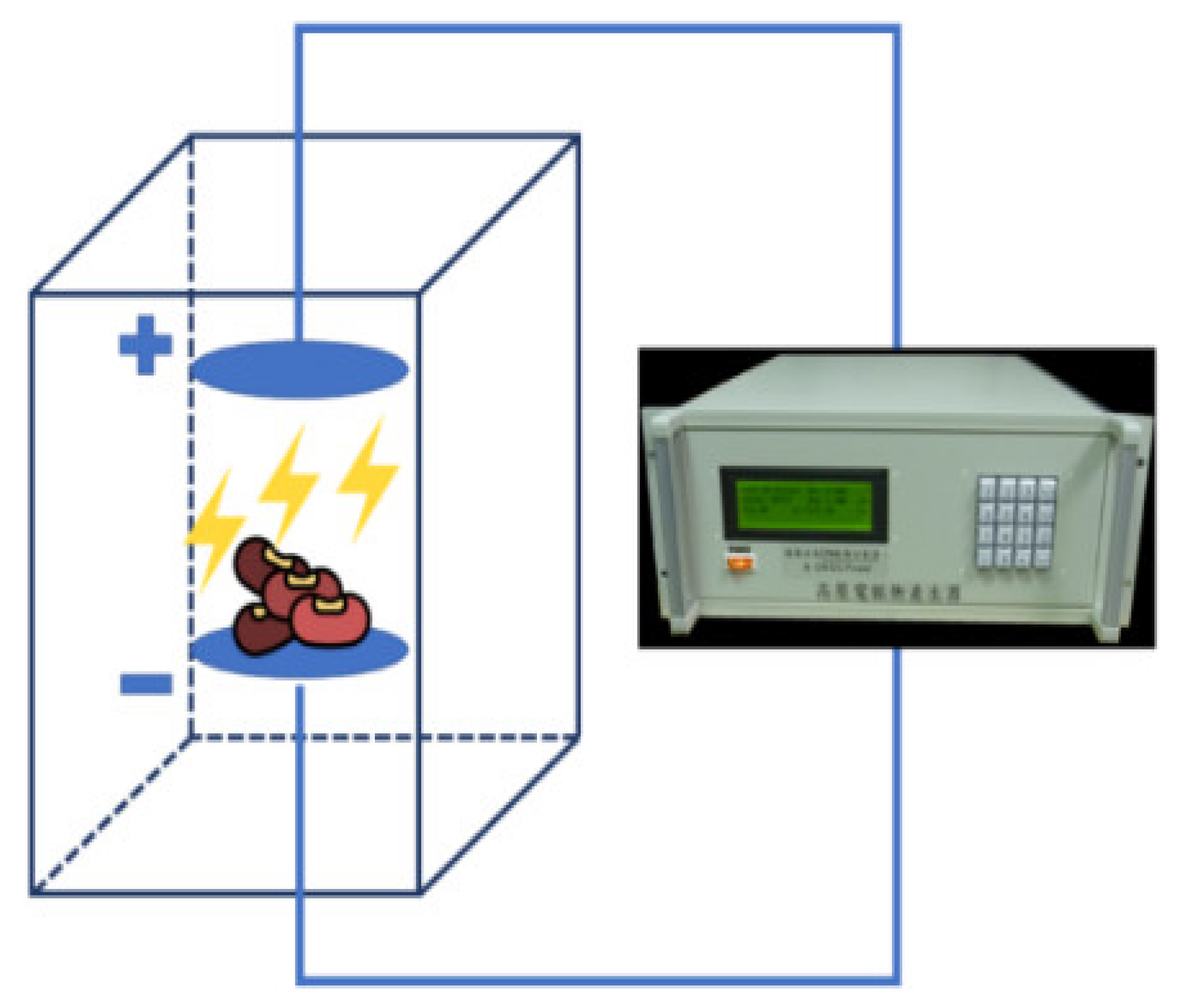
2.3. High-Voltage Electric Field (HVEF) Treatment
2.4. Seed Germination and Hydration Determinations
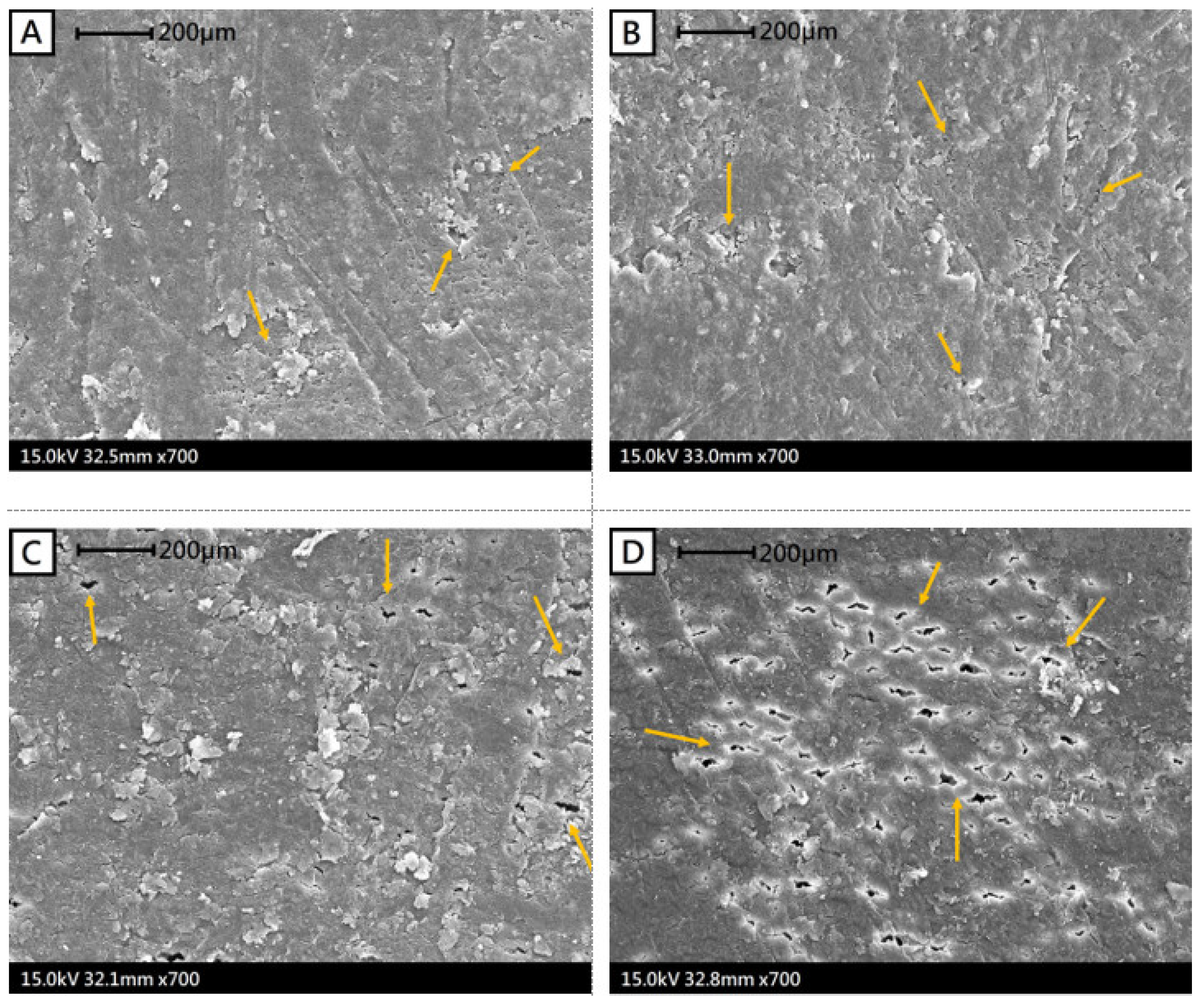
2.5. Scanning Electron Microscopy Examinations
2.6. GABA and Glutamic Acid (Glu) Measurements
2.7. Glutamate Decarboxylase (GAD) and Diamine Oxidase Activities (DAO) Determination
2.8. Microbial Analyses
2.9. Data Analysis
3. Results
3.1. Seed Treatment Effects on Germination and Sprout Growth
3.2. Seed Treatment Effects on Seed Hydration
3.3. Seed Treatment Effects on Biochemical Traits
3.4. Seed Treatment Effects on Microbial Loads in Seeds and Sprouts
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouche, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ruiz, R.; Poirot, E.; Flores-Mosquera, M. GABA, a non-protein amino acid ubiquitous in food matrices. Cogent Food Agric. 2018, 4, 1534323. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef]
- Joseph, P.; Srivastava, S.K. Photoregulation of Diamine Oxidase from Pea Seedlings. J. Plant Physiol. 1995, 146, 108–114. [Google Scholar] [CrossRef]
- Signorelli, S.; Dans, P.D.; Coitiño, E.L.; Borsani, O.; Monza, J. Connecting proline and γ-aminobutyric acid in stressed plants through non-enzymatic reactions. PLoS ONE 2015, 10, e0115349. [Google Scholar] [CrossRef] [Green Version]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A.; Xanthakis, E. The principles of high voltage electric field and its application in food processing: A review. Food Res. Int. 2016, 89, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Ramos-Ruiz, R.; Martinez, F.; Knauf-Beiter, G. The effects of GABA in plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Sita, K.; Kumar, V. Role of Gamma Amino Butyric Acid (GABA) against abiotic stress tolerance in legumes: A review. Plant Physiol. Rep. 2020, 25, 654–663. [Google Scholar] [CrossRef]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef]
- Poojary, M.M.; Dellarosa, N.; Roohinejad, S.; Koubaa, M.; Tylewicz, U.; Gomez-Galindo, F.; Saraiva, J.A.; Rosa, M.D.; Barba, F.J. Influence of Innovative Processing on γ -Aminobutyric Acid (GABA) Contents in Plant Food Materials. Compr. Rev. Food Sci. Food Saf. 2017, 16, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Swieca, M.; Gawlik-Dzikia, U.; Jakubczyka, A.; Bochnaka, J.; Sikoraa, M.; Suliburskab, J. Nutritional