Abstract
Recent investigations carried out all over the world have disclosed the capacity of a multitude of endophytic fungal species/strains to produce bioactive compounds which are the same or structurally related to those synthesized by their host plants. This intriguing phenomenon has implemented huge research activity aimed at ascertaining the nature of the biological processes underlying this convergence, as well as to characterize the genetic bases of the biosynthetic schemes. Insights on these basic issues may support the biotechnological exploitation of wild strains, and their eventual improvement through genome editing. Aspects concerning the use of next generation sequencing technologies for the comparative elucidation of the biosynthetic pathways operating in endophytic fungi and their host plants are reviewed in this paper in view of applicative perspectives. Our literature search yielded 21 references dealing with at least 26 strains which have been the subject of experimental activities involving massively parallel genome and transcriptome sequencing methods in the last eight years.
1. Introduction
Endophytic fungi are known to be able to develop inside tissues of healthy plants during their life cycle without inducing obvious disease symptoms. After having been almost completely neglected for over a century, in the last decades, the concept of endophytism has been re-evaluated concomitantly to the increasing awareness of the important ecological role played by these microbial symbionts of plants, and of the opportunity to exploit their unique biocenotic aptitudes and biosynthetic properties [1,2,3].
One of the possible biotechnological applications concerning this component of biodiversity derives from the capacity to synthesize bioactive products originally known as plant secondary metabolites. This intriguing aptitude was first reported for Taxomyces andreanae, an endophyte of the Pacific yew tree (Taxus brevifolia) producing taxol in axenic cultures [4]. Since, it has been documented for more and more strains recovered from plants from any environment and climatic area [5,6,7,8], which represents an evident indication that, rather than being accidental, it is a common outcome of the intimate interactions established within these symbiotic communities. The possibility of the pharmaceutical industry effectively using endophytic fungal strains for a more economic production of some drugs clearly depends on a more thorough understanding of this biological phenomenon, particularly with insights on the conditions enabling the transfer of biosynthetic capacities between phylogenetically distant organisms. In this respect, genetic aspects have a basic relevance, starting from assessments concerning the share of a common gene base.
Next-generation sequencing (NGS) methods enable faster and cheaper nucleotide sequencing than the traditional Sanger method, opening a new era in genomics and molecular biology. Compared to the Sanger capillary electrophoresis sequencing method, NGS technologies provide higher throughput data at lower costs and better support genome research [9]. These new sequencing methods share three major advantages. First, NGS libraries are prepared in a cell-free system instead of requiring bacterial cloning of DNA fragments. Second, they produce thousands to millions of sequencing reactions in parallel. Finally, base interrogation is performed cyclically and in parallel, so that the sequencing output is detected without electrophoresis. As a result, the enormous number of reads generated by NGS enables genome sequencing at an unprecedented speed [10].
During the past decade, NGS technologies have evolved through the incorporation of revolutionary innovations to tackle the complexities of genomes [11]. Short-read sequencing approaches, such as sequencing by synthesis, ion semiconductor, and nanoball sequencing, maximize the number of bases sequenced in a short time, generating a wealth of data that can help understand complex phenotypes. Otherwise, long-read sequencing techniques aim to sequence longer contiguous DNA sectors, which is required to resolve structurally complex regions. Third generation long-read sequencing has the potential to overcome many limitations of the short-read approaches, e.g., the resolution of repeated sequences and large genomic rearrangements [11,12].
The advancement in genome sequencing technologies has boosted genomics research by enabling many small laboratories to achieve sequencing of entire genomes. In addition, gene expression studies frequently shifted from microarrays to NGS-based methods, enabling the identification and quantification of transcripts without a preliminary knowledge of a particular gene, and providing information on alternative splicing and sequence variation [13].
Currently, efficient and modern NGS technologies are used in studies concerning endophytes due to their precision, sensitivity, and specificity. Hopefully, together with other approaches such as metabolomics, metagenomics, proteomics, and bioinformatics, they will fill a gap in fungal endophyte research. To date, NGS technologies have been employed in the field of endophytic fungi for taxonomic identification and studies on their ecology (resistance to heavy metals and high salinity, communication with other fungi and bacteria, etc.) [14,15,16,17,18,19], interactions with hosts, lifestyle and functional heterogeneity [20,21,22,23,24,25,26], biosynthetic pathways of secondary metabolites [27,28,29,30,31,32,33], bioprospecting for biofuel production [34,35], evolution [36], and many other aspects.
This paper provides an overview of published projects that utilized genome and transcriptome NGS technology in endophytic fungi to elucidate biosynthetic pathways underlying bioactive metabolite production. Additionally, as we are interested in fungal products previously reported as plant metabolites, we have restricted our analysis to research aiming at exploring genetic pathways in endophytic fungi able to synthesize plant bioactive metabolites. Benefits, shortcomings, and future opportunities of the employed strategies are discussed.
2. Do Endophytic Fungi Possess Pathway Genes Encoding Plant Bioactive Metabolites?
As previously introduced, endophytic fungi are a polyphyletic group of microorganisms colonizing healthy plant tissues, which developed different symbiotic relationships with their hosts through evolutionary processes [6]. As a consequence of this long-term association implying mutually beneficial relationships, endophytes may play a role in plant metabolism and boost their own biosynthetic activity or may access genetic information to produce bioactive compounds which are analogues to those directly produced by the host plants [5].
However, our knowledge on genetic mechanisms which have eventually allowed endophytic fungi to gain foreign DNA is scanty. One of the hypotheses is horizontal gene transfer (HGT), which is regarded as an important evolutionary mechanism in prokaryotes and is also considered as a possible mechanism for the transmission of genetic material between phylogenetically distant species [37]. Based on evidence of physical clustering of genes for specialized plant metabolic pathways provided by an increasing number of reports [38], HGT is thought to be responsible for the transfer of whole gene clusters from host plants, conferring novel biosynthetic abilities to the associated fungi. According to this hypothesis, many endophytes have developed the capacity to synthesize bioactive products originally known from their hosts, which has triggered the expectation to exploit endophytes as an alternative and sustainable source of important plant metabolites for relieving our dependence on plants.
Unfortunately, this expectation remains unfulfilled. One of the main reasons consists in the attenuation or loss of the biosynthetic capacity by the endophytic strains upon subculturing [39,40]. It has been postulated that it could be consequential to the lack of host stimulation and/or silencing of genes in axenic cultures [41]. However, attempts to reverse the attenuation by adding host tissue extracts to the culturing medium have been unsuccessful [40,42].
Sachin and coworkers considered three alternative hypotheses to explain, firstly, the capacity by endophytic fungi to produce plant secondary metabolites and, secondly, its attenuation in sub-culturing with reference to the observed absence of a key structural gene (here, the strictosidine synthase gene, STR) in endophytes able to produce terpenoid indole alkaloids [43]. The first hypothesis considers that the function of the missing enzyme might be rendered by another enzyme (encoded by a gene analogue), thus the orthologue gene might be simply absent from the genome of an endophyte. The second hypothesis postulates that endophytic fungi might harbor the missing gene on certain extra-chromosomal elements (ECEs) in the cytoplasm, such as plasmids, which might have been transferred from the host during evolutionary interactions. The third alternative is an extension of the second, saying that the missing gene is included in a plasmid carried by endohyphal bacteria. Like in the former hypothesis, the bacterial plasmid could have acquired the genes through horizontal gene transfer from the host plant. In fact, there are many reports of fungi carrying ECEs, including plasmids [44,45], and of the presence of endohyphal bacteria in fungi [46,47,48]. The two latter hypotheses assume that the attenuation of the secondary metabolite production occurring in cultures results from the steady decay in the number or loss of the endohyphal bacteria or plasmids bearing the genes.
In the current post-genomic era, dilemmas concerning the genetic mechanisms enabling production of plant secondary metabolites by endophytic fungi might have a chance to be resolved. The development of sequencing and bioinformatics methods, as well as the availability of multiple strategies to comprehensively study genomes and transcriptomes [9,10,11], should allow the elucidation of biosynthetic pathways underlying the synthesis of bioactive secondary metabolites, including those operating in endophytic fungi.
3. Gene Discovery and Gene Expression Analysis in the Pre-NGS Era
In the pre-genomic era, several molecular approaches have been developed to screen endophytic fungi for potential producers of valuable secondary metabolites. Screening for molecular markers was (and still is) a popular method which enables detection of key biosynthetic genes in the endophyte genomes by a polymerase chain reaction (PCR) amplification. This technique is based on the design of degenerate PCR primers from sequences of known biosynthetic genes of plants to detect the presence of a certain gene in fungal genomes or environmental DNA, and to obtain its nucleotide sequence. This method was successfully used for identification of a ts (encoding taxadiene synthase) and phenylpropanoyl transferase gene in T. andreanae [49], ts and bapt (encoding C-13 phenylpropanoyl side chain-CoA acyltransferase) in Guignardia mangiferae, Fusarium proliferatum, and Colletotrichum gloeosporioides isolated from Taxus x media [50], dbat (encoding 10-deacetylbaccatin III-10-O-acetyl transferase) in endophytic fungi isolated from Salacia oblonga [51], and bapt in endophytic fungi isolated from Taxus baccata [52]. The presence of genes encoding for hyoscyamine 6β-hydroxylase, putrescine N-methyltransferase and tropinone reductase 1 was reported by the PCR-based molecular marker screening approach in endophytic fungi isolated from Datura metel producing tropane alkaloids [53].
