Review: Research Progress of Dairy Sheep Milk Genes
Abstract
1. Introduction
2. Descriptions of Some Famous Dairy Sheep Breeds
2.1. East Friesian Sheep
2.2. Lacaune Sheep
2.3. Sarda Sheep
2.4. Latxa Sheep
2.5. Awassi Sheep
2.6. Assaf Sheep
3. Comparison of Cow, Goat, and Sheep Milk Contents
4. Genes Affecting Sheep Milk Performance
5. SLC2A2 Gene—Transport Nutrition from Blood to Milk
6. CSN2 Gene—Benefit for the Lactose Intolerance
7. SCD Gene—Promote Unsaturated Fatty Acids Yield
8. SOCS2—An Indicator of Mastitis in Mammary Gland
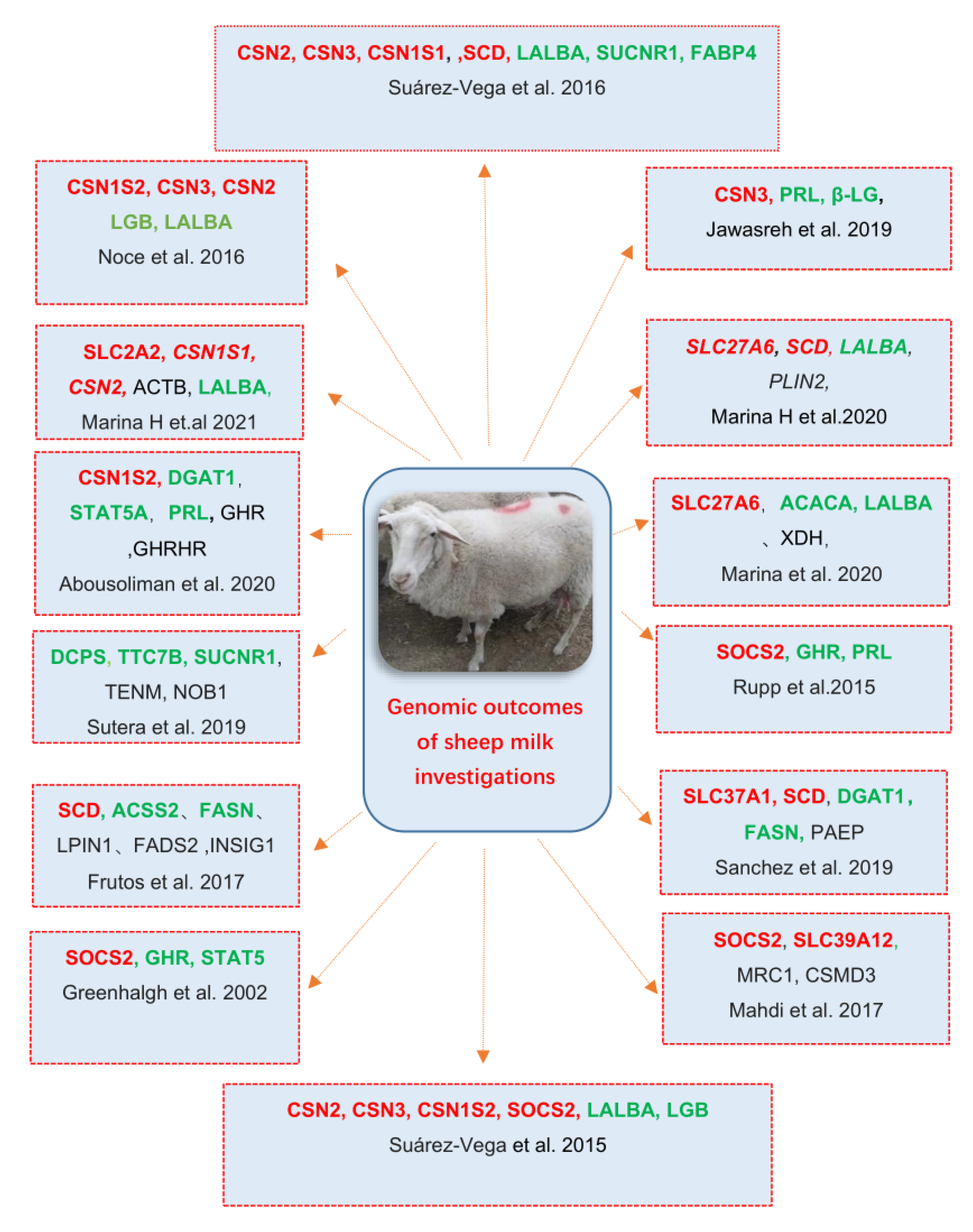
9. Other Detected Genes Related to Milk Traits
10. Personal Insight into Future Sheep Milk Research
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pulina, G.; Milan, M.J.; Lavin, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Invited review: Current production trends, farm structures, and economics of the dairy sheep and goat sectors. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef]
- Leroy, B.P.; Russell, S.R.; Bennett, J.; High, K.A.; Drack, A.V.; Yu, Z.F.; Chung, D.; Reape, K.Z.; Maguire, A.M.; Simunovic, M. Five-year update for the Phase III voretigene neparvovec study in biallelic RPE65 mutation-associated inherited retinal disease. Clin. Exp. Ophthalmol. 2022, 49, 966–967. [Google Scholar]
- Michailidou, S.; Gelasakis, A.; Banos, G.; Arsenos, G.; Argiriou, A. Comparative Transcriptome Analysis of Milk Somatic Cells During Lactation Between Two Intensively Reared Dairy Sheep Breeds. Front. Genet. 2021, 12, 1293. [Google Scholar] [CrossRef]
- Legarra, A.; Ramon, M.; Ugarte, E.; Perez-Guzman, M.D. Economic weights of fertility, prolificacy, milk yield and longevity in dairy sheep. Animal 2007, 1, 193–203. [Google Scholar] [CrossRef]
- Legarra, A.; Baloche, G.; Barillet, F.; Astruc, J.M.; Soulas, C.; Aguerre, X.; Arrese, F.; Mintegi, L.; Lasarte, M.; Maeztu, F.; et al. Within- and across-breed genomic predictions and genomic relationships for Western Pyrenees dairy sheep. breeds Latxa, Manech, and Basco-Bearnaise. J. Dairy Sci. 2014, 97, 3200–3212. [Google Scholar] [CrossRef]
- Ogorevc, J.; Kunej, T.; Razpet, A.; Dovc, P. Database of cattle candidate genes and genetic markers for milk production and mastitis. Anim. Genet. 2009, 40, 832–851. [Google Scholar] [CrossRef]
- Kessler, E.C.; Bruckmaier, R.M.; Gross, J.J. Immunoglobulin G content and colostrum composition of different goat and sheep breeds in Switzerland and Germany. J. Dairy Sci. 2019, 102, 5542–5549. [Google Scholar] [CrossRef]