quality of fresh and stored legumes sprouts-effect of Lactobacillus plantarum 299v enrichment. Food Chem. 2019, 288, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.T.; Olatunde, O.O.; Adekoya, A.E.; Idowu, S. Germination: An alternative source to promote phytonutrients in edible seeds. Food Qual. Saf. 2020, 4, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Tiansawang, K.; Luangpituksai, P.; Varanyanond, W.; Hansawasdi, C. GABA (γ-aminobutyric acid) production, antioxidant activity in some germinated dietary seeds and the effect of cooking on their GABA content. Food Sci. Technol. 2016, 36, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Gan, R.; Lui, W.; Wu, K.; Chan, C.; Dai, S.-H.; Sui, Z.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Liu, R.; Cai, Z.; Xu, B. Characterization and quantifcation of favonoids and saponins in adzuki bean (Vigna angularis L.) by HPLC-DAD-ESI-MSn analysis. Chem. Cent. J. 2017, 11, 93–110. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Benguo Liu, B.; Xueling Zheng, X. Bioactive ingredients in adzuki bean sprouts. J. Medicinal Plants Res. 2011, 5, 5894–5898. [Google Scholar]
- Rifna, E.J.; Ramanan, K.R.; Mahendran, R. Emerging technology applications for improving seed germination. Trends Food Sci. Technol. 2019, 86, 95–108. [Google Scholar] [CrossRef]
- Siddique, A.; Kumar, P. Physiological and biochemical basis of pre-sowing soaking seed treatment-an overview. Plant Arch. 2018, 18, 1933–1937. [Google Scholar]
- Samia El-Safy, F.; Rabab, H.A.S.; Ensaf Mukhtar, Y.Y. The Impact of Soaking and Germination on Chemical Composition, Carbohydrate Fractions, Digestibility, Antinutritional Factors and Minerals Content of Some Legumes and Cereals Grain Seeds. Alex. Sci. Exch. J. 2013, 34, 499–513. [Google Scholar]
- Coffignieza, F.; Briffaza, A.; Mestresa, C.; Akissoéb, L.; Bohuonc, P.; Maâtaouid, M.E. Impact of soaking process on the microstructure of cowpea seeds in relationto solid losses and water absorption. Food Res. Int. 2019, 119, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.; Kim, I.; Dhungana, S.K.; Park, Y.; Kim, J.; Shin, D. Yield and quality characteristics of Korean red bean sprouts produced with different time of seed soaking. Food Sci. Biotechnol. 