In the past decade, a microarray-based technique allowed for transcriptome profiling in the high-throughput manner, thus it became an important step towards global transcriptomic analysis. A microarray-based technique was used to perform comparative transcript profiling in the grass fungal endophytes Neotyphodium coenophialum, Neotyphodium lolii, and Epichloë festucae [54], which allowed to investigate genome-specific gene expression, novel endophyte genes, and general plant–endophyte interactions through revealing factors supporting the establishment and maintenance of the mutualistic association. The microarray studies performed in filamentous fungi, reviewed by Breakspear and Momany [55], have helped revealing genes which play a role in the biosynthesis of both useful and detrimental secondary metabolites. There are no reports, however, on microarray studies performed in endophytic fungi, which would be employed for the discovery and analysis of structural genes involved in fungal biosynthesis of plant bioactive metabolites.
4. Global Genome and Transcriptome Analysis for the Discovery of Plant-Like Secondary Metabolites Biosynthetic Genes in Endophytic Fungi
Despite the astonishing number of species of endophytic fungi which are thought to exist [56,57,58,59], we do not know a lot about the genomics and transcriptomics of this diverse kingdom. Databases containing genomes and transcriptomes of fungi are still poor in resources, even those dedicated to fungal research such as ENSEMBL Fungi (1014 complete genomes) [60], Mycocosm, the branch of Joint Genome Institute (JGI) [61], holding the “1000 fungal genomes” project and e-Fungi, run by the University of Exeter (UK) [62], with only 34 complete Fungal Genomes. Up to date, JGI offers 6 studies of gene expression profiling and only 228 records of endophytic fungi genomes.
Richer resources are found in the GenBank and the initiative named International Nucleotide Sequence Database Collaboration (INSDC)—joint genomic resource database of the National Center of Biotechnological Information (NCBI), the Bioinformatics Institute (EBI), and the DNA Database of Japan (DDBJ). The Sequence Read Archive (SRA) is the NCBI database that stores sequence data obtained from next generation sequence technology. Thorough SRA database search revealed 2279 records for endophytic fungi, without taking into account data from uncultured fungi and metagenomics projects [63]. Out of these, 2230 records reported endophytic fungi genome sequencing and 49 deposited endophytic fungi transcriptomes. Interestingly, all sequences were generated using various Illumina platforms, except for one record of Neocosmospora (= Fusarium) solani whole genome (WGS) sequenced with Oxford Nanopore (MinION) platform.
From the thorough survey of the SRA, PubMed, and Google Scholar databases we defined a list of accessible records of genomes or transcriptomes of endophytic fungi, which allowed to explore their genetic pathways and resulted in discovering fungal structural genes potentially involved in the biosynthesis of plant bioactive metabolites (Table 1).

Table 1.
Endophytic fungi producing plant-like bioactive secondary metabolites which have been the subject of next generation sequencing projects for the discovery of plant-like structural genes.
4.1. Taxol
Discovered in 1970 from bark of Taxus brevifolia, taxol (Figure 1), a diterpenoid also known as paclitaxel, is the best-selling natural anticancer drug [85]. However, the low yield at which taxol can be extracted and the unpredictable availability of natural sources represent a problem for the pharmaceutical industry. Hence, endophytic fungi from Taxus spp. appeared to represent a more reliable source for industrial production of this drug, considering their fast growth, low costs, independence of environmental constraints, and possible improvements through genetic manipulation. The list of endophytic fungi recovered from different hosts and their taxol-producing capacities was recently updated by El-Sayed et al. [86]. However, the exploitation of endophytic fungi for industrial production of taxol has been impaired by reduction of yields depending on the downregulation in the expression of the encoding genes which occurs after subculturing and storage. Therefore, several next generation sequencing attempts were made to enable genome mining and transcriptome analysis to identify the taxol biosynthethic pathway and its rate-limiting steps in endophytic fungi.
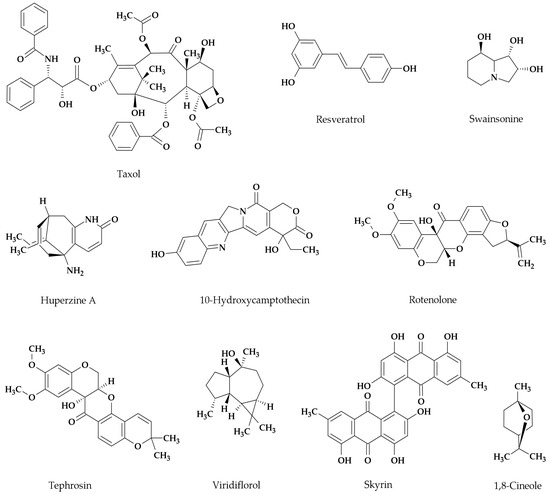
Figure 1.
Chemical structures of some plant bioactive products from endophytic fungi reviewed in this paper.
Following genome sequencing of Penicillium aurantiogriseum NRRL 62431, gene candidates potentially involved in paclitaxel biosynthesis by this strain were identified through comparison with the 13 paclitaxel biosynthetic genes known in Taxus [64]. A protein search performed against the genome of NRRL 62431 revealed putative homologues to seven Taxus genes encoding phenylalanine aminomutase (PAM), geranylgeranyl diphosphate synthase (GGPPS), and five taxane hydroxylases (T2OH, T5OH, T7OH, T10OH, and T13OH). Due to the low homology between the fungal paclitaxel biosynthetic gene candidates and those encoded by its host plant, it was postulated that gene candidates for taxol biosynthesis in NRRL 62431 evolved independently, and that in this case HGT is an unlikely explanation.
In 2018, the transcriptome of strain MD2 of Cladosporium cladosporioides undergoing drawdown in taxol production after subculturing was investigated for the first time with an RNA-sequencing technology to explore aspects related to gene expression [65]. KEGG analysis indicated that 40 unigenes in MD2 transcriptome were related to taxol biosynthesis and were homologous to the plant genes for taxadiene synthase (TS), taxadiene 5-alpha-hydroxylase (T5αH), taxane 13-alpha-hydroxylase (T13αH), and 2-alpha-hydroxytaxane 2-O-benzoyltransferase (TBT). However, no homologues to TAT and T10βH were detected in the transcriptome of this strain, suggesting that, unlike Taxus, it might not have this branch. Thus, the existence of a partial biosynthetic pathway from GGPP to 10-deacetylbaccatin III (10-DAB) in the MD2 transcriptome was speculated. Surprisingly, homologues of genes encoding enzymes functioning in the biosynthetic steps from 10-DAB to taxol, such as C-13 phenylpropanoid side chain-CoA acyltransferase (BAPT), 10-deacetylbaccatin III-10-O-acetyltransferase (DBAT), and 3′-N-debenzoyltaxol N-benzoyltransferase, were not detected. It is not known whether this was due to their low sequence homology with the host-counterparts or to the lack of transcription.
Another transcriptome sequencing and analysis of two taxol-producing strains of Aspergillus aculeatinus was performed in 2020, but the results were inconclusive as the exerted biosynthesis pathway was not clear [66]. Genes from the mevalonate (MVA) and nonmevalonate (MEP) pathways, involved in the biosynthesis of terpenoids, were expressed, including those encoding 1-deoxyxylulose 5-phosphate reductoisomerase (DXR), 3-hydroxy-3-methylglutaryl-coenzyme A synthase (HMGS), 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HDR), and 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HMDR). In addition, genes encoding for delta(2)-isopentenyl pyrophosphate transferase (IPPS), geranyl pyrophosphate synthase (GPPS), and GGPPS were also found. Moreover, a unique sequence was found to correspond to taxane 10-β-hydroxylase (T10βH) involved in the synthesis of taxol by Ozonium sp. BT2. A comparison between the transcriptomes of the original strain A. aculeatinus Tax-6 and its mutant strain BT-2 (producing higher taxol yield) revealed that most genes involved in the MVA pathway in BT-2 were upregulated. Surprisingly, transcripts related to the conversion of GGPP to taxa-4(5)-11(12)-diene, representing the key step in taxol production, were absent in both strains.
4.2. Resveratrol
Resveratrol (Figure 1) is a phenolic compound known for multiple beneficial functions, including neuroprotection, without major adverse effects [87]. High quantities of this compound are required for applications in functional food [88]. At present resveratrol is mainly extracted from plant by-products, with limited availability [67,89].