- Morrissey, A.D.; Cameron, A.W.N.; Caddy, D.J.; Tilbrook, A.J. Predicting milk yield in sheep used for dairying in Australia. J. Dairy Sci. 2007, 90, 5056–5061. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Le, V.H.; Nguyen, D.V.; Malau-Aduli, B.S.; Nichols, P.D.; Malau-Aduli, A.E.O. Supplementing Grazing Dairy Ewes with Plant-Derived Oil and Rumen-Protected EPA plus DHA Pellets Enhances Health-Beneficial n-3 Long-Chain Polyunsaturated Fatty Acids in Sheep Milk. Eur. J. Lipid Sci. Technol. 2018, 120, 1700256. [Google Scholar] [CrossRef]
- Kominakis, A.; Hager-Theodorides, A.L.; Saridaki, A.; Antonakos, G.; Tsiamis, G. Genome-wide population structure and evolutionary history of the Frizarta dairy sheep. Animal 2017, 11, 1680–1688. [Google Scholar] [CrossRef]
- Drouilhet, L.; Lecerf, F.; Bodin, L.; Fabre, S.; Mulsant, P. Fine mapping of the FecL locus influencing prolificacy in Lacaune sheep. Anim. Genet. 2009, 40, 804–812. [Google Scholar] [CrossRef]
- Jimenez, L.E.R.; Hernandez, J.C.A.; Palacios, C.; Abecia, J.A.; Naranjo, A.; Avalos, J.O.; Gonzalez-Ronquillo, M. Milk Production of Lacaune Sheep with Different Degrees of Crossing with Manchega Sheep in a Commercial Flock in Spain. Animals 2020, 10, 520. [Google Scholar] [CrossRef]
- Barillet, F.; Marie, C.; Jacquin, M.; Lagriffoul, G.; Astruc, J.M. The French Lacaune dairy sheep breed: Use in France and abroad in the last 40 years. Livest. Prod. Sci. 2001, 71, 17–29. [Google Scholar] [CrossRef]
- Van Belzen, N. Achieving Sustainable Production of Milk: Volume 1: Milk Composition, Genetics and Breeding; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; Volume 8, pp. 1–339. [Google Scholar]
- Alba, D.F.; Favaretto, J.A.; Marcon, H.; Saldanha, T.F.; Leal, K.W.; Campigoto, G.; Souza, C.F.; Baldissera, M.D.; Bianchi, A.E.; Vedovatto, M.; et al. Vegetable biocholine supplementation in pre- and postpartum Lacaune sheep: Effects on animal health, milk production and quality. Small Rumin. Res. 2020, 190, 106165. [Google Scholar] [CrossRef]
- Bittante, G.; Cipolat-Gotet, C.; Pazzola, M.; Dettori, M.L.; Vacca, G.M.; Cecchinato, A. Genetic analysis of coagulation properties, curd firming modeling, milk yield, composition, and acidity in Sarda dairy sheep. J. Dairy Sci. 2017, 100, 385–394. [Google Scholar] [CrossRef]
- Dettori, M.L.; Pazzola, M.; Paschino, P.; Amills, M.; Vacca, G.M. Association between the GHR, GHRHR, and IGF1 gene polymorphisms and milk yield and quality traits in Sarda sheep. J. Dairy Sci. 2018, 101, 9978–9986. [Google Scholar] [CrossRef]
- Juste, R.A.; Villoria, M.; Leginagoikoa, I.; Ugarte, E.; Minguijon, E. Milk production losses in Latxa dairy sheep associated with small ruminant lentivirus infection. Prev. Vet. Med. 2020, 176, 104886. [Google Scholar] [CrossRef]
- Granado-Tajada, I.; Rodriguez-Ramilo, S.T.; Legarra, A.; Ugarte, E. Inbreeding, effective population size, and coancestry in the Latxa dairy sheep breed. J. Dairy Sci. 2020, 103, 5215–5226. [Google Scholar] [CrossRef]