2020, 29, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.Y.; Burritt, D.J.; Oey, I. Electropriming of wheatgrass seeds using pulsed electric fields enhances antioxidant metabolism and the bioprotective capacity of wheatgrass shoots. Sci. Rep. 2016, 6, 25306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panggabean, S.E.; Kamson, W.; Simanjuntak, A.P.; Rahmawati, N. The effect of giving electric field to the metabolism of andaliman (Zanthoxylum acanthopodium DC) seeds which contributes to accelerating germination. Earth Environ. Sci. 2019, 260, 012134. [Google Scholar] [CrossRef]
- Zhao, S.; Garcia, D.; Zhao, Y.; Huang, D. Hydro-electro hybrid priming promotes carrot (Daucus carota L.) seed germination by activating lipid utilization and respiratory metabolism. Int. J. Mol. Sci. 2021, 22, 11090. [Google Scholar] [CrossRef]
- Yang, Y.; Meier, F.; Lo, J.A.; Yuan, W.; Sze, V.L.P.; Chung, H.; Yuk, H. Overview of recent events in the microbiological safety of sprouts and new intervention technologies. Compr. Rev. Food Sci. Food Saf. 2013, 12, 265–280. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Q.; Zhao, D.; Han, X.; Hao, J. Systematic application of slightly acidic electrolyzed water (SAEW) for natural microbial reduction of buckwheat sprouts. LWT—Food Sci. Technol. 2019, 108, 14–20. [Google Scholar] [CrossRef]
- Qi, M.; Zhao, R.; Liu, Q.; Yan, H.; Zhang, Y.; Wang, S. Antibacterial activity and mechanism of high voltage electrostatic field (HVEF) against Staphylococcus aureus in medium plates and food systems. Food Control 2021, 120, 107566. [Google Scholar] [CrossRef]
- Ellis, R.A.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Li, X.; Kim, Y.B.; Uddin, M.R.; Lee, S.; Kim, S.; Park, S.U. Influence of light on the free amino acid content and γ-aminobutyric acid synthesis in Brassica juncea seedlings. J. Agric. Food Chem. 2013, 61, 8624–8631. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, H.; Chen, F.; Wang, X. Purification and characterization of glutamate decarboxylase from rice germ. Food Chem. 2007, 101, 1670–1676. [Google Scholar] [CrossRef]
- Xing, S.G.; Jun, Y.B.; Hau, Z.W.; Liang, L.Y. Higher accumulation of g-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol. Biochem. 2007, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.Y. Changes in microstructure, germination, sprout growth, phytochemical and microbial quality of ultrasonication treated adzuki bean seeds. Agronomy 2021, 116, 1093. [Google Scholar] [CrossRef]