De novo transcriptome sequencing of Alternaria sp. MG1, an endophytic strain isolated from grape which is able to synthesize resveratrol from glucose, identified 20 genes for glycolysis, 10 for phenylalanine biosynthesis, and 4 genes for phenylpropanoid and stilbenoid biosynthesis [67]. Phenylalanine ammonia-lyase (PAL), cytochrome P-450 CYP73A, 4-coumarate coenzyme A ligase (4CL), and chalcone synthase (CHS) were annotated for converting l-phenylalanine into resveratrol. Resveratrol synthase (also known as stilbene synthase, STS), the key enzyme catalyzing the last step of biosynthesis of this compound in plants, was not annotated; thus CHS, an isoenzyme of resveratrol synthase, was identified for its involvement in biosynthesis by this strain. The identified enzymes indicated that the reasonable biosynthesis pathway from glucose to resveratrol occurred via glycolysis, phenylalanine, and phenylpropanoid biosyntheses, and stilbenoid pathways.
Apart from producing resveratrol, Alternaria sp. MG1 has been found to produce its analogues pterostilbene and piceatannol. Pterostilbene is a dimethyl ether that exhibits cancer chemopreventive, antioxidant, antifungal, and hypolipidemic properties [90]. Piceatannol has displayed antitumor and immunosuppressive properties, a stronger antioxidant activity, and it is considered a potential antiarrhythmic agent [91]. Whole genome sequencing of strain MG1 has outlined its high metabolic diversity and led to the discovery of 21 proteins in the phenylpropanoid pathways, each encoded by more than one gene, some of which are involved in the biosynthesis of stilbenes, flavonoids, and lignins, and are indicative of the resveratrol biosynthesis pathway [68]. Particularly, the key genes encoding 4-coumarate coenzyme A ligase (4CL) and cinnamate 4-hydroxylase (C4H) were identified in the upstream metabolic pathway. As in the previous study, STS was not annotated, while CHS could be identified. The enzymes catalyzing conversion of resveratrol into pterostilbene and piceatannol were also successfully annotated in MG1, respectively as trans-resveratrol di-O-methyltransferase (ROMT) and piceatannol synthase (which is a cytochrome P450 enzyme, CYP1B1).
Additional enzymes could be annotated, indicating that strain MG1 may provide diverse gene resources for the production of functional compounds, especially those normally produced by plants at low levels. Those are the key enzymes involved in the flavonoid biosynthesis pathway from p-coumaric CoA to naringenin chalcone, and shikimate O-hydroxycinnamoyltransferase (HCT), the enzyme responsible for driving metabolic flux from p-coumaric CoA to the lignin biosynthesis pathway; conversely, ferulate-5-hydroxylase, which is involved in the lignin biosynthetic pathway in Vitis vinifera, was not found [68].
4.3. Swainsonine
Swainsonine (Swn), an indolizidine alkaloid (Figure 1), is known as a mannosidase inhibitor that impacts glycoprotein processing and induces lysosomal storage disease. Due to this activity, Swn is under consideration in cancer chemotherapy [92]. It was originally observed in several plant species due to its toxicity to livestock, but later found to be also produced by fungi [93]. Some controversy exists concerning the valid taxonomic name of these fungi; hence, herewith we use Embellisia oxytropis, which is the currently accepted epithet in Mycobank [94].
The first attempt towards elucidation of Swn biosynthetic pathway revealed the existence of multiple genes in the genome of the producing fungi [69]. Five genes were annotated potentially operating in Swn biosynthesis, namely saccharopine dehydrogenase (SDH), thought to be a key gene of this pathway, saccharopine oxidase (FAP2), polyketide synthase (PKS), pyrroline-5-carboxylate reductase (P5CR), and cytochrome P450 (P450).
Later on, the comparative genomics of the known Swn producers (Table 1) enabled identifying the Swn gene cluster [70]. Genome sequence analyses revealed that these fungi share orthologous Swn gene clusters, which include a multifunctional swnK gene containing predicted adenylylation and acyltransferase domains along with their associated thiolation domains, two reductase domains, and a b-ketoacyl synthase domain.
Recent transcriptome analysis of the wild type E. oxytropis and a mutant disrupted at the saccharopine reductase gene enabled screening of differential genes involved in Swn biosynthesis [71]. The biosynthetic pathway of Swn in the fungus was defined to include two branches of P6C and P2C. Delta1-piperideine-2-carboxylate reductase, lysine 6-dehydrogenase, and saccharopine oxidase/l-pipecolate oxidase were part of P6C, while 1-piperidine-2-carboxylate/1-pyrroline-2-carboxylate reductase (NAD(P)H) and delta1-piperideine-2-carboxylate reductase were part of P2C, and saccharopine reductase was involved in both branches. The transcriptome data indicated that hydroxylation of 1-indolizidineone into Swn may be catalyzed by hydroxymethylglutaryl-CoA lyase rather than by PKS and P450, as earlier WGS studies proposed.
4.4. Huperzine A
Huperzine A (Hup), a lycopodium alkaloid originally extracted from Huperzia serrata (Figure 1), is an effective inhibitor of acetylcholinesterase; thus, it has attracted intense attention as a therapeutic drug for Alzheimer’s disease [95]. However, production of Hup from plants in large quantities is currently insufficient because the producing plants are rare and their content of Hup is extremely low. Multiple studies have shown that this compound is produced by various endophytic fungi [96]. In 2014, genome of the endophytic fungus Shiraia sp. Slf14 was sequenced, disclosing the presence of a putative Hup biosynthetic gene cluster [72].
De novo RNA sequencing of transcriptome of the Hup-producing endophytic fungus C. gloeosporioides ES026 has revealed a gene encoding copper amine oxidase (CAO), responsible for the conversion of cadaverine to 5-aminopentanal in Hup biosynthesis [73]. This gene was also found in roots, stems, and leaves of H. serrata. A correlation was observed between the expression of CAO and the quantity of Hup extracted from strain ES026. Thus, it was postulated that CAO plays a key regulatory role in the biosynthesis of Hup. The next attempt of de novo RNA-seq of ES026 transcriptome enabled annotation of two key genes for Hup biosynthesis, encoding lysine decarboxylase (LDC) and again CAO, for conversion of l-lysine to 5-aminopentanal [74].
Whole genome and transcriptome analysis of two other Hup-producing endophytes, Penicillium polonicum hy4 and C. gloeosporioides Cg01, was performed to elucidate Hup biosynthesis [75]. Based on the proposed HupA biosynthetic pathway proposed in plants, 1 LDC gene, 6 CAO genes, 27 PKS genes, 2 BBE genes, 111 P450s, and 35 2OGDs were identified in hy4, while 2 LDC genes, 12 CAO genes, 47 PKS genes, 4 BBE genes, 280 P450s, and 39 2OGDs were found in Cg01. Orthologous and phylogenetic analyses between hy4 and Cg01 were performed. The presence of differentially expressed genes was observed between a group where production was induced with added extracts of H. serrata and the control group. Among these genes, those displaying a similar expression pattern in Cg01 and hy4 were selected. This enabled identification of 2 LDCs, 1 CAO4, and 3 PKS genes in Cg01 as candidates for further validation.
Strain hy4 of P. polonicum was also subjected to the mitochondrial genome sequencing [97]. The study of P. polonicum hy4 mitogenome enabled detailed phylogenetic analysis and provided valuable information on mitochondrial evolution.
4.5. Plant Growth Factors and Phytohormones
Studies on the interaction between Arabidopsis thaliana and the endophytic fungus Harpophora (= Falciphora) oryzae revealed an important role of fungus-derived phytohormones, indole-3-acetic acid (IAA) and indole-3-carboxaldehyde (ICA), in the stimulation of lateral root growth [76]. Comparative genomic analysis resulted in the recognition of orthologue genes involved in the biosynthetic pathway of IAA and ICA in H. oryzae genome. Both pathways share ASB1, IGS, TSB1 genes, while TAM1, YUC3, and IAD1 were identified specifically for IAA biosynthesis and CYP79B3, CYP71A13, and CYP71B6 were specific for ICA production. Interestingly, the upregulation of these genes was only observed after treatment with Arabidopsis exudate, suggesting dependence of IAA and ICA production by H. oryzae on plant auxin signaling and transport, and that lateral root growth promotion by the fungus derives by an auxin-dependent mechanism.
Although the biosynthesis of plant hormones is well known in many endophytic bacteria and fungi, there is still an information gap concerning genomics and transcriptomics. To address this need, a broad study was conducted on the genome and transcriptome of strain WP1 of Rhodotorula graminis, an endophyte isolated from Populus trichocarpa [77], revealing the absence of genes for the biosynthesis of IAA via indole-3-pyruvate and indole-3-acetate. However, other enzymes were found which are putatively involved in the formation of IAA from l-tryptophan via tryptamine: a monoamine oxidase, an aromatic-l-amino-acid decarboxylase, and an indol-3-acetalaldehyde dehydrogenase. Moreover, 15 proteins were annotated in strain WP1 as primarily involved in the biosynthesis of other plant hormones, i.e., gibberellic acid, jasmonic acid, and aminobutyric acid.