- Ugarte, E.; Urarte, E.; Arrese, F.; Arranz, J.; Silio, L.; Rodriguez, C. Genetic parameters and trends for milk production of blond-faced Latxa sheep using Bayesian analysis. J. Dairy Sci. 1996, 79, 2268–2277. [Google Scholar] [CrossRef]
- Ruiz, R.; Oregui, L.M.; Herrero, M. Comparison of models for describing the lactation curve of latxa sheep and an analysis of factors affecting milk yield. J. Dairy Sci. 2000, 83, 2709–2719. [Google Scholar] [CrossRef]
- Talafha, A.Q.; Ababneh, M.M. Awassi sheep reproduction and milk production: Review. Trop. Anim. Health Prod. 2011, 43, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Pollott, G.E.; Gootwine, E. Reproductive performance and milk production of Assaf sheep in an intensive management system. J. Dairy Sci. 2004, 87, 3690–3703. [Google Scholar] [CrossRef]
- Kridli, R.T.; Abdullah, A.Y.; Shaker, M.M.; Al-Smadi, N.A. Reproductive performance and milk yield in Awassi ewes following crossbreeding. Small Rumin. Res. 2007, 71, 103–108. [Google Scholar] [CrossRef]
- Galal, S.; Gursoy, O.; Shaat, I. Awassi sheep as a genetic resource and efforts for their genetic improvement-A review. Small Rumin. Res. 2008, 79, 99–108. [Google Scholar] [CrossRef]
- Milan, M.J.; Frendi, F.; Gonzalez-Gonzalez, R.; Caja, G. Cost structure and profitability of Assaf dairy sheep farms in Spain. J. Dairy Sci. 2014, 97, 5239–5249. [Google Scholar] [CrossRef]
- Gootwine, E.; Goot, H. Lamb and milk production of Awassi and East-Friesian sheep and their crosses under Mediterranean environment. Small Rumin. Res. 1996, 20, 255–260. [Google Scholar] [CrossRef]
- Gootwine, E.; Pollott, G.E. Factors affecting milk production in improved Awassi dairy ewes. Anim. Sci. 2000, 71, 607–615. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Lagriffoul, G.; Paccard, P.; Guillet, I.; Chilliard, Y. Composition of goat and sheep milk products: An update. Small Rumin. Res. 2008, 79, 57–72. [Google Scholar] [CrossRef]
- Park, Y.W.; Juarez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Scintu, M.F.; Piredda, G. Typicity and biodiversity of goat and sheep milk products. Small Rumin. Res. 2007, 68, 221–231. [Google Scholar] [CrossRef]
- Caboni, P.; Murgia, A.; Porcu, A.; Manis, C.; Ibba, I.; Contu, M.; Scano, P. A metabolomics comparison between sheep’s and goat’s milk. Food Res. Int. 2019, 119, 869–875. [Google Scholar] [CrossRef]
- Ceballos, L.S.; Morales, E.R.; Adarve, G.D.T.; Castro, J.D.; Martinez, L.P.; Sampelayo, M.R.S. Composition of goat and cow milk produced under similar conditions and analyzed by identical methodology. J. Food Compos. Anal. 2009, 22, 322–329. [Google Scholar] [CrossRef]
