Multiple Factors at Regional, Landscape, and Local Scales Determine Spider Assemblage Composition in Pomegranate Orchards
Abstract
1. Introduction
2. Methods
2.1. Study Sites
2.2. Spider and Insect Sampling
2.3. Spider Identification and Functional Groups
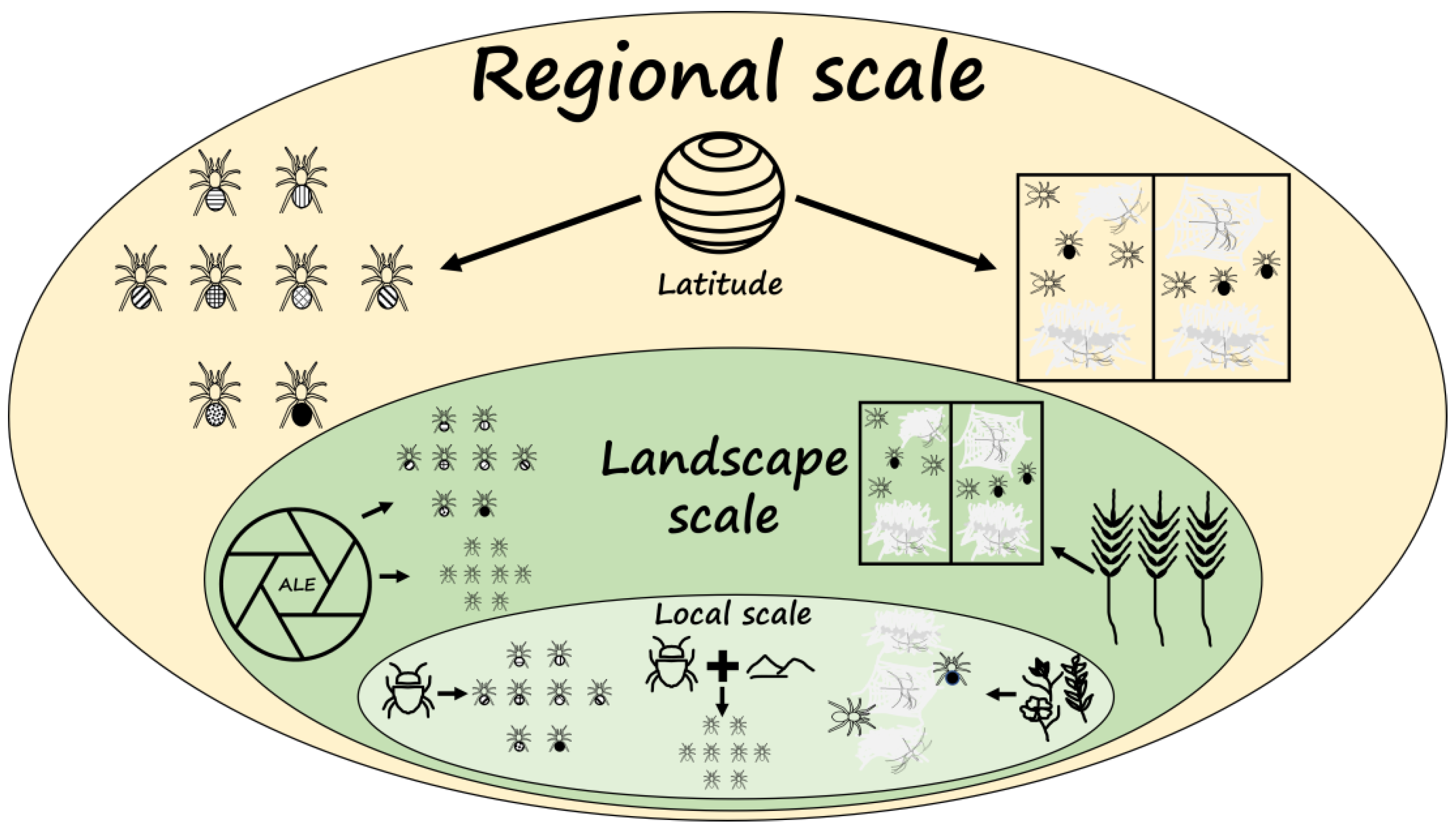
2.4. Environmental Variables at Regional, Landscape, and Local Scales Used in Data Analyses
2.5. Statistical Analysis
2.6. Limitations and Novelty of the Study Design
3. Results
3.1. Similarity of the Spider Assemblage Composition among Orchards
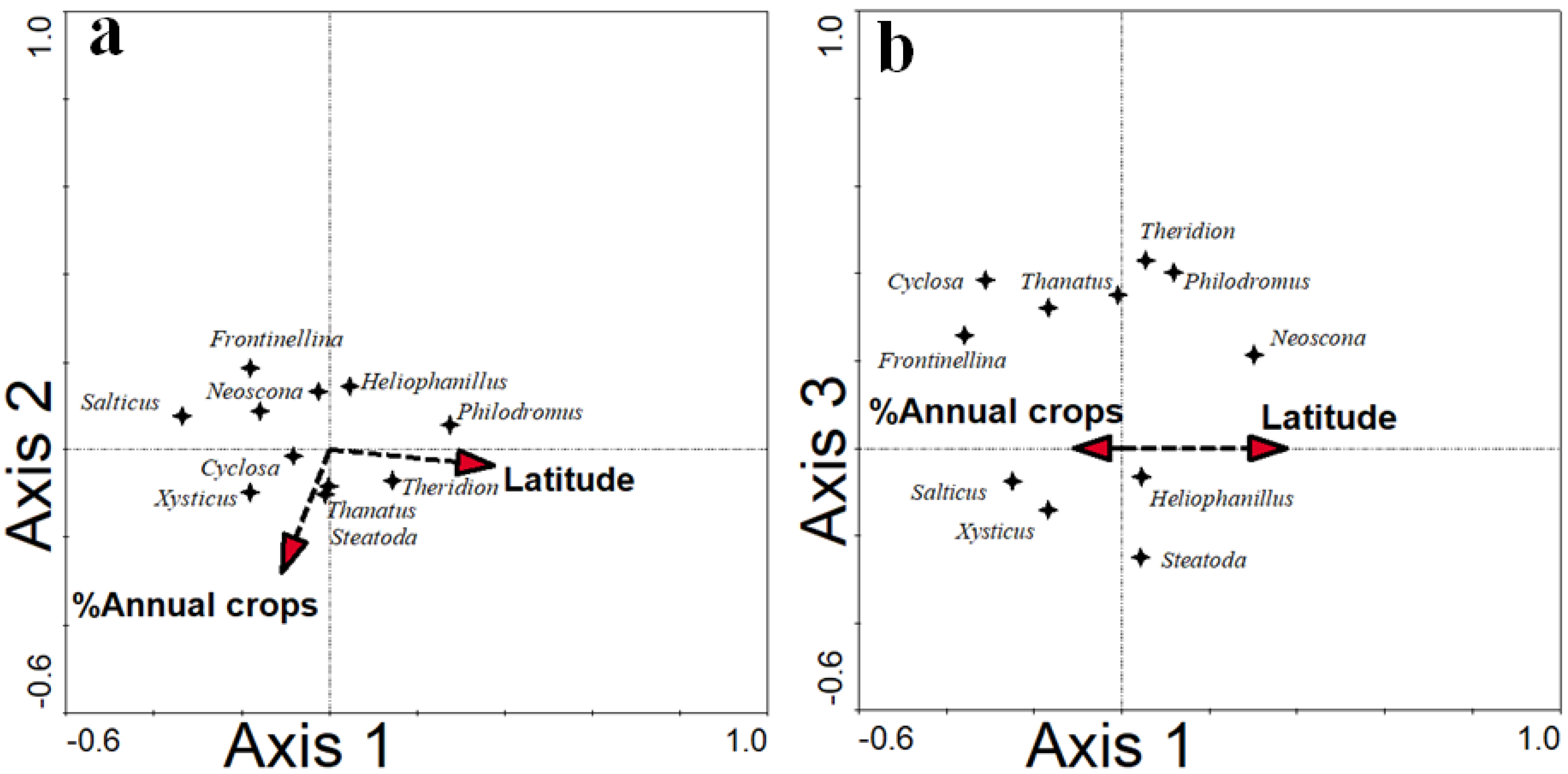
3.2. Environmental Variables and the Composition of Spider Assemblages in Pomegranate Orchards
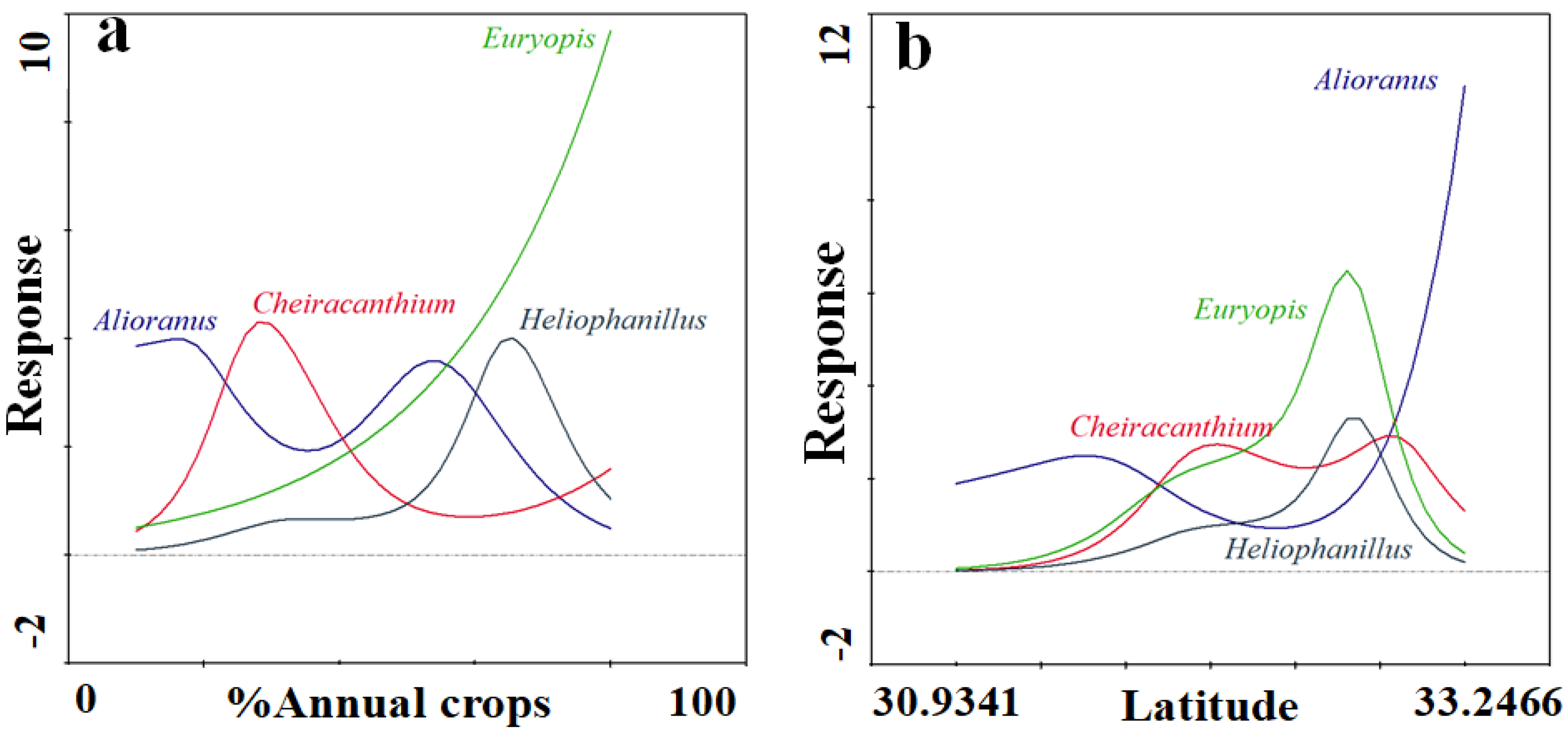
3.3. Functional Groups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Landscape Variables | Local Variables | ||||||
|---|---|---|---|---|---|---|---|
| Non-Crop | Annual Crops | Orchards | Human-Dominated | Insect Abundance | %Plant Cover | Plant Species Richness | |
| Kefar Yuval | 1.5 | 51 | 15 | 4.5 | 4 (0, 12) | 17 (4, 30) | 4 (2, 4) |
| Dishon | 4.5 | 19 | 2 | 0.5 | 28 (8, 37) | 2 (1, 2) | 3 (3, 3) |
| Evron | 0 | 26 | 39 | 17 | 4 (0, 6) | 11 (6, 16) | 5 (3, 7) |
| Sede Ya’aqov | 4 | 62 | 7 | 7 | 59 (16, 213) | 20 (14, 27) | 9 (4, 13) |
| Giv’at ‘Ada | 3 | 75 | 13 | 0.5 | 42 (17, 71) | 4 (3, 6) | 5 (2, 7) |
| Giv’at Hayyim W. | 3 | 50 | 16 | 11 | 13 (7, 39) | 29 (14, 29) | 8 (6, 8) |
| Giv’at Hayyim M. | 0 | 32 | 63 | 1 | 7 (3, 13) | 6 (3, 9) | 9 (4, 13) |
| Hazor | 5 | 37 | 26 | 9.5 | 8 (2, 17) | 5 (1, 8) | 8 (6, 10) |
| Zor’a | 7 | 30 | 20 | 7.5 | 16 (0, 26) | 1 (1, 4) | 4 (3, 4) |
| Lakhish | 0 | 13 | 18 | 0 | 5 (0, 13) | 0 (0, 0) | 1 (0, 1) |
| Mishmar HaNegev | 2.5 | 60 | 10 | 4 | 30 (6, 140) | 15 (2, 15) | 3 (5, 5) |
| Be’er Milka | 8 | 15 | 7 | 0 | 15 (7, 154) | 15 (10, 20) | 3 (2, 3) |
| Family | Genus | Functional Group | Number of Individuals |
|---|---|---|---|
