Disentangling the Benefits of Organic Farming for Beetle Communities (Insecta: Coleoptera) in Traditional Fruit Orchards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Sampling
2.3. Data Analysis
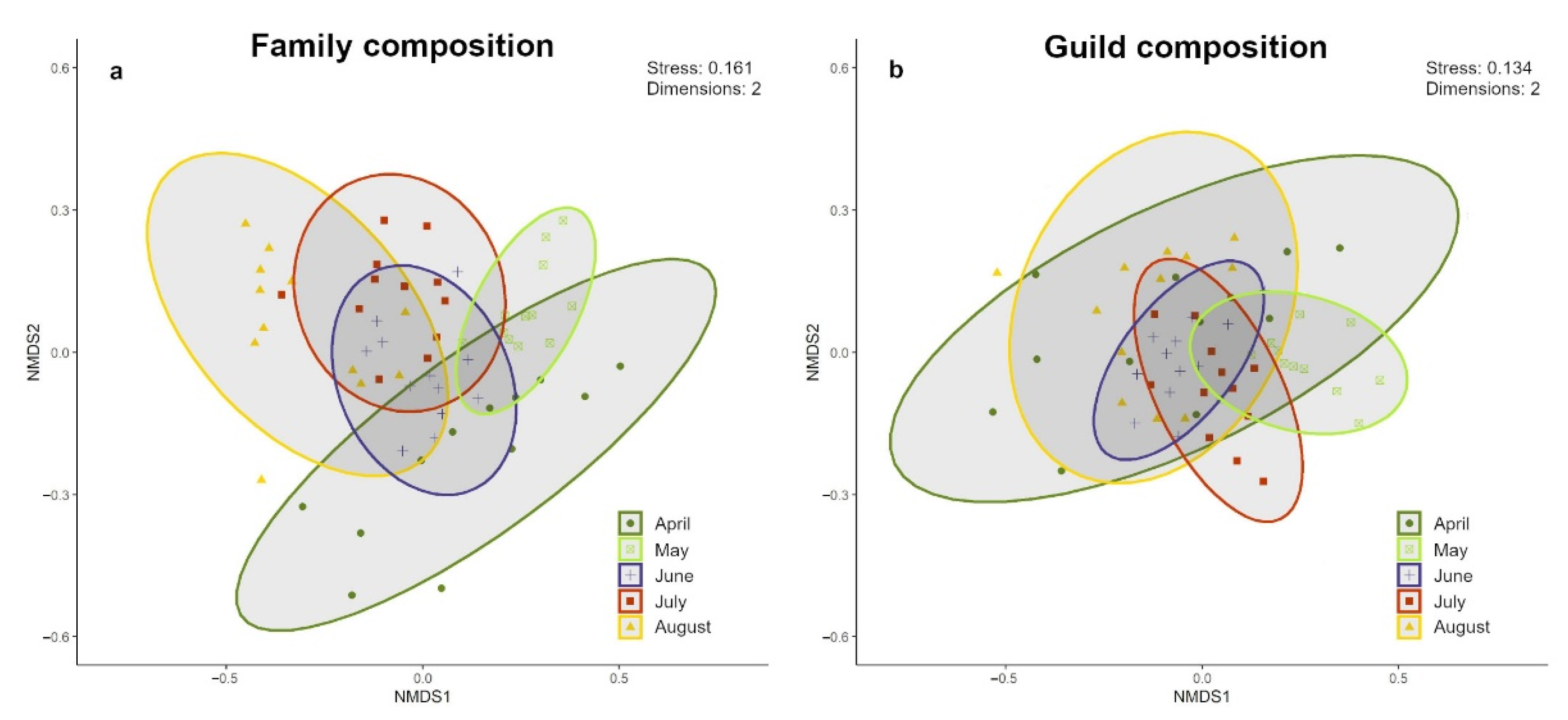
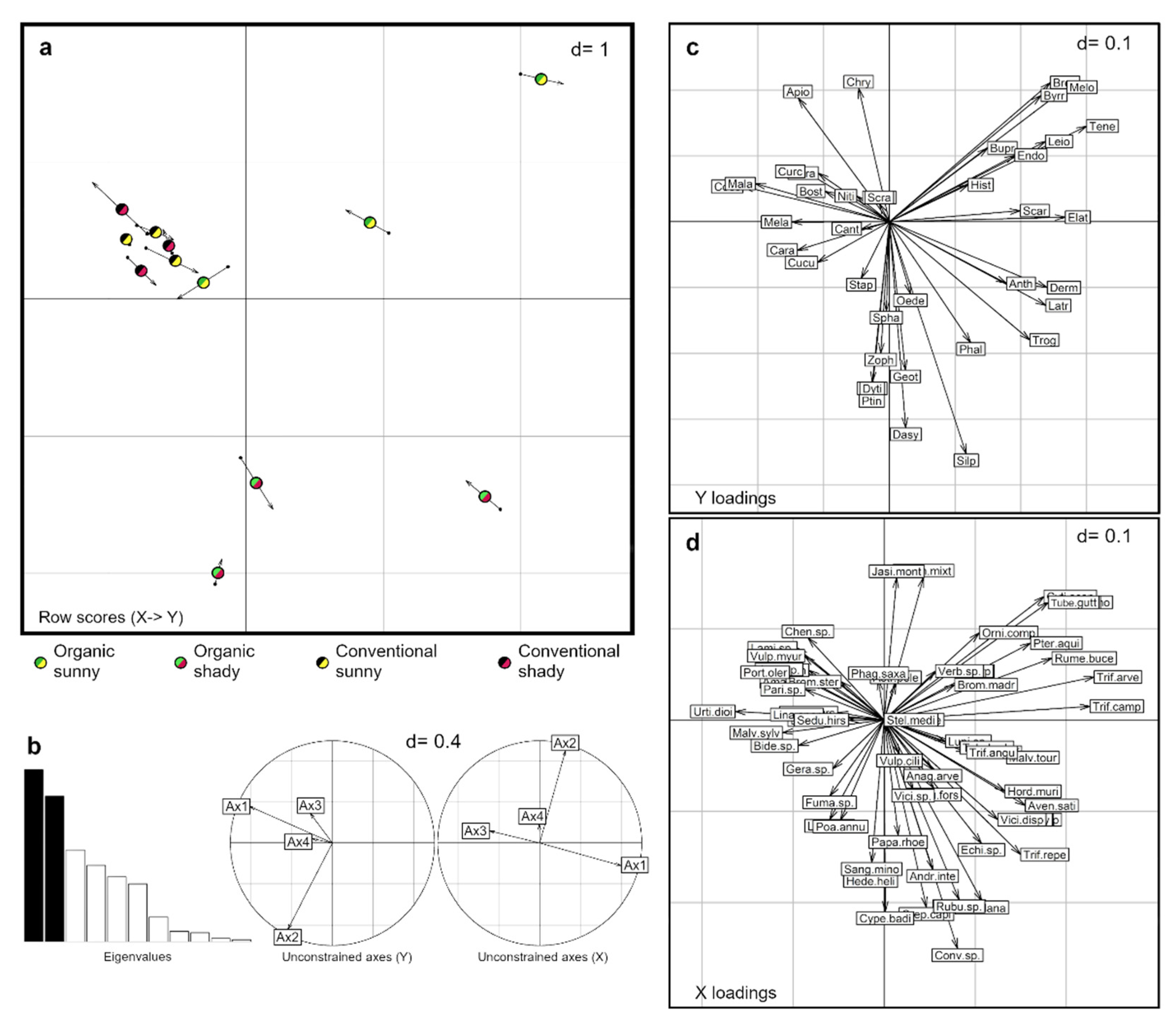
3. Results
3.1. Taxonomical Response
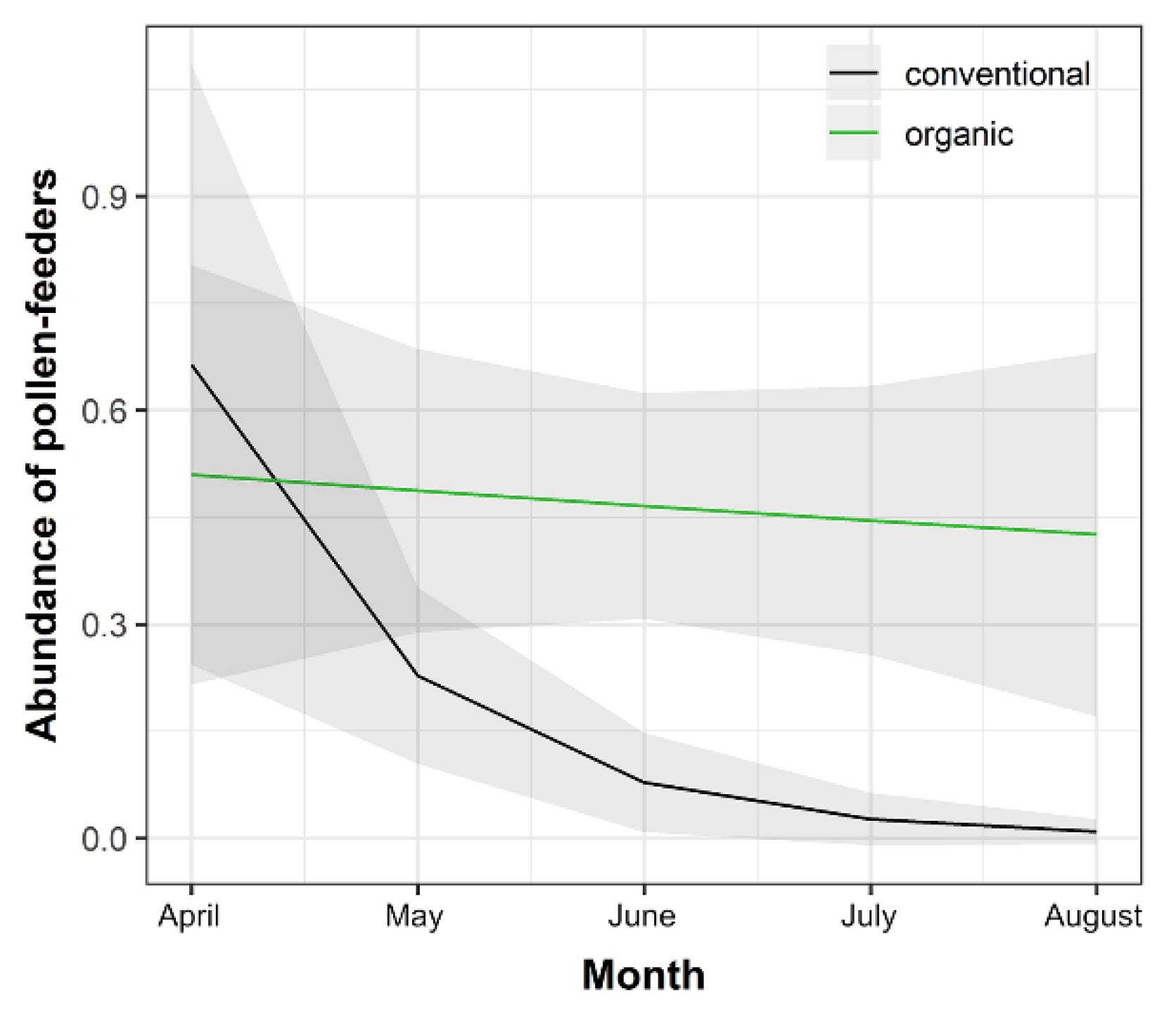
3.2. Trophic Guild Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape Perspectives on Agricultural Intensification and Biodiversity—Ecosystem Service Management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Hof, A.R.; Bright, P.W. The Impact of Grassy Field Margins on Macro-Invertebrate Abundance in Adjacent Arable Fields. Agric. Ecosyst. Environ. 2010, 139, 280–283. [Google Scholar] [CrossRef]
- Ramankutty, N.; Mehrabi, Z.; Waha, K.; Jarvis, L.; Kremen, C.; Herrero, M.; Rieseberg, L.H. Trends in Global Agricultural Land Use: Implications for Environmental Health and Food Security. Annu. Rev. Plant Biol. 2018, 69, 789–815. [Google Scholar] [CrossRef] [Green Version]
- Raven, P.H.; Wagner, D.L. Agricultural Intensification and Climate Change Are Rapidly Decreasing Insect Biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar] [CrossRef] [PubMed]
- Bignal, E.M.; McCracken, D.I. The Nature Conservation Value of European Traditional Farming Systems. Environ. Rev. 2000, 8, 149–171. [Google Scholar] [CrossRef]
- Hadjicharalampous, E.; Kalburtji, K.L.; Mamolos, A.P. Soil Arthropods (Coleoptera, Isopoda) in Organic and Conventional Agroecosystems. Environ. Manage. 2002, 29, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ramos, N.; Baños-Picón, L.; Tormos, J.; Asís, J.D. The Complementarity between Ecological Infrastructure Types Benefits Natural Enemies and Pollinators in a Mediterranean Vineyard Agroecosystem. Ann. Appl. Biol. 2019, 175, 193–201. [Google Scholar] [CrossRef]
- Altieri, M.A. Linking Ecologists and Traditional Farmers in the Search for Sustainable Agriculture. Front. Ecol. Environ. 2004, 2, 35–42. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Biodiveristy and Pest Management in Agroecosystems; Haworth Press: New York, NY, USA, 2004; ISBN 1-56022-922-5. [Google Scholar]