- Daetwyler, H.D.; Capitan, A.; Pausch, H.; Stothard, P.; Van Binsbergen, R.; Brondum, R.F.; Liao, X.P.; Djari, A.; Rodriguez, S.C.; Grohs, C.; et al. Whole-genome sequencing of 234 bulls facilitates mapping of monogenic and complex traits in cattle. Nat. Genet. 2014, 46, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, M.; Laudadio, V.; Dario, C.; Tufarelli, V. Investigating the genetic polymorphism of sheep milk proteins: A useful tool for dairy production. J. Sci. Food Agric. 2014, 94, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Amigo, L.; Recio, I.; Ramos, M. Genetic polymorphism of ovine milk proteins: Its influence on technological properties of milk—A review. Int. Dairy J. 2000, 10, 135–149. [Google Scholar] [CrossRef]
- Mohan, G.; Kumar, A.; Khan, S.H.; Kumar, N.A.; Kapila, S.; Lathwal, S.S.; Sodhi, M.; Niranjan, S.K. Casein (CSN) gene variants and parity affect the milk protein traits in crossbred (Bos taurus x Bos indicus) cows in sub-tropical climate. Trop. Anim. Health Prod. 2021, 53, 289. [Google Scholar] [CrossRef]
- Brenaut, P.; Bangera, R.; Bevilacqua, C.; Rebours, E.; Cebo, C.; Martin, P. Validation of RNA isolated from milk fat globules to profile mammary epithelial cell expression during lactation and transcriptional response to a bacterial infection. J. Dairy Sci. 2012, 95, 6130–6144. [Google Scholar] [CrossRef]
- Gu, M.; Cosenza, G.; Iannaccone, M.; Macciotta, N.P.P.; Guo, Y.; Di Stasio, L.; Pauciullo, A. The single nucleotide polymorphism g.133A>C in the stearoyl CoA desaturase gene (SCD) promoter affects gene expression and quali-quantitative properties of river buffalo milk. J. Dairy Sci. 2019, 102, 442–451. [Google Scholar] [CrossRef]
- Tong, J.J.; Thompson, I.M.; Zhao, X.; Lacasse, P. Effect of 17beta-estradiol on milk production, hormone secretion, and mammary gland gene expression in dairy cows. J. Dairy Sci. 2018, 101, 2588–2601. [Google Scholar] [CrossRef]
- Jacometo, C.B.; Zhou, Z.; Luchini, D.; Trevisi, E.; Correa, M.N.; Loor, J.J. Maternal rumen-protected methionine supplementation and its effect on blood and liver biomarkers of energy metabolism, inflammation, and oxidative stress in neonatal Holstein calves. J. Dairy Sci. 2016, 99, 6753–6763. [Google Scholar] [CrossRef]
- Sekhar, G.N.; Watson, C.P.; Fidanboylu, M.; Sanderson, L.; Thomas, S.A. Delivery of antihuman African trypanosomiasis drugs across the blood-brain and blood-CSF barriers. Adv. Pharm. 2014, 71, 245–275. [Google Scholar]
- Ma, X.Y.; Liang, S.S.; Liang, A.X.; Rushdi, H.E.; Deng, T.X. Evolutionary Analysis of OAT Gene Family in River and Swamp Buffalo: Potential Role of SLCO3A1 Gene in Milk Performance. Genes 2021, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lino, A.M.; Alvarez-Fernandez, I.; Blanco-Paniagua, E.; Merino, G.; Alvarez, A.I. Transporters in the Mammary Gland-Contribution to Presence of Nutrients and Drugs into Milk. Nutrients 2019, 11, 2372. [Google Scholar] [CrossRef] [PubMed]