4.6. Other Plant Metabolites Produced by Endophytic Fungi
4.6.1. 10-Hydroxycamptothecin
The anticancer terpenoid alkaloids camptothecin (CPT) and 10-hydroxycamptothecin (10-HCPT) (Figure 1), originally described from the bark of the happy tree (Camptotheca acuminata), are also well-known products of endophytic fungi, such as N. solani and Xylaria sp. [5]. However, genes involved in the biosynthesis of camptothecin in fungi remained unknown until 2017 [78]. The transcriptome analysis of the endophytic strain Xylaria sp. M71 treated with salicylic acid revealed 13 functional genes mapped to MVA, which might be putatively involved in the production of 10-HCPT, which encode 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), 10-hydroxygeraniol oxidoreductase (10-HGO), acetyl-CoA C-acetyltransferase (AACT), geranylgeranyl pyrophosphate synthase (GGPS), 3-hydroxymethyl- 3-methylglutaryl-CoA lyase (HMGCL), mevalonate kinase (MK), geraniol-10-hydroxylase (G10H), and secologanin synthase (SCS).
The biosynthesis pathway of terpenoid alkaloids is conserved both in plants and fungi. However, while plants can utilize a number of pathways to produce CPT, i.e., MEP and the 1-deoxy-d-xylulose 5-phosphate pathway, genes associated with MEP were not identified in M71, suggesting that the biosynthesis of 10-HCPT occurs via MVA, similarly to the CPT-producing fungus Ganoderma lucidum [78].
4.6.2. Rotenoids: Rotenolone and Tephrosin
The two rotenoids rotenolone and tephrosin (Figure 1), previously reported in plants belonging to the genera Derris and Tephrosia, were found to be produced by the endophytic fungus Pseudofusicoccum stromaticum isolated from the timber tree Myracrodruon urundeuva [79]. In plants, rotenoids are formed from flavonoids in a multi-step pathway where chalcone isomerase (CHI) and CHS play a role as key enzymes [98]. During the investigation of the P. stromaticum genome, the gene encoding for a CHI-like protein was identified, elucidating the ability of this fungus to produce plant-related rotenoids, while no CHS homologue could be found. Protein alignment analysis and maximum likelihood phylogenetic reconstruction analysis have revealed that fungal CHI-like enzymes have more than one substrate specificities since they recognize chalconoids other than (2S)-naringenin [79].
4.6.3. Viridiflorol
Serendipita indica (previously known as Piriformospora indica) is an endophytic fungus widely used in the studies on plant–fungi interactions. This fungus presents a characteristic biphasic colonization style during migration to the host plant. Nonetheless, S. indica demonstrates a unique ability to biosynthesize viridiflorol (Figure 1), an antifungal sesquiterpene which is also reported from extracts of many plant species. In 2021, Ntana et al. [80] investigated the genome of S. indica searching for the specific genes associated with the production of sesquiterpenes. The study resulted in the discovery of SiTPS, a putative terpenoid synthase gene. Functional characterization of this enzyme showed that it participates in the synthesis of viridiflorol. Until present, only two viridiflorol synthases have been identified in plants [99]. After AaVS from Cyclocybe (= Agrocybe) aegerita [100], SiTPS is the second fungal enzyme to be identified and characterized as a viridiflorol synthase.
4.6.4. Skyrin
Genome sequencing of Cyanodermella asteris, a non-lichenized representative of the fungal class Lecanoromycetes isolated as an endophyte from the Chinese medicinal plant Aster tataricus, provided an interesting insight into the biosynthesis of skyrin (Figure 1). A gene cluster of five genes was identified through genetic and in silico analyses to possibly encode enzymes catalyzing biosynthesis of skyrin [81]. The proposed pathway of skyrin biosynthesis in C. asteris would go via atrochrysone carboxylic acid (ACA) and emodin. A putative gene encoding ACA-synthase has been identified, as well as genes encoding two dehydrogenases and a dehydratase potentially involved in the multistep conversion of ACA to emodin and a gene encoding for a monooxygenase that putatively produces skyrin from two emodin molecules.
Genome analysis of C. asteris also revealed an intriguing aspect, that is a very high gene density (363 genes/Mb). Higher gene density was observed in the genome of another endophytic fungus, the previously mentioned S. indica [21]. Generally, higher gene density is more specific to endophytic species which may lead to the conclusion that a more “compressed” genome somehow supports the endophytic lifestyle.
4.6.5. 1,8-Cineole
In 2015, Shaw et al. described a new fungal 1,8-cineole synthase gene, hyp3, in the genome of isolate E7406B of Hypoxolon sp. [82]. It was the first monoterpene synthase discovered in fungi to be structurally and biochemically characterized, exhibiting the same specificity and mechanism for water capture as observed in unrelated plants species. The whole genome sequencing of isolate E7406B enabled identification of thirteen terpene synthases, out of which two were excluded and four were proven to conduct terpene biosynthesis. Only hyp3 demonstrated a strong affinity for GPP substrate and production specificity of 1,8-cineole (Figure 1) as a major monoterpene product of this enzyme.
Phylogenetic analysis of 1,8-cineole synthases in bacteria, plants, and fungi revealed interesting relationships between these proteins, suggesting convergent evolution. However, terpene synthases from strain E7406B do not belong in a monophyletic clade and are related with ascomycete terpene synthases, while hyp3 is specifically related with fungal aristolochene synthases. These results suggest independent evolution of this pathway in fungi and argue against hypothetical horizontal gene transfer.
4.6.6. Polyphenols, Flavonoids, Terpenoids, Isoquinoline Alkaloids
An interesting attempt towards elucidation of multiple pathway genes was made recently by Zou et al. [83]. The maidenhair tree (Ginkgo biloba) is a natural resource for flavonoids and terpenes. However, limited insights have considered the role of endophytic fungi in this plant, and it is unclear whether a genetic exchange has eventually occurred between G. biloba and its endophytic associates. In a study considering functional gene profiles and repetitive sequences carried out to focus on this issue, 25 endophytic fungal and bacterial strains were isolated from roots, which included two Aspergillus spp. The discovery that several genes from polyphenolic, flavonoid, terpenoid, and isoquinoline alkaloid biosynthetic pathways are present in both Ginkgo and the endophytic Aspergillus strains, suggests that the latter may participate in plant metabolism in a complementary manner.
The fungal endophytic strains Penicillium citrinum TDPEF34, and Geotrichum candidum TDPEF20, respectively from healthy and brittle leaf diseased date palms, proved to be very effective in assays against some pathogenic fungi and bacteria [84]. Ethyl acetate extract of G. candidum exhibited strong antioxidant activity, probably due to high polyphenol and flavonoid content. Four publicly available genomes of P. citrinum and G. candidum were also screened to identify secondary metabolite gene clusters, and 48 putative secondary metabolite gene clusters resulted. However, none of them showed significant similarity to known secondary metabolite clusters. This led to the conclusion, that there is a potential for the discovery of novel metabolites in these endophytic fungi.
5. Functional Verification of Structural Genes Discovered in Endophytic Fungi
Although more and more plant bioactive metabolites are being found to be produced by endophytic fungi, the real challenge consists in the discovery of the underlying biosynthetic pathways, which in turn would enable us to consider genetic engineering of fungal producers and for synthetic biology approaches to create systems able to synthetize these compounds on a large scale and in a sustainable manner.
After the discovery of a structural gene by an NGS approach, its function needs to be verified and proven experimentally. This could be done either by a gene disruption approach through a knock-out mutant, by inducing stable or transient gene overexpression, or via gene activation by using different triggers to activate silent pathways.
Several successful examples of functional verification of a structural gene function exist in the field of fungal endophytes able to produce plant metabolites. The role of swnK was understood after inactivating it in Metarhizium robertsii through homologous gene replacement to generate a ΔswnK mutant unable to produce Swn, then complementing the mutant with the wild-type gene to restore this biosynthetic aptitude [70]. The role of gene hyp3 encoding 1,8-cineole synthase in Hypoxylon sp. was determined by heterologous expression in Escherichia coli overexpression strain [82]. Likewise, functional analysis of the copper amine oxidase and lysine decarboxylase genes from the above-mentioned endophytic strain ES026 has been done by constructing a stable, high yielding Hup system in E. coli resulting from the overexpression of CgCAO and CgLDC [74]. Afterwards, the function of two LDC genes from C. gloeosporioides Cg01 was validated by the split-marker homologous recombination knockout method. It has been found that knock-out of single candidate biosynthetic-genes did not influence Hup production, while knock-out of both LDC genes led to increased Hup production, which revealed the existence of differences in Hup biosynthesis between endophytes and plants [75].