- Fischer, J.; Hartel, T.; Kuemmerle, T. Conservation Policy in Traditional Farming Landscapes. Conserv. Lett. 2012, 5, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Horak, J.; Peltanova, A.; Podavkova, A.; Safarova, L.; Bogusch, P.; Romportl, D.; Zasadil, P. Biodiversity Responses to Land Use in Traditional Fruit Orchards of a Rural Agricultural Landscape. Agric. Ecosyst. Environ. 2013, 178, 71–77. [Google Scholar] [CrossRef]
- Horak, J. Fragmented Habitats of Traditional Fruit Orchards Are Important for Dead Wood-Dependent Beetles Associated with Open Canopy Deciduous Woodlands. Naturwissenschaften 2014, 101, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Atauri, J.A.; de Lucio, J.V. The Role of Landscape Structure in Species Richness Distribution of Birds, Amphibians, Reptiles and Lepidopterans in Mediterranean Landscapes. Landsc. Ecol. 2001, 16, 147–159. [Google Scholar] [CrossRef]
- González-Bernaldez, F. Ecological Consequences of the Abandonment of Traditional Land Use Systems in Central Spain. Options Méditerranéennes 1991, 15, 23–29. [Google Scholar]
- Nielsen, A.; Steffan-Dewenter, I.; Westphal, C.; Messinger, O.; Potts, S.G.; Roberts, S.P.M.; Settele, J.; Szentgyörgyi, H.; Vaissière, B.E.; Vaitis, M.; et al. Assessing Bee Species Richness in Two Mediterranean Communities: Importance of Habitat Type and Sampling Techniques. Ecol. Res. 2011, 26, 969–983. [Google Scholar] [CrossRef]
- Petanidou, T.; Lamborn, E. A Land for Flowers and Bees: Studying Pollination Ecology in Mediterranean Communities. Plant Biosyst. 2005, 139, 279–294. [Google Scholar] [CrossRef]
- Ponti, L.; Gutierrez, A.P.; Altieri, M.A. Preserving the Mediterranean Diet Through Holistic Strategies for the Conservation of Traditional Farming Systems. In Biocultural Diversity in Europe; Springer International Publishing: Cham, Switzerland, 2016; pp. 453–469. ISBN 9783319263151. [Google Scholar]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ Warning to Humanity on Insect Extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable Pest Regulation in Agricultural Landscapes: A Review on Landscape Composition, Biodiversity and Natural Pest Control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent Negative Effects of Pesticides on Biodiversity and Biological Control Potential on European Farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Persson, A.S.; Olsson, O.; Rundlöf, M.; Smith, H.G. Land Use Intensity and Landscape Complexity-Analysis of Landscape Characteristics in an Agricultural Region in Southern Sweden. Agric. Ecosyst. Environ. 2010, 136, 169–176. [Google Scholar] [CrossRef]
- Rader, R.; Birkhofer, K.; Schmucki, R.; Smith, H.G.; Stjernman, M.; Lindborg, R. Organic Farming and Heterogeneous Landscapes Positively Affect Different Measures of Plant Diversity. J. Appl. Ecol. 2014, 51, 1544–1553. [Google Scholar] [CrossRef]
- Brühl, C.A.; Zaller, J.G. Biodiversity Decline as a Consequence of an Inappropriate Environmental Risk Assessment of Pesticides. Front. Environ. Sci. 2019, 7, 2013–2016. [Google Scholar] [CrossRef] [Green Version]
- Rouaux, J.; Cabrera, N.; Martínez, A.S.; Posse, M.C.; Luna, M.G. Diversity and Phenology of Epigeal Coleoptera Assemblages in Lettuce and Tomato Crops in Northern Buenos Aires Province, Argentina. An. Acad. Bras. Cienc. 2020, 92, 1–15. [Google Scholar] [CrossRef]
- Feber, R.E.; Johnson, P.J.; Bell, J.R.; Chamberlain, D.E.; Firbank, L.G.; Fuller, R.J.; Manley, W.; Mathews, F.; Norton, L.R.; Townsend, M.; et al. Organic Farming: Biodiversity Impacts Can Depend on Dispersal Characteristics and Landscape Context. PLoS ONE 2015, 10, e0135921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehinde, T.; von Wehrden, H.; Samways, M.; Klein, A.-M.; Brittain, C. Organic Farming Promotes Bee Abundance in Vineyards in Italy but Not in South Africa. J. Insect Conserv. 2018, 22, 61–67. [Google Scholar] [CrossRef]
- Puech, C.; Baudry, J.; Joannon, A.; Poggi, S.; Aviron, S. Organic vs. Conventional Farming Dichotomy: Does It Make Sense for Natural Enemies? Agric. Ecosyst. Environ. 2014, 194, 48–57. [Google Scholar] [CrossRef]