- Hadsell, D.L.; Hadsell, L.A.; Rijnkels, M.; Carcamo-Bahena, Y.; Wei, J.; Williamson, P.; Grusak, M.A. In silico mapping of quantitative trait loci (QTL) regulating the milk ionome in mice identifies a milk iron locus on chromosome 1. Mamm. Genome 2018, 29, 632–655. [Google Scholar] [CrossRef] [PubMed]
- Marina, H.; Pelayo, R.; Suarez-Vega, A.; Gutierrez-Gil, B.; Esteban-Blanco, C.; Arranz, J.J. Genome-wide association studies (GWAS) and post-GWAS analyses for technological traits in Assaf and Churra dairy breeds. J. Dairy Sci. 2021, 104, 11850–11866. [Google Scholar] [CrossRef]
- Crisa, A.; Marchitelli, C.; Pariset, L.; Contarini, G.; Signorelli, F.; Napolitano, F.; Catillo, G.; Valentini, A.; Moioli, B. Exploring polymorphisms and effects of candidate genes on milk fat quality in dairy sheep. J. Dairy Sci. 2010, 93, 3834–3845. [Google Scholar] [CrossRef]
- Schweigel-Rontgen, M. The families of zinc (SLC30 and SLC39) and copper (SLC31) transporters. Curr. Top. Membr. 2014, 73, 321–355. [Google Scholar]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef]
- Sanchez, M.P.; Ramayo-Caldas, Y.; Wolf, V.; Laithier, C.; El Jabri, M.; Michenet, A.; Boussaha, M.; Taussat, S.; Fritz, S.; Delacroix-Buchet, A.; et al. Sequence-based GWAS, network and pathway analyses reveal genes co-associated with milk cheese-making properties and milk composition in Montbeliarde cows. Genet. Sel. Evol. 2019, 51, 34. [Google Scholar] [CrossRef]
- Flis, Z.; Molik, E. Importance of Bioactive Substances in Sheep’s Milk in Human Health. Int. J. Mol. Sci. 2021, 22, 4364. [Google Scholar] [CrossRef]
- Cieslak, J.; Wodas, L.; Borowska, A.; Pawlak, P.; Czyzak-Runowska, G.; Wojtowski, J.; Puppel, K.; Kuczynska, B.; Mackowski, M. 5’-flanking variants of equine casein genes (CSN1S1, CSN1S2, CSN2, CSN3) and their relationship with gene expression and milk composition. J. Appl. Genet. 2019, 60, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Perez, C.M.; Alvarez-Cepeda, A.A.; Rosendo-Ponce, A.; Alonso-Morales, R.A.; Gayosso-Vazquez, A.; Torres-Hernandez, G.; Rosales-Martinez, F. Kappa-casein genotyping in tropical milking Criollo and its association to milk production and composition. Trop. Anim. Health Prod. 2020, 52, 3885–3888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Li, C.; Cai, W.T.; Liu, S.L.; Yin, H.W.; Shi, S.L.; Zhang, Q.; Zhang, S.L. Genome-Wide Association Study for Milk Protein Composition Traits in a Chinese Holstein Population Using a Single-Step Approach. Front. Genet. 2019, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Woodford, K.; Kukuljan, S.; Ho, S. Milk Intolerance, Beta-Casein and Lactose. Nutrients 2015, 7, 7285–7297. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Q.; Xu, L.M.; Lu, X.; Yelland, G.W.; Ni, J.Y.; Clarke, A. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr. J. 2016, 15, 35. [Google Scholar]