6. Third-Generation Sequencing Technology for Fungal Genomics
Advances in technologies for genome sequencing have generated a huge quantity of high-throughput and high-dimensional biological data [101]. Sequencing technology is rapidly evolving, and many options are now available for massively parallel DNA sequencing. By combining them with complementary methods, such as RNA-seq, hybrid sequencing, or ChIP-Seq, a greater insight into the genetic bases of biosynthetic pathways is now possible [12].
Although the enormous numbers of reads generated by NGS have enabled genome sequencing at an unprecedented speed, this technology is not free from limitations. A drawback of NGS sequencing consists in relatively short reads, making genome assembly more difficult, with a need for the development of novel alignment algorithms [10]. Genomes of many organisms (especially of plant and fungi) are highly complex with many repetitive elements, copy number, and structural variations which are relevant for evolution and adaptation. Many of these elements are so long that they cannot be resolved through short-read paired-end technologies [11]. Therefore, other NGS methods have been developed, out of which the so-called third generation methods could be especially promising for the field of fungal genomics. Third-generation methods allow for the detection of single molecules, but sequencing occurs in real time as an additional common feature [102]. Reads in excess of several kilobases are delivered by such long-read sequencing, which allows for the resolution of these large structural features. These long reads can span repetitive regions with a single continuous read, which eliminates ambiguity in the position or size of genomic elements. Long reads can also be used in transcriptomic research, considering that they can span entire mRNA transcripts, thus allowing to identify the precise connectivity of exons and discern gene isoforms [11].
Out of the third-generation sequencing technologies, two main types of long-read technologies in particular seem to be most suitable for fungal genomics, that is the single-molecule real-time sequencing approaches delivered by Pacific Biosciences (PacBio) [103] and Oxford Nanopore [104]. The advantages of nanopore sequencing for studying plant genomes, especially in the context of addressing challenges for producing high-quality, highly contiguous plant genome assemblies was recently demonstrated by Oxford Nanopore Technology [105]. Furthermore, there are more and more examples of using the advantages of the third-generation technology in the field of fungal genomics. The accurate long-read sequences obtained through the PacBio Sequel Systems were used to assemble the draft genomes of fungal endophytes isolated from G. biloba [83]. The genome of an endophytic strain of Amphirosellinia nigrospora (JS-1675), isolated from Pteris cretica, was recently sequenced with the PacBio RS II technology [106]. This strain produces an oxygenated cyclohexanone, which is particularly effective against the agent of bacterial wilt of tomato, Ralstonia solanacearum, and various phytopathogenic fungi. Finally, genome sequence of an endophytic strain of Gaeumannomyces sp. (JS-464), producing a new glycosylated anthraquinone and two new C-glycosylated dialkylresorcinol derivatives, was generated using both the HiSeq 2000 (Illumina) and PacBio RSII platform [107].
7. Future Prospects
The examination of literature in the field highlights the need to enlarge our acquisitions concerning the genetic bases enabling many endophytic fungi to synthesize bioactive metabolites common to their host plants. In fact, at the current stage of knowledge some clues lean toward an independent evolutionary development of the biosynthetic capacities, while other inferences support the exchange of genetic elements through modalities that need to be experimentally demonstrated. Aside from the general motivation to get to a rational and unequivocal elucidation of this intriguing biological phenomenon, research in the field is also pursuing applicative purposes in the aim to support industrial production of drugs and other biotechnologically relevant compounds derived from plants. In this respect, new indications could derive from follow-up studies concerning some valuable phytochemicals which have been recently reported as products of endophytic strains. This is the case of asiaticoside, a triterpenoid saponin possessing memory enhancement properties, which is extracted from the Asiatic pennywort (Centella asiatica) and reported as a product of an associated endophytic strain of C. gloeosporioides [108], and of the myrtucommulones, displaying several notable bioactivities, which were originally extracted from myrtle (Myrtus communis) and later found in axenic culture of its endophyte Neofusicoccum australe [109,110]. In the above perspective, studies are currently in progress in our laboratories at the Wroclaw Medical University concerning tanshinones, a class of norditerpenoid quinones originally extracted from danshen (Salvia miltiorrhiza) with applicative potential in the treatment of cardiovascular and tumor diseases [111,112]. These compounds have been also found in Salvia yangii (formerly Perovskia atriplicifolia) [113] and Salvia abrotanoides (formerly Perovskia abrotanoides) [114], in considerably different assortments [115]. Tanshinones have also been found as products of endophytic fungi isolated from S. miltiorrhiza and S. abrotanoides [8,116,117,118]. Although genome sequencing of S. miltiorrhiza has been recently accomplished [119], the biosynthetic pathway of tanshinones has been only partially elucidated. Indeed, comparative genomics and transcriptomics approaches of tanshinone-producing endophytic fungi and their hosts can bring novel information and widen our knowledge about the genetic background of biosynthesis of these valuable metabolites.
Developments in genome sequencing technologies have revolutionized genomics research concerning the endophytic microbiota. They are expected to directly contribute to drug discovery through genome mining, by increasing our capacity to identify novel compounds encoded by silent biosynthetic gene clusters. As illustrated in this paper, they could also be very effective for the discovery of gene homologues and pathway elucidation of specific bioactive products, including those originally known from the host plants.
Author Contributions
Conceptualization, M.B.; investigation, M.B., B.P. and R.N.; writing—original draft preparation, M.B., B.P. and R.N.; writing—review and editing, M.B. and R.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal im-portance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayanan, T.S.; Shaanker, R.U. Can fungal endophytes fast-track plant adaptations to climate change? Fungal Ecol. 2021, 50, 101039. [Google Scholar] [CrossRef]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest tree microbiomes and associated fungal endophytes: Functional roles and impact on forest health. Forests 2019, 10, 42. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Nicoletti, R.; Fiorentino, A. Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture 2015, 5, 918–970. [Google Scholar] [CrossRef]
- Jia, M.; Chen, L.; Xin, H.-L.; Zheng, C.-J.; Rahman, K.; Han, T.; Qin, L.-P. A friendly relationship between endophytic fungi and medicinal plants: A systematic review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef]
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; Van Staden, J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2019, 39, 107462. [Google Scholar] [CrossRef]
- Zimowska, B.; Bielecka, M.; Abramczyk, B.; Nicoletti, R. Bioactive products from endophytic fungi of sages (Salvia spp.). Agriculture 2020, 10, 543. [Google Scholar] [CrossRef]
- Park, S.T.; Kim, J. Trends in next-generation sequencing and a new era for whole genome sequencing. Int. Neurourol. J. 2016, 20 (Suppl. S2), S76–S83. [Google Scholar] [CrossRef]
- van Dijk, E.L.; Auger, H.; Jaszczyszyn, Y.; Thermes, C. Ten years of next-generation sequencing technology. Trends Genet. 2014, 30, 418–426. [Google Scholar] [CrossRef]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of age: Ten years of next-generation sequencing technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.R.; Cowley, M.J.; Davis, R.L. Next-generation sequencing and emerging technologies. Semin. Thromb. Hemost. 2019, 45, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Peršoh, D. Factors shaping community structure of endophytic fungi–evidence from the Pinus-Viscum-system. Fungal Divers. 2013, 60, 55–69. [Google Scholar] [CrossRef]
- Buée, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009, 184, 449–456. [Google Scholar] [CrossRef]
- Jumpponen, A.; Jones, K.L. Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol. 2009, 184, 438–448. [Google Scholar] [CrossRef]
- Öpik, M.; Metsis, M.; Daniell, T.J.; Zobel, M.; Moora, M. Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol. 2009, 184, 424–437. [Google Scholar] [CrossRef]
- Bullington, L.S.; Larkin, B.G. Using direct amplification and next-generation sequencing technology to explore foliar endophyte communities in experimentally inoculated western white pines. Fungal Ecol. 2015, 17, 170–178. [Google Scholar] [CrossRef]
- Liu, K.-H.; Ding, X.-W.; Rao, M.P.N.; Zhang, B.; Zhang, Y.-G.; Liu, F.-H.; Liu, B.-B.; Xiao-Wei, D.; Li, W.-J. Morphological and transcriptomic analysis reveals the osmoadaptive response of endophytic fungus Aspergillus montevidensis ZYD4 to high salt stress. Front. Microbiol. 2017, 8, 1789. [Google Scholar] [CrossRef]