- Altieri, M.A.; Farrell, J.G.; Hecht, S.B.; Liebman, M.; Magdoff, F.; Murphy, B.; Norgaard, R.B.; Sikor, T.O. Agroecology; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9780429495465. [Google Scholar]
- Katayama, N.; Osada, Y.; Mashiko, M.; Baba, Y.G.; Tanaka, K.; Kusumoto, Y.; Okubo, S.; Ikeda, H.; Natuhara, Y. Organic Farming and Associated Management Practices Benefit Multiple Wildlife Taxa: A Large-Scale Field Study in Rice Paddy Landscapes. J. Appl. Ecol. 2019, 56, 1970–1981. [Google Scholar] [CrossRef]
- Rosas-Ramos, N.; Baños-Picón, L.; Tormos, J.; Asís, J.D. Natural Enemies and Pollinators in Traditional Cherry Orchards: Functionally Important Taxa Respond Differently to Farming System. Agric. Ecosyst. Environ. 2020, 295, 106920. [Google Scholar] [CrossRef]
- Rosas-Ramos, N.; Baños-Picón, L.; Tormos, J.; Asís, J.D. Farming System Shapes Traits and Composition of Spider Assemblages in Mediterranean Cherry Orchards. PeerJ 2020, 8, e8856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, V.; Kostadinova, E.; Rancheva, E.; Yancheva, C. Causal Relationship between Biodiversity of Insect Population and Agro-Management in Organic and Conventional Apple Orchard. Org. Agric. 2018, 8, 355–370. [Google Scholar] [CrossRef]
- Reiff, J.M.; Kolb, S.; Entling, M.H.; Herndl, T.; Möth, S.; Walzer, A.; Kropf, M.; Hoffmann, C.; Winter, S. Organic Farming and Cover-Crop Management Reduce Pest Predation in Austrian Vineyards. Insects 2021, 12, 220. [Google Scholar] [CrossRef]
- Gkisakis, V.D.; Bàrberi, P.; Kabourakis, E.M. Olive Canopy Arthropods under Organic, Integrated, and Conventional Management. The Effect of Farming Practices, Climate and Landscape. Agroecol. Sustain. Food Syst. 2018, 42, 843–858. [Google Scholar] [CrossRef]
- Happe, A.; Alins, G.; Blüthgen, N.; Boreux, V.; Bosch, J.; García, D.; Hambäck, P.A.; Klein, A.; Martínez-Sastre, R.; Miñarro, M.; et al. Predatory Arthropods in Apple Orchards across Europe: Responses to Agricultural Management, Adjacent Habitat, Landscape Composition and Country. Agric. Ecosyst. Environ. 2019, 273, 141–150. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/#home (accessed on 22 January 2019).