- Ramakrishnan, M.; Eaton, T.K.; Sermet, O.M.; Savaiano, D.A. Milk Containing A2 beta-Casein ONLY, as a Single Meal, Causes Fewer Symptoms of Lactose Intolerance than Milk Containing A1 and A2 beta-Caseins in Subjects with Lactose Maldigestion and Intolerance: A Randomized, Double-Blind, Crossover Trial. Nutrients 2020, 12, 3855. [Google Scholar] [CrossRef]
- Skrzypczak, E.; Babicz, M.; Pastwa, M. Effect of Prolactin Receptor (PRLR) and Beta-Casein (CSN2) Gene Polymorphism on the Chemical Composition of Milk Sows. Folia Biol. 2015, 63, 135–144. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Sun, D.; Zhang, S.; Liu, L.; Alim, M.A.; Zhang, Q. A post-GWAS confirming the SCD gene associated with milk medium- and long-chain unsaturated fatty acids in Chinese Holstein population. Anim. Genet. 2016, 47, 483–490. [Google Scholar] [CrossRef]
- Li, C.; Sun, D.; Zhang, S.; Wang, S.; Wu, X.; Zhang, Q.; Liu, L.; Li, Y.; Qiao, L. Genome wide association study identifies 20 novel promising genes associated with milk fatty acid traits in Chinese Holstein. PLoS ONE 2014, 9, e96186. [Google Scholar] [CrossRef]
- Suarez-Vega, A.; Gutierrez-Gil, B.; Arranz, J.J. Transcriptome expression analysis of candidate milk genes affecting cheese-related traits in 2 sheep breeds. J. Dairy Sci. 2016, 99, 6381–6390. [Google Scholar] [CrossRef]
- Suarez-Vega, A.; Gutierrez-Gil, B.; Klopp, C.; Tosser-Klopp, G.; Arranz, J.J. Comprehensive RNA-Seq profiling to evaluate lactating sheep mammary gland transcriptome. Sci. Data 2016, 3, 160051. [Google Scholar] [CrossRef] [PubMed]
- Pauciullo, A.; Cosenza, G.; Steri, R.; Coletta, A.; La Battaglia, A.; Di Berardino, D.; Macciotta, N.P.P.; Ramunno, L. A single nucleotide polymorphism in the promoter region of river buffalo stearoyl CoA desaturase gene (SCD) is associated with milk yield. J. Dairy Res. 2012, 79, 429–435. [Google Scholar] [CrossRef][Green Version]
- Rupp, R.; Senin, P.; Sarry, J.; Allain, C.; Tasca, C.; Ligat, L.; Portes, D.; Woloszyn, F.; Bouchez, O.; Tabouret, G.; et al. A Point Mutation in Suppressor of Cytokine Signalling 2 (Socs2) Increases the Susceptibility to Inflammation of the Mammary Gland while Associated with Higher Body Weight and Size and Higher Milk Production in a Sheep Model. PLoS Genet. 2015, 11, e1005629. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Naka, T. Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. 