- Knapp, D.G.; Németh, J.B.; Barry, K.; Hainaut, M.; Henrissat, B.; Johnson, J.; Kuo, A.; Lim, J.H.P.; Lipzen, A.; Nolan, M.; et al. Comparative genomics provides insights into the lifestyle and reveals functional heterogeneity of dark septate endophytic fungi. Sci. Rep. 2018, 8, 6321. [Google Scholar] [CrossRef]
- Zuccaro, A.; Lahrmann, U.; Güldener, U.; Langen, G.; Pfiffi, S.; Biedenkopf, D.; Wong, P.; Samans, B.; Grimm, C.; Basiewicz, M.; et al. Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathog. 2011, 7, e1002290. [Google Scholar] [CrossRef] [PubMed]
- Gazis, R.; Kuo, A.; Riley, R.; LaButti, K.; Lipzen, A.; Lin, J.; Amirebrahimi, M.; Hesse, C.N.; Spatafora, J.W.; Henrissat, B.; et al. The genome of Xylona heveae provides a window into fungal endophytism. Fungal Biol. 2015, 120, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Day, R.; Zhang, N.; Dupont, P.-Y.; Cox, M.P.; Schardl, C.; Minards, N.; Truglio, M.; Moore, N.; Harris, D.R.; et al. Host tissue environment directs activities of an Epichloë endophyte, while it induces systemic hormone and defense responses in its native perennial ryegrass host. Mol. Plant-Microbe Interact. 2017, 30, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yang, S.; Meng, L.; Wang, B.-G. The plant hormone abscisic acid regulates the growth and metabolism of endophytic fungus Aspergillus nidulans. Sci. Rep. 2018, 8, 6504. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Liu, T.; Zhang, W.; Li, S.; Zhu, M.; Li, H.; Kong, Y.; Xu, L. Disclosure of the molecular mechanism of wheat leaf spot disease caused by Bipolaris sorokiniana through comparative transcriptome and metabolomics analysis. Int. J. Mol. Sci. 2019, 20, 6090. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Chen, Y.; Dai, C.-C. De novo transcriptome assembly of Phomopsis liquidambari provides insights into genes associated with different lifestyles in rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 121. [Google Scholar] [CrossRef]
- Bhargavi, S.D.; Praveen, V.K.; Savitha, J. Bioinformatic comparative analysis of lovastatin gene cluster in endophytic fungi and a soil fungus, Aspergillus terreus. MOJ Proteom. Bioinform. 2014, 1, 26. [Google Scholar]
- Bhargavi, S.D.; Praveen, V.K.; Kumar, M.A.; Savitha, J. Comparative study on whole genome sequences of Aspergillus terreus (soil fungus) and Diaporthe ampelina (endophytic fungus) with reference to lovastatin production. Curr. Microbiol. 2017, 75, 84–91. [Google Scholar] [CrossRef]
- Cheng, J.-T.; Cao, F.; Chen, X.-A.; Li, Y.-Q.; Mao, X.-M. Genomic and transcriptomic survey of an endophytic fungus Calcarisporium arbuscula NRRL 3705 and potential overview of its secondary metabolites. BMC Genom. 2020, 21, 424. [Google Scholar] [CrossRef]
- Savitha, J.; Bhargavi, S.D.; Praveen, V.K. Complete genome sequence of the endophytic fungus Diaporthe (Phomopsis) ampelina. Genome Announc. 2016, 4, e00477-16. [Google Scholar] [CrossRef]
- Vignolle, G.A.; Mach, R.L.; Mach-Aigner, A.R.; Derntl, C. Novel approach in whole genome mining and transcriptome analysis reveal conserved RiPPs in Trichoderma spp. BMC Genom. 2020, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Liu, L.; Xiang, M.; Wang, W.; Sun, X.; Che, Y.; Guo, L.; Liu, G.; Guo, L.; et al. Genomic and transcriptomic analysis of the endophytic fungus Pestalotiopsis fici reveals its lifestyle and high potential for synthesis of natural products. BMC Genom. 2015, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Bai, J.; Yan, D.; Bao, X.; Li, W.; Liu, B.; Zhang, D.; Qi, X.; Yu, D.; Hu, Y. Genome mining combined metabolic shunting and OSMAC strategy of an endophytic fungus leads to the production of diverse natural products. Acta Pharm. Sin. B 2020, 11, 572–587. [Google Scholar] [CrossRef]
- Gianoulis, T.A.; Griffin, M.A.; Spakowicz, D.J.; Dunican, B.F.; Alpha, C.J.; Sboner, A.; Sismour, A.M.; Kodira, C.; Egholm, M.; Church, G.M.; et al. Genomic analysis of the hydrocarbon-producing, cellulolytic, endophytic fungus Ascocoryne sarcoides. PLoS Genet. 2012, 8, e1002558. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Davis, R.W.; Tran-Gyamfi, M.B.; Kuo, A.; LaButti, K.; Mihaltcheva, S.; Hundley, H.; Chovatia, M.; Lindquist, E.; Barry, K.; et al. Characterization of four endophytic fungi as potential consolidated bioprocessing hosts for conversion of lignocellulose into advanced biofuels. Appl. Microbiol. Biotechnol. 2017, 101, 2603–2618. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.N.; Zhang, X.L.; Liu, X.Z.; Zhang, Y.J. Complete mitochondrial genome of the endophytic fungus Pestalotiopsis fici: Features and evolution. Appl. Microbiol. Biotechnol. 2017, 101, 1593–1604. [Google Scholar] [CrossRef]
- Chandra, S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 2012, 95, 47–59. [Google Scholar] [CrossRef]
- Tiwari, P.; Bae, H. Horizontal gene transfer and endophytes: An implication for the acquisition of novel traits. Plants 2020, 9, 305. [Google Scholar] [CrossRef]
- Li, J.Y.; Sidhu, R.S.; Ford, E.J.; Long, D.M.; Hess, W.M.; Strobel, G.A. The induction of taxol production in the endophytic fungus Periconia sp. from Torreya grandifolia. J. Ind. Microbiol. Biotechnol. 1998, 20, 259–264. [Google Scholar] [CrossRef]
- Gurudatt, P.S.; Priti, V.; Shweta, S.; Ramesha, B.T.; Ravikanth, G.; Vasudeva, R.; Amna, T.; Deepika, S.; Ganeshaiah, K.N.; Uma Shaanker, R.; et al. Attenuation of camptothecin production and negative relation between hyphal biomass and camptothecin content in endophytic fungal strains isolated from Nothapodytes nimmoniana Grahm (Icacinaceae). Curr. Sci. 2010, 98, 1006–1010. [Google Scholar]
- Priti, V.; Ramesha, B.T.; Singh, S.; Ravikanth, G.; Ganeshaiah, K.N.; Suryanarayanan, T.S.; Shaanker, R.U. How promising are endophytic fungi as alternative sources of plant secondary metabolites? Curr. Sci. 2009, 97, 477–478. [Google Scholar]
- Kusari, S.; Zühlke, S.; Spiteller, M. Effect of artificial reconstitution of the interaction between the plant Camptotheca acuminata and the fungal endophyte Fusarium solani on camptothecin biosynthesis. J. Nat. Prod. 2011, 74, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Sachin, N.; Manjunatha, B.L.; Kumara, P.M.; Ravikanth, G.; Shweta, S.; Suryanarayanan, T.S.; Ganeshaiah, K.N.; Shaanker, R.U. Do endophytic fungi possess pathway genes for plant secondary metabolites? Curr. Sci. 2013, 104, 178–182. [Google Scholar]
- Griffiths, A.J.F. Natural plasmids of filamentous fungi. Microbiol. Rev. 1995, 59, 673–685. [Google Scholar] [CrossRef]
- Walthert, T.C.; Kennell, J.C. Linear mitochondrial plasmids of F. oxysporum are novel, telomere-like retroelements. Mol. Cell 1999, 4, 229–238. [Google Scholar] [CrossRef]
- Bertaux, J.; Schmid, M.; Hutzler, P.; Hartmann, A.; Garbaye, J.; Frey-Klett, P. Occurrence and distribution of endobacteria in the plant-associated mycelium of the ectomycorrhizal fungus Laccaria bicolor S238N. Environ. Microbiol. 2005, 7, 1786–1795. [Google Scholar] [CrossRef]
- Partida-Martinez, L.P.; Hertweck, C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature 2005, 437, 884–888. [Google Scholar] [CrossRef]
- Kobayashi, D.Y.; Crouch, J.A. Bacterial/fungal interactions: From pathogens to mutualistic endosymbionts. Annu. Rev. Phytopathol. 2009, 47, 63–82. [Google Scholar] [CrossRef]
- Staniek, A.; Woerdenbag, H.J.; Kayser, O. Taxomyces andreanae: A presumed paclitaxel producer demystified? Planta Med. 2009, 75, 1561–1566. [Google Scholar] [CrossRef]
- Xiong, Z.-Q.; Yang, Y.-Y.; Zhao, N.; Wang, Y. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojap yew, Taxus x media. BMC Microbiol. 2013, 13, 71. [Google Scholar] [CrossRef]
- Roopa, G.R.; Madhusudhan, M.; Sunil, K.; Lisa, N.; Calvin, R.; Poornima, R.; Zeinab, N.; Kini, R.; Prakash, H.; Geetha, N. Identification of taxol-producing endophytic fungi isolated from Salacia oblonga through genomic mining approach. J. Genet. Eng. Biotechnol. 2015, 13, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Garyali, S.; Kumar, A.; Reddy, M.S. Diversity and antimitotic activity of taxol-producing endophytic fungi isolated from Himalayan yew. Ann. Microbiol. 2013, 64, 1413–1422. [Google Scholar] [CrossRef]
- Naik, T.; Vanitha, S.C.; Rajvanshi, P.K.; Chandrika, M.; Kamalraj, S.; Jayabaskaran, C. Novel microbial sources of tropane alkaloids: First report of production by endophytic fungi isolated from Datura metel L. Curr. Microbiol. 2017, 75, 206–212. [Google Scholar] [CrossRef]
- Felitti, S.; Shields, K.; Ramsperger, M.; Tian, P.; Sawbridge, T.; Webster, T.; Logan, E.; Erwin, T.; Forster, J.; Edwards, D. Transcriptome analysis of Neotyphodium and Epichloë grass endophytes. Fungal Genet. Biol. 2006, 43, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Breakspear, A.; Momany, M. The first fifty microarray studies in filamentous fungi. Microbiology 2007, 153, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Schmit, J.P.; Mueller, G.M. An estimate of the lower limit of global fungal diversity. Biodivers. Conserv. 2006, 16, 99–111. [Google Scholar] [CrossRef]
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5, 1–17. [Google Scholar] [CrossRef]
- Dreyfuss, M.; Chapela, I. Potential of fungi in the discovery of novel, low-molecular weight pharmaceuticals. In Discovery of Novel Natural Products with Therapeutic Potential; Elsevier: Amsterdam, The Netherlands, 1994; pp. 49–80. [Google Scholar] [CrossRef]
- ENSEMBL Fungi. Available online: https://fungi.ensembl.org/index.html (accessed on 15 November 2021).