- Duelli, P.; Obrist, M.K. In Search of the Best Correlates for Local Biodiversity in Cultivated Areas: In Search of the Best Correlates for Local Biodiversity in Cultivated Areas. Biodivers. Conserv. 1998, 7, 297–309. [Google Scholar] [CrossRef]
- Wardhaugh, C.W.; Stork, N.E.; Edwards, W. Feeding Guild Structure of Beetles on Australian Tropical Rainforest Trees Reflects Microhabitat Resource Availability. J. Anim. Ecol. 2012, 81, 1086–1094. [Google Scholar] [CrossRef]
- Bernardes, A.C.C.; Oliveira, O.C.C.; Silva, R.A.; Albuquerque, P.M.C.; Rebêlo, J.M.M.; Viana, J.H.; Siqueira, G.M. Abundance and Diversity of Beetles (Insecta: Coleoptera) in Land Use and Management Systems. Rev. Bras. Cienc. Do Solo 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Shah, P.A.; Brooks, D.R.; Ashby, J.E.; Perry, J.N.; Woiwod, I.P. Diversity and Abundance of the Coleopteran Fauna from Organic and Conventional Management Systems in Southern England. Agric. For. Entomol. 2003, 5, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Pizzolotto, R.; Mazzei, A.; Bonacci, T.; Scalercio, S.; Iannotta, N.; Brandmayr, P. Ground Beetles in Mediterranean Olive Agroecosystems: Their Significance and Functional Role as Bioindicators (Coleoptera, Carabidae). PLoS ONE 2018, 13, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecheur, E.; Piqueray, J.; Monty, A.; Dufrêne, M.; Mahy, G. The Influence of Ecological Infrastructures Adjacent to Crops on Their Carabid Assemblages in Intensive Agroecosystems. PeerJ 2020, 8, e8094. [Google Scholar] [CrossRef] [PubMed]
- Báldi, A. Using Higher Taxa as Surrogates of Species Richness: A Study Based on 3700 Coleoptera, Diptera, and Acari Species in Central-Hungarian Reserves. Basic Appl. Ecol. 2003, 4, 589–593. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, S.S.; Ortega, J.C.G.; Ribas, L.G.; dos Santos Ribas, L.G.; Bini, L.M. Higher Taxa Are Sufficient to Represent Biodiversity Patterns. Ecol. Indic. 2020, 111, 105994. [Google Scholar] [CrossRef]
- Grimbacher, P.S.; Catterall, C.P.; Kitching, R.L. Detecting the Effects of Environmental Change above the Species Level with Beetles in a Fragmented Tropical Rainforest Landscape. Ecol. Entomol. 2008, 33, 66–79. [Google Scholar] [CrossRef]
- González, E.; Salvo, A.; Valladares, G. Arthropods on Plants in a Fragmented Neotropical Dry Forest: A Functional Analysis of Area Loss and Edge Effects. Insect Sci. 2014, 22, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Vinson, M.R.; Hawkins, C.P. Biodiversity of Stream Insects: Variation at Local, Basin, and Regional Scales. Annu. Rev. Entomol. 1998, 43, 271–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora, J.; Verdú, J.R.; Galante, E. Species Richness in Mediterranean Agroecosystems: Spatial and Temporal Analysis for Biodiversity Conservation. Biol. Conserv. 2007, 134, 113–121. [Google Scholar] [CrossRef]
- González-Megías, A.; Gómez, J.M.; Sánchez-Piñero, F. Spatio-Temporal Change in the Relationship between Habitat Heterogeneity and Species Diversity. Acta Oecologica 2011, 37, 179–186. [Google Scholar] [CrossRef]
- Council Regulation (EC) No 834/2007 of 28 June 2007. 2007; Organic production and labelling of organic products and repealing regulation (EEC) No 2092/91.