| Araneidae | Cyclosa Menge, 1866 | Orb-web weavers | 15 |
| Cyrtophora Simon, 1864 | Orb-web weavers | 2 | |
| Larinioides Caporiacco, 1934 | Orb-web weavers | 1 | |
| Neoscona Simon, 1864 | Orb-web weavers | 85 | |
| Cheiracanthidae | Cheiracanthium C. L. Koch, 1839 | Active hunters | 147 |
| Clubionidae | Clubiona Latreille, 1804 | Active hunters | 48 |
| Gnaphosidae | Aphantaulax Simon, 1878 | Active hunters | 8 |
| Micaria Westring, 1851 | Active hunters | 6 | |
| Linyphiidae | Agyneta Hull, 1911 | Sheet-web weavers | 1 |
| Alioranus Simon, 1926 | Sheet-web weavers | 223 | |
| Frontinellina van Helsdingen, 1969 | Sheet-web weavers | 12 | |
| Lepthyphantes Menge, 1866 | Sheet-web weavers | 1 | |
| Miturgidae | Zora C. L. Koch, 1847 | Active hunters | 1 |
| Philodromidae | Philodromus Walckenaer, 1826 | Ambush hunters | 41 |
| Thanatus C. L. Koch, 1837 | Ambush hunters | 56 | |
| Pisauridae | Pisaura Simon, 1886 | Active hunters | 4 |
| Salticidae | Aelurillus Simon, 1885 | Active hunters | 3 |
| Heliophanillus Prószyński, 1989 | Active hunters | 97 | |
| Macaroeris Wunderlich, 1992 | Active hunters | 1 | |
| Pseudicius Simon, 1885 | Active hunters | 8 | |
| Salticus Latreille, 1804 | Active hunters | 29 | |
| Synageles Simon, 1876 | Active hunters | 2 | |
| Segestriidae | Segestria Latreille, 1804 | Tangle-web weavers | 1 |
| Tetragnathidae | Tetragnatha Latreille, 1804 | Orb-web weavers | 9 |
| Theridiidae | Dipoena Thorell, 1869 | Tangle-web weavers | 2 |
| Euryopis Menge, 1868 | Tangle-web weavers | 190 | |
| Kochiura Archer, 1950 | Tangle-web weavers | 6 | |
| Latrodectus Walckenaer, 1805 | Tangle-web weavers | 1 | |
| Steatoda Sundevall, 1833 | Tangle-web weavers | 61 | |
| Theridion Walckenaer, 1805 | Tangle-web weavers | 30 | |
| Thomisidae | Ozyptila Simon, 1864 | Ambush hunters | 3 |
| Synaema Simon, 1864 | Ambush hunters | 2 | |
| Thomisus Walckenaer, 1805 | Ambush hunters | 2 | |
| Xysticus C. L. Koch, 1835 | Ambush hunters | 55 | |