- Mycocosm. Available online: https://mycocosm.jgi.doe.gov/mycocosm/home (accessed on 15 November 2021).
- e-Fungi. Available online: http://www.cs.man.ac.uk/~cornell/eFungi/index.html (accessed on 15 November 2021).
- Sequence Read Archive (SRA). Available online: https://www.ncbi.nlm.nih.gov/sra (accessed on 15 November 2021).
- Yang, Y.; Zhao, H.; Barrero, R.A.; Zhang, B.; Sun, G.; Wilson, I.W.; Xie, F.; Walker, K.D.; Parks, J.W.; Bruce, R.; et al. Genome sequencing and analysis of the paclitaxel-producing endophytic fungus Penicillium aurantiogriseum NRRL 62431. BMC Genom. 2014, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.-Y.; Mo, X.-C.; Xi, X.-Y.; Zhou, L.; De, G.; Ke, Y.-S.; Liu, P.; Song, F.-J.; Jin, W.-W.; Zhang, P. Transcriptome analysis of a taxol-producing endophytic fungus Cladosporium cladosporioides MD2. AMB Express 2018, 8, 41. [Google Scholar] [CrossRef]
- Qiao, W.; Tang, T.; Ling, F. Comparative transcriptome analysis of a taxol-producing endophytic fungus, Aspergillus aculeatinus Tax-6, and its mutant strain. Sci. Rep. 2020, 10, 10558. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Shi, J.; Gao, Z.; Zhang, Y. Transcriptome analysis reveals the genetic basis of the resveratrol biosynthesis pathway in an endophytic fungus (Alternaria sp. MG1) isolated from Vitis vinifera. Front. Microbiol. 2016, 7, 1257. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ye, C.; Che, J.; Xu, X.; Shao, D.; Jiang, C.; Liu, Y.; Shi, J. Genomic sequencing, genome-scale metabolic network reconstruction, and in silico flux analysis of the grape endophytic fungus Alternaria sp. MG1. Microb. Cell Factories 2019, 18, 13. [Google Scholar] [CrossRef]
- Lu, H.; Quan, H.; Ren, Z.; Wang, S.; Xue, R.; Zhao, B. The genome of Undifilum oxytropis provides insights into swainsonine biosynthesis and locoism. Sci. Rep. 2016, 6, 30760. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Donzelli, B.; Creamer, R.; Baucom, D.L.; Gardner, D.R.; Pan, J.; Moore, N.; Krasnoff, S.B.; Jaromczyk, J.W.; Schardl, C.L. Swainsonine biosynthesis genes in diverse symbiotic and pathogenic fungi. G3 Genes Genomes Genet. 2017, 7, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, P. Transcriptome profiles of Alternaria oxytropis provides insights into swainsonine biosynthesis. Sci. Rep. 2019, 9, 6021. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.; Zhang, Z.; Yan, R.; Zhu, D. Whole-genome shotgun assembly and analysis of the genome of Shiraia sp. strain Slf14, a novel endophytic fungus producing huperzine A and hypocrellin A. Genome Announc. 2014, 2, e00011-14. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, W.; Zhang, X.; Xia, Q.; Zhao, X.; Ahn, Y.; Ahmed, N.; Cosoveanu, A.; Wang, M.; Wang, J.; et al. De novo RNA sequencing and transcriptome analysis of Colletotrichum gloeosporioides ES026 reveal genes related to biosynthesis of huperzine A. PLoS ONE 2015, 10, e0120809. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Jan, S.; Yang, Q.; Wang, M. Expression and functional analysis of the lysine decarboxylase and copper amine oxidase genes from the endophytic fungus Colletotrichum gloeosporioides ES026. Sci. Rep. 2017, 7, 2766. [Google Scholar] [CrossRef]
- Kang, X.; Liu, C.; Shen, P.; Hu, L.; Lin, R.; Ling, J.; Xiong, X.; Xie, B.; Liu, D. Genomic characterization provides new insights into the biosynthesis of the secondary metabolite huperzine A in the endophyte Colletotrichum gloeosporioides Cg01. Front. Microbiol. 2019, 9, 3237. [Google Scholar] [CrossRef]
- Sun, X.; Wang, N.; Li, P.; Jiang, Z.; Liu, X.; Wang, M.; Su, Z.; Zhang, C.; Lin, F.; Liang, Y. Endophytic fungus Falciphora oryzae promotes lateral root growth by producing indole derivatives after sensing plant signals. Plant Cell Environ. 2019, 43, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Firrincieli, A.; Eotillar, R.; Esalamov, A.; Eschmutz, J.; Ekhan, Z.; Redman, R.S.; Fleck, N.D.; Elindquist, E.; Grigoriev, I.V.; Doty, S.L. Genome sequence of the plant growth promoting endophytic yeast Rhodotorula graminis WP1. Front. Microbiol. 2015, 6, 978. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, K.; Zhang, Y.; Liu, F. De novo transcriptome assembly and characterization of the 10-hydroxycamptothecin-producing Xylaria sp. M71 following salicylic acid treatment. J. Microbiol. 2017, 55, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Sobreira, A.C.M.; Pinto, F.D.C.L.; Florêncio, K.G.D.; Wilke, D.V.; Staats, C.C.; Streit, R.D.A.S.; Freire, F.D.C.D.O.; Pessoa, O.D.L.; Trindade-Silva, A.E.; Canuto, K.M. Endophytic fungus Pseudofusicoccum stromaticum produces cyclopeptides and plant-related bioactive rotenoids. RSC Adv. 2018, 8, 35575–35586. [Google Scholar] [CrossRef]
- Ntana, F.; Bhat, W.; Johnson, S.; Jørgensen, H.; Collinge, D.; Jensen, B.; Hamberger, B. A Sesquiterpene synthase from the endophytic fungus Serendipita indica catalyzes formation of viridiflorol. Biomolecules 2021, 11, 898. [Google Scholar] [CrossRef]
- Jahn, L.; Schafhauser, T.; Wibberg, D.; Rückert, C.; Winkler, A.; Kulik, A.; Weber, T.; Flor, L.; van Pée, K.H.; Kalinowski, J.; et al. Linking secondary metabolites to biosynthesis genes in the fungal endophyte Cyanodermella asteris: The anti-cancer bisan-thraquinone skyrin. J. Biotechnol. 2017, 257, 233–239. [Google Scholar] [CrossRef]
- Shaw, J.J.; Berbasova, T.; Sasaki, T.; Jefferson-George, K.; Spakowicz, D.; Dunican, B.F.; Portero, C.E.; Narváez-Trujillo, A.; Strobel, S.A. Identification of a fungal 1,8-cineole synthase from Hypoxylon sp. with specificity determinants in common with the plant synthases. J. Biol. Chem. 2015, 290, 8511–8526. [Google Scholar] [CrossRef]
- Zou, K.; Liu, X.; Hu, Q.; Zhang, D.; Fu, S.; Zhang, S.; Huang, H.; Lei, F.; Zhang, G.; Miao, B.; et al. Root endophytes and Ginkgo biloba are likely to share and compensate secondary metabolic processes, and potentially exchange genetic information by LTR-RTs. Front. Plant Sci. 2021, 12, 704985. [Google Scholar] [CrossRef]
- Ben Mefteh, F.; Daoud, A.; Bouket, A.C.; Thissera, B.; Kadri, Y.; Cherif-Silini, H.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Oszako, T.; et al. Date palm trees root-derived endophytes as fungal cell factories for diverse bioactive metabolites. Int. J. Mol. Sci. 2018, 19, 1986. [Google Scholar] [CrossRef]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- El-Sayed, A.S.; El Sayed, M.T.; Rady, A.M.; Zein, N.; Enan, G.; Shindia, A.; El-Hefnawy, S.; Sitohy, M.; Sitohy, B. Exploiting the biosynthetic potency of taxol from fungal endophytes of conifers plants; Genome mining and metabolic manipulation. Molecules 2020, 25, 3000. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1071–1113. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Hubbard, B.P. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1209–1218. [Google Scholar] [CrossRef]
- Kiselev, K.V. Perspectives for production and application of resveratrol. Appl. Microbiol. Biotechnol. 2011, 90, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Shimizu, N.; Shimizu, Y.; Akagi, M.; Eshita, Y.; Ozaki, S.-I.; Nakajima, N.; Ishihara, K.; Masuoka, N.; Hamada, H.; et al. Synthesis of glycosides of resveratrol, pterostilbene, and piceatannol, and their anti-oxidant, anti-allergic, and neuroprotective activities. Biosci. Biotechnol. Biochem. 2014, 78, 1123–1128. [Google Scholar] [CrossRef]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Santos, F.M.; Latorre, A.O.; Hueza, I.M.; Sanches, D.S.; Lippi, L.L.; Gardner, D.R.; Spinosa, H.S. Increased antitumor efficacy by the combined administration of swainsonine and cisplatin in vivo. Phytomedicine 2011, 18, 1096–1101. [Google Scholar] [CrossRef]
- Cook, D.; Gardner, D.R.; Pfister, J.A. Swainsonine-containing plants and their relationship to endophytic fungi. J. Agric. Food Chem. 2014, 62, 7326–7334. [Google Scholar] [CrossRef]
- Mycobank. Available online: www.mycobank.org (accessed on 6 December 2021).