- Avinent, L.; Llácer, G. Adaptación de Un Aspirador de Jardín Para La Captura de Insectos. Bol. Sanid. Veg. Plagas 1995, 21, 329–335. [Google Scholar]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. User’s Guide and Application. Available online: http://purl.oclc.org/estimates (accessed on 7 August 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Rundlöf, M.; Edlund, M.; Smith, H.G. Organic Farming at Local and Landscape Scales Benefits Plant Diversity. Ecography (Cop.). 2010, 33, 514–522. [Google Scholar] [CrossRef]
- Nascimbene, J.; Marini, L.; Paoletti, M.G. Organic Farming Benefits Local Plant Diversity in Vineyard Farms Located in Intensive Agricultural Landscapes. Environ. Manag. 2012, 49, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Yvoz, S.; Cordeau, S.; Zuccolo, C.; Petit, S. Crop Type and Within-Field Location as Sources of Intraspecific Variations in the Phenology and the Production of Floral and Fruit Resources by Weeds. Agric. Ecosyst. Environ. 2020, 302, 107082. [Google Scholar] [CrossRef]
- Chouangthavy, B.; Sanguansub, S.; Das, A. Sustainable Organic Farming Supports Diversity of Coleopteran Beetles as a Good Indicator Taxon: A Case Study from Central Lao PDR. Org. Agric. 2021, 11, 615–624. [Google Scholar] [CrossRef]
- Hole, D.G.; Perkins, A.J.; Wilson, J.D.; Alexander, I.H.; Grice, P.V.; Evans, A.D. Does Organic Farming Benefit Biodiversity? Biol. Conserv. 2005, 122, 113–130. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A Global Quantitative Synthesis of Local and Landscape Effects on Wild Bee Pollinators in Agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, E.M.; Kennedy, C.M.; Kremen, C.; Batáry, P.; Berendse, F.; Bommarco, R.; Bosque-Pérez, N.A.; Carvalheiro, L.G.; Snyder, W.E.; Williams, N.M.; et al. A Global Synthesis of the Effects of Diversified Farming Systems on Arthropod Diversity within Fields and across Agricultural Landscapes. Glob. Chang. Biol. 2017, 23, 4946–4957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcel, M.; Andersson, G.K.S.; Pålsson, J.; Tasin, M. Organic Management in Apple Orchards: Higher Impacts on Biological Control than on Pollination. J. Appl. Ecol. 2018, 55, 2779–2789. [Google Scholar] [CrossRef] [Green Version]
- Rahmann, G. Biodiversity and Organic Farming: What Do We Know? Agric. For. Res. 2011, 61, 189–208. [Google Scholar]
- Stein-Bachinger, K.; Gottwald, F.; Haub, A.; Schmidt, E. To What Extent Does Organic Farming Promote Species Richness and Abundance in Temperate Climates? A Review. Org. Agric. 2021, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, J.; Ahnström, J.; Weibull, A.-C. The Effects of Organic Agriculture on Biodiversity and Abundance: A Meta-Analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Botías, C.; David, A.; Hill, E.M.; Goulson, D. Contamination of Wild Plants near Neonicotinoid Seed-Treated Crops, and Implications for Non-Target Insects. Sci. Total Environ. 2016, 566, 269–278. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Mcfield, M.; et al. Effects of Neonicotinoids and Fipronil on Non-Target Invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef] [Green Version]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat Management to Conserve Natural Enemies of Arthropod Pests in Agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Langellotto, G.A.; Denno, R.F. Responses of Invertebrate Natural Enemies to Complex-Structured Habitats: A Meta-Analytical Synthesis. Oecologia 2004, 139, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Crisp, P.N.; Dickinson, K.J.M.; Gibbs, G.W. Does Native Invertebrate Diversity Reflect Native Plant Diversity? A Case Study from New Zealand and Implications for Conservation. Biol. Conserv. 1998, 83, 209–220. [Google Scholar] [CrossRef]
- Asteraki, E.; Hart, B.; Ings, T.; Manley, W. Factors Influencing the Plant and Invertebrate Diversity of Arable Field Margins. Agric. Ecosyst. Environ. 2004, 102, 219–231. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Dosdall, L.M.; Spence, J.R.; Willenborg, C.J. Field Density and Distribution of Weeds Are Associated with Spatial Dynamics of Omnivorous Ground Beetles (Coleoptera: Carabidae). Agric. Ecosyst. Environ. 2017, 236, 134–141. [Google Scholar] [CrossRef]