| Titanoecidae | Titanoeca Thorell, 1870 | Other-web weavers | 2 |
| Uloboridae | Uloborus Latreille, 1806 | Orb-web weavers | 1 |
| Zodariidae | Zodarion Walckenaer, 1826 | Active hunters | 1 |
References
- D’Alberto, C.F.; Hoffmann, A.A.; Thomson, L.J. Limited benefits of non-crop vegetation on spiders in Australian vineyards: Regional or crop differences? BioControl 2012, 57, 541–552. [Google Scholar] [CrossRef]
- Gonçalves, F.; Nunes, C.; Carlos, C.; López, Á.; Oliveira, I.; Crespí, A.; Teixeira, B.; Pinto, R.; Costa, C.A.; Torres, L. Do soil management practices affect the activity density, diversity, and stability of soil arthropods in vineyards? Agric. Ecosyst. Environ. 2020, 294, 106863. [Google Scholar] [CrossRef]
- Nardi, D.; Lami, F.; Pantini, P.; Marini, L. Using species-habitat networks to inform agricultural landscape management for spiders. Biol. Conserv. 2019, 239, 108275. [Google Scholar] [CrossRef]
- Öberg, S. Influence of landscape structure and farming practice on body condition and fecundity of wolf spiders. Basic Appl. Ecol. 2009, 10, 614–621. [Google Scholar] [CrossRef]
- Karp, D.S.; Chaplin-Kramer, R.; Meehan, T.D.; Martin, E.A.; DeClerck, F.; Grab, H.; Gratton, C.; Hunt, L.; Larsen, A.E.; Martínez-Salinas, A.; et al. Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl. Acad. Sci. USA 2018, 115, E7863–E7870. [Google Scholar] [CrossRef]
- Martin, E.A.; Seo, B.; Park, C.R.; Reineking, B.; Steffan-Dewenter, I. Scale-dependent effects of landscape composition and configuration on natural enemy diversity, crop herbivory, and yields. Ecol. Appl. 2016, 26, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, G.; Steward, P.R.; Benton, T.G.; Kunin, W.E.; Potts, S.G.; Biesmeijer, J.C.; Sait, S.M. Comparison of pollinators and natural enemies: A meta-analysis of landscape and local effects on abundance and richness in crops. Biol. Rev. 2013, 88, 1002–1021. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.J.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Lu, Z.X.; Zhu, P.Y.; Gurr, G.M.; Zheng, X.S.; Read, D.M.; Heong, K.L.; Yang, Y.J.; Xu, H.X. Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: Prospects for enhanced use in agriculture. Insect Sci. 2014, 21, 1–12. [Google Scholar] [CrossRef]
- Shapira, I.; Gavish-Regev, E.; Sharon, R.; Harari, A.R.; Kishinevsky, M.; Keasar, T. Habitat use by crop pests and natural enemies in a Mediterranean vineyard agroecosystem. Agric. Ecosyst. Environ. 2018, 267, 109–118. [Google Scholar] [CrossRef]
- Thomson, L.J.; Hoffmann, A.A. Natural enemy responses and pest control: Importance of local vegetation. Biol. Control. 2010, 52, 160–166. [Google Scholar] [CrossRef]
- Sackett, T.E.; Buddle, C.M.; Vincent, C. Comparisons of the composition of foliage-dwelling spider assemblages in apple orchards and adjacent deciduous forest. Can. Entomol. 2008, 140, 338–347. [Google Scholar] [CrossRef]
- Lefebvre, M.; Franck, P.; Toubon, J.F.; Bouvier, J.C.; Lavigne, C. The impact of landscape composition on the occurrence of a canopy dwelling spider depends on orchard management. Agric. Ecosyst. Environ. 2016, 215, 20–29. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; O’Rourke, M.E.; Blitzer, E.J.; Kremen, C. A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol. Lett. 2011, 14, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Isaia, M.; Bona, F.; Badino, G. Influence of landscape diversity and agricultural practices on spider assemblage in Italian vineyards of Langa Astigiana (Northwest Italy). Environ. Entomol. 2006, 35, 297–307. [Google Scholar] [CrossRef]
- Salman, I.N.; Gavish-Regev, E.; Saltz, D.; Lubin, Y. The agricultural landscape matters: Spider diversity and abundance in pomegranate orchards as a case study. BioControl 2019, 64, 583–593. [Google Scholar] [CrossRef]