- Nonogakia, H.; Basselb, G.W.; Bewley, J.D. Germination—Still a mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Xu, W.; Song, Z.; Luan, X.; Ding, C.; Cao, Z.; Ma, X. Biological effects of high-voltage electric field treatment of naked oat seeds. Appl. Sci. 2019, 9, 3829. [Google Scholar] [CrossRef] [Green Version]
- Xi, G.; Liu, K.; Xu, Y.; Gao, Y. Effects comparison of seeds germinating treated by extremely low frequency PEF and HVEF. Trans. Chin. Soc. Agric. Eng. 2013, 29, 265–271, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Oliveira, A.L.; Colnaghi, B.G.; da Silva, E.Z.; Gouvêa, I.R.; Vieira, R.L.; Augusto, P.E.D. Modelling the effect of temperature on the hydration kinetic of adzuki beans (Vigna angularis). J. Food Eng. 2013, 118, 417–420. [Google Scholar] [CrossRef]
- Kramer, C.; Soltani, N.; Swanton, C.J.; Robinson, D.E.; Sikkema, P.H. Control of volunteer adzuki bean (Vigna angularis) with pre and postemergence herbicides in corn (Zea mays). Can. J. Plant Sci. 2010, 90, 925–932. [Google Scholar] [CrossRef]
- Mahajan, T.S.; Pandey, O.P. Effect of electric field (at different temperatures) on germination of chickpea seed. Afr. J. Biotechnol. 2014, 13, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Jiang, J.; Li, J.; Shen, M.; He, X.; Shao, H.; Dong, Y. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef] [Green Version]
- Holc, M.; Gselman, P.; Primc, G.; Vesel, A.; Mozetič, M.; Recek, N. Wettability and Water Uptake Improvement in Plasma-Treated Alfalfa Seeds. Agriculture 2022, 12, 96. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P. Changes in γ-aminobutyric acid content and related enzyme activities in Jindou 25 soybean (Glycine max L.) seeds during germination. LWT—Food Sci. Technol. 2014, 55, 341–346. [Google Scholar] [CrossRef]
- Chiu, K.Y. Ultrasonication-enhanced microbial safety of sprouts produced from selected crop species. J. Appl. Bot. Food Qual. 2015, 88, 120–126. [Google Scholar] [CrossRef]
- Miyahira, R.F.; Antunes, A.E.C. Bacteriological safety of sprouts: A brief review. Int. J. Food Microbiol. 2021, 352, 109266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Zhao, Z.; Liu, W.; Chen, Y.; Yang, G.; Xia, X.; Yanfei, C. The application of slightly acidic electrolyzed water in pea sprout production to ensure food safety, biological and nutritional quality of the sprout. Food Control 2019, 104, 83–90. [Google Scholar] [CrossRef]