- Zangara, A. The psychopharmacology of huperzine A: An alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer’s disease. Pharmacol. Biochem. Behav. 2003, 75, 675–686. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Wang, Z.-Q.; Shu, S.-H.; Wang, W.-J.; Xu, H.-J.; Ahn, Y.-J.; Wang, M.; Hu, X. Ethanol and methanol can improve huperzine A production from endophytic Colletotrichum gloeosporioides ES026. PLoS ONE 2013, 8, e61777. [Google Scholar] [CrossRef]
- Kang, X.; Liu, C.; Liu, D.; Zeng, L.; Shi, Q.; Qian, K.; Xie, B. The complete mitochondrial genome of huperzine A-producing endophytic fungus Penicillium polonicum. Mitochondrial DNA Part B 2016, 1, 202–203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crombie, L.; Whiting, D.A. Review article number 135 biosynthesis in the rotenoid group of natural products: Applications of isotope methodology. Phytochemistry 1998, 49, 1479–1507. [Google Scholar] [CrossRef]
- Padovan, A.; Keszei, A.; Köllner, T.G.; Degenhardt, J.; Foley, W.J. The molecular basis of host plant selection in Melaleuca quinquenervia by a successful biological control agent. Phytochemistry 2010, 71, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Shukal, S.; Chen, X.; Zhang, C. Systematic engineering for high-yield production of viridiflorol and amorphadiene in auxotrophic Escherichia coli. Metab. Eng. 2019, 55, 170–178. [Google Scholar] [CrossRef]
- Marx, V. The big challenges of big data. Nature 2013, 498, 255–260. [Google Scholar] [CrossRef]
- Schadt, E.E.; Turner, S.; Kasarskis, A. A window into third-generation sequencing. Hum. Mol. Genet. 2010, 19, R227–R240. [Google Scholar] [CrossRef]
- Eid, J.; Fehr, A.; Gray, J.; Luong, K.; Lyle, J.; Otto, G.; Peluso, P.; Rank, D.; Baybayan, P.; Bettman, B.; et al. Real-time DNA sequencing from single polymerase molecules. Science 2009, 323, 133–138. [Google Scholar] [CrossRef]
- Clarke, J.; Wu, H.-C.; Jayasinghe, L.; Patel, A.; Reid, S.; Bayley, H. Continuous base identification for single-molecule nanopore DNA sequencing. Nat. Nanotechnol. 2009, 4, 265–270. [Google Scholar] [CrossRef]
- Oxford Nanopore Technologies Closing the Gap in Plant Genomes. White Pap. 2020. Available online: https://nanoporetech.com/resource-centre/closing-gap-plant-genomes-white-paper (accessed on 16 November 2021).
- Jeon, J.; Park, S.-Y.; Kim, J.A.; Yu, N.H.; Park, A.R.; Kim, J.-C.; Lee, Y.-H.; Kim, S. Draft Genome Sequence of Amphirosellinia nigrospora JS-1675, an endophytic fungus from Pteris cretica. Microbiol. Resour. Announc. 2019, 8, e00069-19. [Google Scholar] [CrossRef]
- Kim, J.A.; Jeon, J.; Kim, K.-T.; Choi, G.; Park, S.-Y.; Lee, H.-J.; Shim, S.-H.; Lee, Y.-H.; Kim, S. Draft genome sequence of an endophytic fungus, Gaeumannomyces sp. strain JS-464, isolated from a reed plant, Phragmites communis. Genome Announc. 2017, 5, e00734-17. [Google Scholar] [CrossRef]
- Gupta, S.; Bhatt, P.; Chaturvedi, P. Determination and quantification of asiaticoside in endophytic fungus from Centella asiatica (L.) Urban. World J. Microbiol. Biotechnol. 2018, 34, 111. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Ferranti, P.; Caira, S.; Misso, G.; Castellano, M.; Di Lorenzo, G.; Caraglia, M. Myrtucommulone production by a strain of Neofusicoccum australe endophytic in myrtle (Myrtus communis). World J. Microbiol. Biotechnol. 2013, 30, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Salvatore, M.M.; Ferranti, P.; Andolfi, A. Structures and bioactive properties of myrtucommulones and related acylphloroglucinols from Myrtaceae. Molecules 2018, 23, 3370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, P.; Ye, M.; Kim, S.-H.; Jiang, C.; Lü, J. Tanshinones: Sources, pharmacokinetics and anti-cancer activities. Int. J. Mol. Sci. 2012, 13, 13621–13666. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, critical pharmacological components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Slusarczyk, S.; Topolski, J.; Domaradzki, K.; Adams, M.; Hamburger, M.; Matkowski, A. Isolation and fast selective determi-nation of nor-abietanoid diterpenoids from Perovskia atriplicifolia roots using LC-ESI-MS/MS with multiple reaction monitoring. Nat. Prod. Commun. 2015, 10, 1149–1152. [Google Scholar]
- Sairafianpour, M.; Christensen, J.; Stærk, D.; Budnik, B.A.; Kharazmi, A.; Bagherzadeh, A.K.; Jaroszewski, J.W. Leishmanicidal, antiplasmodial, and cytotoxic activity of novel diterpenoid 1,2-quinones from Perovskia abrotanoides: New source of tanshinones. J. Nat. Prod. 2001, 64, 1398–1403. [Google Scholar] [CrossRef]
- Bielecka, M.; Pencakowski, B.; Stafiniak, M.; Jakubowski, K.; Rahimmalek, M.; Gharibi, S.; Matkowski, A.; Ślusarczyk, S. Metabolomics and DNA-based authentication of two traditional Asian medicinal and aromatic species of Salvia subg. Perovskia. Cells 2021, 10, 112. [Google Scholar] [CrossRef]
- Ming, Q.; Han, T.; Li, W.; Zhang, Q.; Zhang, H.; Zheng, C.; Huang, F.; Rahman, K.; Qin, L. Tanshinone IIA and tanshinone I production by Trichoderma atroviride D16, an endophytic fungus in Salvia miltiorrhiza. Phytomedicine 2012, 19, 330–333. [Google Scholar] [CrossRef]
- Wei, X.; Jing, M.; Wang, J.; Yang, X. Preliminary study on Salvia miltiorrhiza Bung endophytic fungus. J. Clin. Pediatr. 2010, 22, 241–246. [Google Scholar]
- Teimoori-Boghsani, Y.; Ganjeali, A.; Cernava, T.; Müller, H.; Asili, J.; Berg, G. Endophytic fungi of native Salvia abrotanoides plants reveal high taxonomic diversity and unique profiles of secondary metabolites. Front. Microbiol. 2020, 10, 3013. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cui, G.; Chen, T.; Ma, X.; Wang, R.; Jin, B.; Yang, J.; Kang, L.; Tang, J.; Lai, C.; et al. Expansion within the CYP71D subfamily drives the heterocyclization of tanshinones synthesis in Salvia miltiorrhiza. Nat. Commun. 2021, 12, 685. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).