- Clough, Y.; Kruess, A.; Kleijn, D.; Tscharntke, T. Spider diversity in cereal fields: Comparing factors at local, landscape and regional scales. J. Biogeogr. 2005, 32, 2007–2014. [Google Scholar] [CrossRef]
- Bogya, S.; Markó, V.; Szinetár, C.S. Comparison of pome fruit orchard inhabiting spider assemblages at different geographical scales. Agric. For. Entomol. 1999, 1, 261–269. [Google Scholar] [CrossRef]
- Sanders, D.; Vogel, E.; Knop, E. Individual and species-specific traits explain niche size and functional role in spiders as generalist predators. J. Anim. Ecol. 2015, 84, 134–142. [Google Scholar] [CrossRef]
- Uetz, G.W. Foraging strategies of spiders. Trends Ecol. Evol. 1992, 7, 155–159. [Google Scholar] [CrossRef]
- Uetz, G.W.; Halaj, J.; Cady, A.B. Guild structure of spiders in major crops. J. Arachnol. 1999, 27, 270–280. [Google Scholar]
- Cardoso, P.; Pekár, S.; Jocqué, R.; Coddington, J.A. Global patterns of guild composition and functional diversity of spiders. PLoS ONE 2011, 6, e21710. [Google Scholar] [CrossRef] [PubMed]
- Michalko, R.; Pekár, S.; Entling, M.H. An updated perspective on spiders as generalist predators in biological control. Oecologia 2019, 189, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Marc, P.; Canard, A. Maintaining spider biodiversity in agroecosystems as a tool in pest control. Agric. Ecosyst. Environ. 1997, 62, 229–235. [Google Scholar] [CrossRef]
- Özturk, N.; Danisman, T.; Tufekli, M.; Ulusoy, M.R. Spider fauna of pomegranate and olive orchards in the Eastern Mediterranean Region of Turkey. Türk. Entomol. Bült. 2013, 3, 67–73. [Google Scholar]
- Salman, I.N.; Ferrante, M.; Möller, D.M.; Gavish-Regev, E.; Lubin, Y. Trunk refugia: A simple, inexpensive method for sampling tree trunk arthropods. J. Insect Sci. 2020, 20, 5. [Google Scholar] [CrossRef]
- World Spider Catalog. World Spider Catalog. Version 23.0. Natural History Museum Bern. 2022. Available online: http://wsc.nmbe.ch (accessed on 19 January 2022).