- Padilla, C.; Fraga, N.; Uárez, M. Effect of the soaking time of moringa (Moringa oleífera) seeds on the germination and growth indicators of the plant. Cuba. J. Agric. Sci. 2012, 46, 419–421. [Google Scholar]
- Labbafi, M.R.; Mehrafarin, A.; Naghdi Badi, H.; Ghorbani, M.; Tavakoli, M. Improve germination of caper (Capparis spinosa L.) seeds by different induction treatments of seed dormancy breaking. Trakia J. Sci. 2018, 1, 70–74. [Google Scholar] [CrossRef]
- Butscher, D.; Loon, H.V.; Waskow, A.; von Rohr, P.R.; Schuppler, M. Plasma inactivation of microorganisms on sprout seeds in a dielectric barrier discharge. Int. J. Food Microbiol. 2016, 238, 222–232. [Google Scholar] [CrossRef]
- Yusaf, T.; Al-Juboori, R.A. Alternative methods of microorganism disruption for agricultural applications. Appl. Energy 2014, 114, 909–923. [Google Scholar] [CrossRef]



| Treatments | Germination | MGT | Sprout Fresh Weight | Sprout Length |
|---|---|---|---|---|
| % | days | g 100 seeds−1 | cm | |
| CK | 82.1 ± 2.12 d | 3.87 ± 0.45 a | 203.40 ± 2.91 d | 5.24 ± 0.32 c |
| HVEF | 86.4 ± 0.83 c | 3.43 ± 0.17 b | 227.82 ± 3.52 c | 6.91 ± 0.41 b |
| S | 96.3 ± 0.61 b | 2.08 ± 0.21 c | 284.15 ± 4.71 b | 11.27 ± 0.62 a |
| SHVEF | 100.0 ± 0.00 a | 1.42 ± 0.13 d | 343.72 ± 5.83 a | 12.84 ± 0.83 a |
| Days | |||||
|---|---|---|---|---|---|
| Treatments | 0 | 2 | 4 | 6 | |
| CK | 4.74 ± 0.20 a | 14.22 ± 0.31 d | 70.31 ± 2.32 d | 126.30 ± 2.62 d | |
| GABA | HVEF | 4.92 ± 0.26 a | 24.91 ± 0.92 c | 94.62 ± 4.21 c | 162.12 ± 4.13 c |
| (mg 100 g−1) | S | 5.02 ± 0.17 a | 42.31 ± 0.61 b | 121.11 ± 3.13 b | 187.21 ± 3.82 b |
| SHVEF | 5.49 ± 0.24 a | 94.80 ± 0.82 a | 235.53 ± 4.82 a | 258.13 ± 5.64 a | |
| CK | 58.72 ± 0.41 a | 233.48 ± 7.62 c | 208.15 ± 6.81 c | 296.46 ± 8.42 c | |
| Glu | HVEF | 60.23 ± 0.52 a | 354.21 ± 8.91 c | 316.38 ± 9.42 c | 328.76 ± 6.81 b |
| (mg 100 g−1) | S | 56.85 ± 0.32 a | 445.63 ± 9.23 b | 357.11 ± 8.31 ab | 345.24 ± 7.42 ab |
| SHVEF | 58.24 ± 0.23 a | 488.65 ± 6.71 a | 387.37 ± 7.92 a | 368.46 ± 6.22 a | |
| CK | 0.21 ± 0.00 a | 1.52 ± 0.00 d | 3.41 ± 0.01 c | 4.52 ± 0.01 c | |
| GAD | HVEF | 0.21 ± 0.00 a | 2.21 ± 0.01 c | 4.72 ± 0.02 b | 6.61 ± 0.02 b |
| (U mg−1) | S | 0.22 ± 0.01 a | 2.82 ± 0.01 b | 4.51 ± 0.01 b | 6.22 ± 0.02 b |