- Twardowski, J.P.; Gruss, I.; Hurej, M. Does Vegetation Complexity within Intensive Agricultural Landscape Affect Rove Beetle (Coleoptera: Staphylinidae) Assemblages? Biocontrol Sci. Technol. 2020, 30, 116–131. [Google Scholar] [CrossRef]
- Gulden, R.H.; Sikkema, P.H.; Hamill, A.S.; Tardif, F.J.; Swanton, C.J. Glyphosate-Resistant Cropping Systems in Ontario: Multivariate and Nominal Trait-Based Weed Community Structure. Weed Sci. 2010, 58, 278–288. [Google Scholar] [CrossRef]
- Xue, R.; Yang, Q.; Miao, F.; Wang, X.; Shen, Y. Slope Aspect Influences Plant Biomass, Soil Properties and Microbial Composition in Alpine Meadow on the Qinghai-Tibetan Plateau. J. Soil Sci. Plant 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Fried, G.; Kazakou, E.; Gaba, S. Trajectories of Weed Communities Explained by Traits Associated with Species’ Response to Management Practices. Agric. Ecosyst. Environ. 2012, 158, 147–155. [Google Scholar] [CrossRef]
- Triplehorn, C.A.; Johnson, N.F. Borror and Delong’s Introduction to the Study of Insects; Thomson Brooks/Cole: Belmont, CA, USA, 2005. [Google Scholar]
- Rosa García, R.; Miñarro, M. Role of Floral Resources in the Conservation of Pollinator Communities in Cider-Apple Orchards. Agric. Ecosyst. Environ. 2014, 183, 118–126. [Google Scholar] [CrossRef]
- Vilà, M.; Sardans, J. Plant Competition in Mediterranean-Type Vegetation. J. Veg. Sci. 1999, 10, 281–294. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Castaldi, S.; D’Ascoli, R.; Papa, S.; Carfora, A.; Marzaioli, R.; Fioretto, A. Soil Activities Related to Nitrogen Cycle under Three Plant Cover Types in Mediterranean Environment. Appl. Soil Ecol. 2009, 43, 40–46. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Penas, Á.; del Río, S.; Díaz González, T.E.; Rivas-Sáenz, S. Bioclimatology of the Iberian Peninsula and the Balearic Islands. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 29–80. [Google Scholar]
- Carrié, R.; Ekroos, J.; Smith, H.G. Organic Farming Supports Spatiotemporal Stability in Species Richness of Bumblebees and Butterflies. Biol. Conserv. 2018, 227, 48–55. [Google Scholar] [CrossRef]
- Forrest, J.R.K. Plant-Pollinator Interactions and Phenological Change: What Can We Learn about Climate Impacts from Experiments and Observations? Oikos 2015, 124, 4–13. [Google Scholar] [CrossRef]
- Markó, V.; Elek, Z.; Kovács-Hostyánszki, A.; Kőrösi, Á.; Somay, L.; Földesi, R.; Varga, Á.; Iván, Á.; Báldi, A. Landscapes, Orchards, Pesticides–Abundance of Beetles (Coleoptera) in Apple Orchards along Pesticide Toxicity and Landscape Complexity Gradients. Agric. Ecosyst. Environ. 2017, 247, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Coulis, M. Abundance, Biomass and Community Composition of Soil Saprophagous Macrofauna in Conventional and Organic Sugarcane Fields. Appl. Soil Ecol. 2021, 164, 103923. [Google Scholar] [CrossRef]
- Schauer, B.; Bong, J.; Popp, C.; Obermaier, E.; Feldhaar, H. Dispersal Limitation of Saproxylic Insects in a Managed Forest? A Population Genetics Approach. Basic Appl. Ecol. 2018, 32, 26–38. [Google Scholar] [CrossRef]
- Gerlach, J.; Samways, M.; Pryke, J. Terrestrial Invertebrates as Bioindicators: An Overview of Available Taxonomic Groups. J. Insect Conserv. 2013, 17, 831–850. [Google Scholar] [CrossRef]


| Response Variable | Factor | Estimate | Std. Error | z | p | |
|---|---|---|---|---|---|---|
| Number of beetle families | Intercept | 2.780 | 0.252 | 11.023 | <2 × 10−16 | *** |
| System (organic) | 0.483 | 0.115 | 4.201 | 2.66 × 10−5 | *** | |
| Month | −0.123 | 0.040 | −3.041 | 2.36 × 10−3 | ** | |
| Beetle abundance | Intercept | 7.553 | 0.527 | 14.323 | <2 × 10−16 | *** |
| Month | −0.384 | 0.086 | −4.485 | 7.29 × 10−6 | *** | |
| Plant species richness | Intercept | 2.603 | 0.119 | 21.954 | <2 × 10−16 | *** |
| System (organic) | 0.675 | 0.149 | 4.542 | 5.58 × 10−6 | *** |