- Cardoso, P.; Silva, I.; de Oliveira, N.G.; Serrano, A.R. Indicator taxa of spider (Araneae) diversity and their efficiency in conservation. Biolog. Conserv. 2004, 120, 517–524. [Google Scholar] [CrossRef]
- Kindt, R.; Coe, R. Tree Diversity Analysis: A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry Centre: Nairobi, Kenya, 2005. [Google Scholar]
- Braak, C.T.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Canoco: Ithaca, NY, USA, 2002. [Google Scholar]
- Økland, R.H. Vegetation Ecology: Theory, Methods and Applications with Reference to Fennoscandia. Sommerfeltia; Botanical Garden and Museum, University of Oslo: Oslo, Norway, 1990; Volume 1, pp. 1–238. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Wang, M.; Yu, Z.; Liu, Y.; Wu, P.; Axmacher, J.C. Taxon-and functional group-specific responses of ground beetles and spiders to landscape complexity and management intensity in apple orchards of the North China Plain. Agric. Ecosyst. Environ. 2022, 323, 107700. [Google Scholar] [CrossRef]
- Picchi, M.S.; Bocci, G.; Petacchi, R.; Entling, M.H. Effects of local and landscape factors on spiders and olive fruit flies. Agric. Ecosyst. Environ. 2016, 222, 138–147. [Google Scholar] [CrossRef]
- Rosas-Ramos, N.; Baños-Picón, L.; Tormos, J.; Asís, J.D. Farming system shapes traits and composition of spider assemblages in Mediterranean cherry orchards. PeerJ 2020, 8, e8856. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; He, F. Region effects influence local tree species diversity. Proc. Natl. Acad. Sci. USA 2016, 113, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Zobel, M. The relative of species pools in determining plant species richness: An alternative explanation of species coexistence? Trends Ecol. Evol. 1997, 12, 266–269. [Google Scholar] [CrossRef]
- Klein, M. The geomorphology of Israel. In Monographiae Biologicae; Springer: New York, NY, USA, 1988; Volume 62, pp. 59–78. [Google Scholar]
- Zonstein, S.; Marusik, Y.M. Checklist of the spiders (Araneae) of Israel. Zootaxa 2013, 3671, 1–127. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Valverde, A.; Lobo, J.M. Determinants of local spider (Araneidae and Thomisidae) species richness on a regional scale: Climate and altitude vs. habitat structure. Ecol. Ento. 2007, 32, 113–122. [Google Scholar] [CrossRef]
- Segev, U.; Kigel, J.; Lubin, Y.; Tielbörger, K. Ant abundance along a productivity gradient: Addressing two conflicting hypotheses. PLoS ONE 2015, 10, e0131314. [Google Scholar] [CrossRef]
- Greenstone, M.H. Determinants of web spider species diversity: Vegetation structural diversity vs. prey availability. Oecologia 1984, 62, 299–304. [Google Scholar] [CrossRef]
- Schindler, B.; Gavish-Regev, E.; Keasar, T. Parasitoid wasp community dynamics in vineyards following insecticide application. Front. Environ. Sci. 2022, 1–10. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Tscharntke, T. The role of perennial habitats for Central European farmland spiders. Agric. Ecosyst. Environ. 2005, 105, 235–242. [Google Scholar] [CrossRef]