| SHVEF | 0.23 ± 0.01 a | 6.04 ± 0.01 a | 7.04 ± 0.04 a | 8.13 ± 0.04 a | |
| CK | 1.12 ± 0.00 a | 2.10 ± 0.11 d | 4.02 ± 0.11 c | 5.21 ± 0.21 d | |
| DAO | HVEF | 1.12 ± 0.00 a | 3.11 ± 0.12 c | 4.73 ± 0.20 c | 8.52 ± 0.30 c |
| (U g−1 ) | S | 1.13 ± 0.01 a | 6.82 ± 0.21 b | 10.12 ± 0.31 b | 11.42 ± 0.21 b |
| SHVEF | 1.14 ± 0.00 a | 9.41 ± 0.13 a | 13.92 ± 0.42 a | 14.71 ± 0.32 a |
| Correlation Coefficient (r) | |||||
|---|---|---|---|---|---|
| GABA | Glu | GAD | DAO | Sprout weight | |
| Glu | 0.3727 * | - | - | - | - |
| GAD | 0.8918 ** | 0.6184 ** | - | - | - |
| DAO | 0.8777 ** | 0.5713 ** | 0.8613 ** | - | - |
| Sprout weight | 0.9247 ** | 0.5216 ** | 0.9114 ** | 0.8484 ** | - |
| Treatments | Total Aerobic Bacteria Count | Total Coliform Count | Total Mold Count | |||
|---|---|---|---|---|---|---|
| (log 10 CFU g−1 Fresh Weight) | ||||||
| Seeds | Sprouts | Seeds | Sprouts | Seeds | Sprouts | |
| CK | 1.94 ± 0.13 a | 9.39 ± 0.33 a | 1.08 ± 0.05 a | 6.24 ± 0.11 a | 1.78 ± 0.13 a | 8.94 ± 0.31 a |
| HVEF | 0.95 ± 0.01 c | 2.83 ± 0.21 c | 0.48 ± 0.03 c | 1.32 ± 0.02 c | 0.71 ± 0.03 c | 2.74 ± 0.12 c |
| S | 1.23 ± 0.13 b | 4.91 ± 0.41 b | 0.78 ± 0.11 b | 2.33 ± 0.04 b | 1.04 ± 0.02 b | 3.47 ± 0.21 b |
| SHVEF | 0.31 ± 0.00 d | 1.85 ± 0.13 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.30 ± 0.03 d | 1.48 ± 0.13 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, K.-Y. The Changes in GABA, GAD and DAO Activities, and Microbial Safety of Soaking- and High Voltage Electric Field-Treated Adzuki Bean Sprouts. Agriculture 2022, 12, 469. https://doi.org/10.3390/agriculture12040469
Chiu K-Y. The Changes in GABA, GAD and DAO Activities, and Microbial Safety of Soaking- and High Voltage Electric Field-Treated Adzuki Bean Sprouts. Agriculture. 2022; 12(4):469. https://doi.org/10.3390/agriculture12040469
Chicago/Turabian StyleChiu, Kai-Ying. 2022. "The Changes in GABA, GAD and DAO Activities, and Microbial Safety of Soaking- and High Voltage Electric Field-Treated Adzuki Bean Sprouts" Agriculture 12, no. 4: 469. https://doi.org/10.3390/agriculture12040469
APA StyleChiu, K.-Y. (2022). The Changes in GABA, GAD and DAO Activities, and Microbial Safety of Soaking- and High Voltage Electric Field-Treated Adzuki Bean Sprouts. Agriculture, 12(4), 469. https://doi.org/10.3390/agriculture12040469