| Family Composition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Global Model | Pairwise Comparisons | |||||||||
| Variable | d. f. | MS | R2 | F | p | Month | F | p | ||
| System | 1 | 0.894 | 0.047 | 4.085 | 5.0 × 10−4 | *** | April vs. August | 6.732 | 1.00 × 10−4 | *** |
| Month | 4 | 1.580 | 0.332 | 7.223 | 1.0 × 10−4 | *** | April vs. July | 6.699 | 1.00 × 10−4 | *** |
| Residuals | 54 | 0.219 | 0.621 | April vs. June | 3.474 | 4.00 × 10−4 | *** | |||
| Total | 59 | 1 | April vs. May | 6.043 | 1.00 × 10−4 | *** | ||||
| August vs. July | 4.358 | 0.0033 | ** | |||||||
| August vs. June | 5.844 | 6.00 × 10−4 | *** | |||||||
| August vs. May | 13.786 | 2.00 × 10−4 | *** | |||||||
| July vs. June | 2.934 | 0.0027 | ** | |||||||
| July vs. May | 11.007 | 1.00 × 10−4 | *** | |||||||
| June vs. May | 9.922 | 1.00 × 10−4 | *** | |||||||
| Guild Composition | ||||||||||
| Global Model | Pairwise Comparisons | |||||||||
| Variable | d. f. | MS | R2 | F | p | Month | F | p | ||
| System | 1 | 0.489 | 0.037 | 2.964 | 0.009 | ** | April vs. August | 1.569 | 0.1467 | |
| Month | 4 | 0.979 | 0.294 | 5.934 | 0.000 | *** | April vs. July | 4.348 | 4.00 × 10−4 | *** |
| Residuals | 54 | 0.165 | 0.669 | April vs. June | 2.460 | 0.0293 | * | |||
| Total | 59 | 1.000 | April vs. May | 6.350 | 1.00 × 10−4 | *** | ||||
| August vs. July | 5.033 | 0.0022 | ** | |||||||
| August vs. June | 2.875 | 0.029 | * | |||||||
| August vs. May | 12.653 | 2.00 × 10−4 | *** | |||||||
| July vs. June | 3.303 | 0.0129 | * | |||||||
| July vs. May | 9.936 | 1.00 × 10−4 | *** | |||||||
| June vs. May | 14.267 | 1.00 × 10−4 | *** | |||||||
| Response Variable | Factor | Estimate | Std. Error | z | p | |
|---|---|---|---|---|---|---|
| Predators | Intercept | 6.742 | 0.591 | 11.400 | <2 × 10−4 | *** |
| Month | −0.410 | 0.096 | −4.269 | 1.96 × 10−5 | *** | |
| Phytophages | Intercept | 5.554 | 0.572 | 9.715 | <2 × 10−4 | *** |
| Month | −0.391 | 0.093 | −4.197 | 2.71 × 10−5 | *** | |
| Pollen-feeders | Intercept | 3.872 | 2.637 | 1.468 | 0.142 | |
| System (organic) | −4.366 | 3.018 | −1.447 | 0.148 | ||
| Month | −1.070 | 0.553 | −1.935 | 0.053 | . | |
| System (organic): Month | 1.025 | 0.603 | 1.701 | 0.089 | . | |
| Fungivores | Intercept | 3.002 | 1.098 | 2.734 | 0.00625 | ** |
| System (organic) | 0.954 | 0.505 | 1.890 | 0.05877 | . | |
| Month | −0.540 | 0.182 | −2.965 | 0.00303 | ** | |
| Xylophages | Intercept | 4.949 | 0.487 | 10.169 | <2 × 10−16 | *** |
| System (organic) | −1.490 | 0.564 | −2.643 | 0.00821 | ** | |
| Aspect (sunny) | −1.564 | 0.564 | −2.773 | 0.00555 | ** | |
| Saprophages | Intercept | 3.304 | 0.268 | 12.351 | <2 × 10−16 | *** |
| System (organic) | 0.644 | 0.378 | 1.704 | 0.0883 | . | |
| Mixed feeding habits | Intercept | 8.807 | 2.110 | 4.174 | 2.99 × 10−5 | *** |
| System (organic) | 2.097 | 0.802 | 2.615 | 0.00893 | ** | |
| Month | −2.014 | 0.446 | −4.512 | 6.41 × 10−6 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Ramos, N.; Asís, J.D.; Tobajas, E.; de Paz, V.; Baños-Picón, L. Disentangling the Benefits of Organic Farming for Beetle Communities (Insecta: Coleoptera) in Traditional Fruit Orchards. Agriculture 2022, 12, 243. https://doi.org/10.3390/agriculture12020243
Rosas-Ramos N, Asís JD, Tobajas E, de Paz V, Baños-Picón L. Disentangling the Benefits of Organic Farming for Beetle Communities (Insecta: Coleoptera) in Traditional Fruit Orchards. Agriculture. 2022; 12(2):243. https://doi.org/10.3390/agriculture12020243
Chicago/Turabian StyleRosas-Ramos, Natalia, Josep D. Asís, Estefanía Tobajas, Víctor de Paz, and Laura Baños-Picón. 2022. "Disentangling the Benefits of Organic Farming for Beetle Communities (Insecta: Coleoptera) in Traditional Fruit Orchards" Agriculture 12, no. 2: 243. https://doi.org/10.3390/agriculture12020243
APA StyleRosas-Ramos, N., Asís, J. D., Tobajas, E., de Paz, V., & Baños-Picón, L. (2022). Disentangling the Benefits of Organic Farming for Beetle Communities (Insecta: Coleoptera) in Traditional Fruit Orchards. Agriculture, 12(2), 243. https://doi.org/10.3390/agriculture12020243