- Pluess, T.; Opatovsky, I.; Gavish-Regev, E.; Lubin, Y.; Schmidt-Entling, M.H. Non-crop habitats in the landscape enhance spider diversity in wheat fields of a desert agroecosystem. Agric. Ecosyst. Environ. 2010, 137, 68–74. [Google Scholar] [CrossRef]
- Gardiner, M.M.; Landis, D.A.; Gratton, C.; DiFonzo, C.D.; O’neal, M.; Chacon, J.M.; Wayo, M.T.; Schmidt, N.P.; Mueller, E.E.; Heimpel, G.E. Landscape diversity enhances biological control of an introduced crop pest in the north-central USA. Ecol. Appl. 2009, 19, 143–154. [Google Scholar] [CrossRef]
- Kishinevsky, M.; Keasar, T.; Harari, A.R.; Chiel, E. A comparison of naturally growing vegetation vs. border-planted companion plants for sustaining parasitoids in pomegranate orchards. Agric. Ecosyst. Environ. 2017, 246, 117–123. [Google Scholar] [CrossRef]
- Opatovsky, I.; Chapman, E.G.; Weintraub, P.G.; Lubin, Y.; Harwood, J.D. Molecular characterization of the differential role of immigrant and agrobiont generalist predators in pest suppression. Biol. Cont. 2012, 63, 25–30. [Google Scholar] [CrossRef]
- Opatovsky, I.; Weintraub, P.G.; Musli, I.; Lubin, Y. Use of alternative habitats by spiders in a desert agroecosystem. J. Arachnol. 2017, 45, 129–138. [Google Scholar] [CrossRef]
- Herrmann, J.D.; Opatovsky, I.; Lubin, Y.; Pluess, T.; Gavish-Regev, E.; Entling, M.H. Effects of non-native Eucalyptus plantations on epigeal spider communities in the northern Negev desert, Israel. J. Arachnol. 2015, 43, 101–106. [Google Scholar] [CrossRef]
- Gavish-Regev, E.; Lubin, Y.; Coll, M. Migration patterns and functional groups of spiders in a desert agroecosystem. Ecol. Entomol. 2008, 33, 202–212. [Google Scholar] [CrossRef]
- Bishop, L.; Riechert, S.E. Spider colonization of agroecosystems: Mode and source. Environ. Entomol. 1990, 19, 1738–1745. [Google Scholar] [CrossRef]
- Mansour, F.; Whitecomb, W.H. The spiders of a citrus grove in Israel and their role as biocontrol agents of Ceroplastes floridensis [Homoptera: Coccidae]. Entomophaga 1986, 31, 269–276. [Google Scholar] [CrossRef]
- Líznarová, E.; Pekár, S. Trophic niche and capture efficacy of an ant-eating spider, Euryopis episinoides (Araneae: Theridiidae). J. Arachnol. 2019, 47, 45–51. [Google Scholar] [CrossRef]
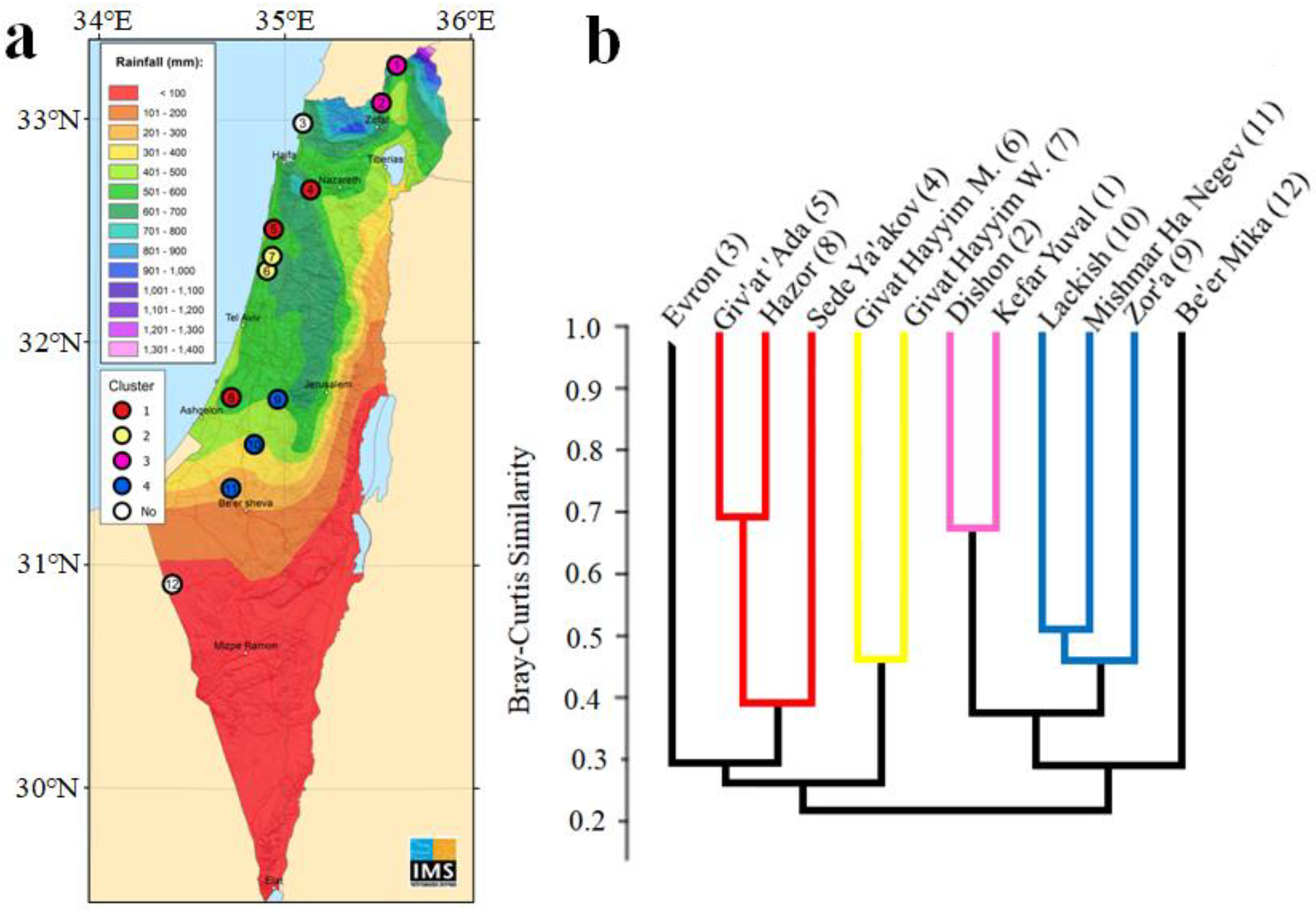
| Site Number | Location | Year Planted (Age at 2015) | Latitude (N) | Longitude (E) | Elevation (m) | Annual Rainfall (mm) |
|---|---|---|---|---|---|---|
| 1 | Kefar Yuval | 2006 (9) | 33°14′48″ | 35°35′53″ | 185.37 | 690 |
| 2 | Dishon | 2006 (9) | 33°4′53″ | 35°31′0″ | 360.3 | 507 |
| 3 | Evron | 2011 (4) | 32°59′29″ | 35°6′1″ | 25.26 | 622 |
| 4 | Sede Ya’aqov | 2001 (14) | 32°41′48″ | 35°8′27″ | 54.03 | 592 |
| 5 | Giv’at ‘Ada | 2008 (7) | 32°31′20″ | 34°56′42″ | 28.12 | 617 |
| 6 | Giv’at Hayyim M | 2006 (9) | 32°23’33″ | 34°55’46″ | 35.29 | 603 |
| 7 | Giv’at Hayyim W | 2006 (9) | 32°24’7″ | 34°56’15″ | 15.21 | 617 |
| 8 | Hazor | 2003 (12) | 31°46′20″ | 34°43′13″ | 39.15 | 561 |
| 9 | Zor’a | 2006 (9) | 31°45′51″ | 34°58′2″ | 188.18 | 484 |
| 10 | Lakhish | 2006 (9) | 31°33′42″ | 34°50′34″ | 287.56 | 383 |
| 11 | Mishmar HaNegev | 2010 (5) | 31°21′51″ | 34°43′7″ | 178.71 | 277 |
| 12 | Be’er Milka | 2008 (7) | 30°55′56″ | 34°24′28″ | 185.75 | 126 |
| Environmental Variables | Trace | Variance Explained (%) | F | p-Value | |
|---|---|---|---|---|---|
| a. Regional Scale | |||||
| Latitude | 0.086 | 3.163 | 2.7 | 1.85 | 0.02 |
| Rainfall | 0.044 | 3.121 | 1.4 | 0.94 | 0.50 |
| b. Landscape scale | |||||
| Semi-natural habitat | 0.067 | 3.145 | 2.1 | 1.45 | 0.10 |
| Annual crops | 0.098 | 3.176 | 3.1 | 2.11 | 0.01 |
| Orchards | 0.074 | 3.152 | 2.4 | 1.60 | 0.07 |
| c. Local scale | |||||
| Age | 0.063 | 2.968 | 2.1 | 1.43 | 0.12 |
| Area | 0.031 | 3.109 | 1.0 | 0.67 | 0.78 |
| Elevation | 0.036 | 3.114 | 1.2 | 0.78 | 0.66 |
| Plant cover | 0.049 | 3.126 | 1.6 | 1.05 | 0.38 |
| Plant richness | 0.064 | 3.141 | 2.0 | 1.37 | 0.15 |
| Insect abundance | 0.04 | 3.118 | 1.3 | 0.86 | 0.51 |
| Eigenvalues | Genus-Environment Correlation | Cumulative Percentage Variance | Sum of all Canonical Values | ||
|---|---|---|---|---|---|
| of Genera | of Genus-Environment Relation | ||||
| Axis 1 | 0.109 | 0.665 | 3.3 | 59.0 | |
| Axis 2 | 0.075 | 0.558 | 5.6 | 100.0 | 0.184 |
| Axis 3 | 0.534 | 0.000 | 22.0 | 0.0 | |
| Axis 4 | 0.324 | 0.000 | 32.0 | 0.0 | |
| Sum of all eigenvalues | 3.261 | ||||
| Environmental Variables | Orb-Web | Active Hunter | Ambush Hunter | Tangle-Web Weavers | Sheet-Web Weavers |
|---|---|---|---|---|---|
| a. Regional | |||||
| Latitude | −0.15 | 0.04 | −0.01 | 0.01 | 0.27 |
| b. Landscape | |||||
| Annual crops | 0.18 | 0.07 | 0.12 | 0.12 | −0.09 |
| Orchards | −0.05 | 0.10 | 0.03 | −0.06 | −0.08 |
| c. Local | |||||
| Age | −0.11 | −0.10 | −0.11 | −0.26 | 0.12 |
| Area | −0.01 | −0.07 | 0.09 | −0.06 | −0.03 |
| Elevation | −0.20 | −0.19 | −0.06 | −0.13 | 0.30 |
| Plant cover | 0.15 | 0.15 | 0.15 | 0.00 | 0.08 |
| Plant richness | 0.32 | 0.43 | 0.33 | −0.05 | −0.17 |
| Insect abundance | 0.22 | 0.08 | 0.08 | 0.15 | −0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salman, I.N.A.; Lubin, Y.; Gavish-Regev, E. Multiple Factors at Regional, Landscape, and Local Scales Determine Spider Assemblage Composition in Pomegranate Orchards. Agriculture 2022, 12, 512. https://doi.org/10.3390/agriculture12040512
Salman INA, Lubin Y, Gavish-Regev E. Multiple Factors at Regional, Landscape, and Local Scales Determine Spider Assemblage Composition in Pomegranate Orchards. Agriculture. 2022; 12(4):512. https://doi.org/10.3390/agriculture12040512
Chicago/Turabian StyleSalman, Ibrahim N. A., Yael Lubin, and Efrat Gavish-Regev. 2022. "Multiple Factors at Regional, Landscape, and Local Scales Determine Spider Assemblage Composition in Pomegranate Orchards" Agriculture 12, no. 4: 512. https://doi.org/10.3390/agriculture12040512
APA StyleSalman, I. N. A., Lubin, Y., & Gavish-Regev, E. (2022). Multiple Factors at Regional, Landscape, and Local Scales Determine Spider Assemblage Composition in Pomegranate Orchards. Agriculture, 12(4), 512. https://doi.org/10.3390/agriculture12040512