2003, 24, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Piessevaux, J.; Lavens, D.; Peelman, F.; Tavernier, J. The many faces of the SOCS box. Cytokine Growth Factor Rev. 2008, 19, 371–381. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Murray, P.J. Cytokine signaling modules in inflammatory responses. Immunity 2008, 28, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Stanford, P.M.; Sutherland, K.; Oakes, S.R.; Naylor, M.J.; Robertson, F.G.; Blazek, K.D.; Kazlauskas, M.; Hilton, H.N.; Wittlin, S.; et al. Socs2 and Elf5 mediate prolactin-induced mammary gland development. Mol. Endocrinol. 2006, 20, 1177–1187. [Google Scholar] [CrossRef]
- Lindeman, G.J.; Wittlin, S.; Lada, H.; Naylor, M.J.; Santamaria, M.; Zhang, J.G.; Starr, R.; Hilton, D.J.; Alexander, W.S.; Ormandy, C.J.; et al. SOCS1 deficiency results in accelerated mammary gland development and rescues lactation in prolactin receptor-deficient mice. Gene Dev. 2001, 15, 1631–1636. [Google Scholar] [CrossRef]
- Marina, H.; Reverter, A.; Gutierrez-Gil, B.; Alexandre, P.A.; Porto-Neto, L.R.; Suarez-Vega, A.; Li, Y.T.; Esteban-Blanco, C.; Arranz, J.J. Gene Networks Driving Genetic Variation in Milk and Cheese-Making Traits of Spanish Assaf Sheep. Genes 2020, 11, 715. [Google Scholar] [CrossRef]
- Garcia-Gamez, E.; Gutierrez-Gil, B.; Sahana, G.; Sanchez, J.P.; Bayon, Y.; Arranz, J.J. GWA Analysis for Milk Production Traits in Dairy Sheep and Genetic Support for a QTN Influencing Milk Protein Percentage in the LALBA Gene. PLoS ONE 2012, 7, e47782. [Google Scholar] [CrossRef]
- Sutera, A.M.; Riggio, V.; Mastrangelo, S.; Di Gerlando, R.; Sardina, M.T.; Pong-Wong, R.; Tolone, M.; Portolano, B. Genome-wide association studies for milk production traits in Valle del Belice sheep using repeated measures. Anim. Genet. 2019, 50, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, X.L.; Tait, R.G.; Bauck, S.; Thomas, D.L.; Murphy, T.W.; Rosa, G.J.M. Genome-wide association study of milk production traits in a crossbred dairy sheep population using three statistical models. Anim. Genet. 2020, 51, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Abousoliman, I.; Reyer, H.; Oster, M.; Murani, E.; Mourad, M.; Rashed, M.A.; Mohamed, I.; Wimmers, K. Analysis of Candidate Genes for Growth and Milk Performance Traits in the Egyptian Barki Sheep. Animals 2020, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Scata, M.C.; Napolitano, F.; Casu, S.; Carta, A.; De Matteis, G.; Signorelli, F.; Annicchiarico, G.; Catillo, G.; Moioli, B. Ovine acyl CoA:diacylglycerol acyltransferase 1- molecular characterization, polymorphisms and association with milk traits. Anim. Genet. 2009, 40, 737–742. [Google Scholar] [CrossRef]
- Khan, M.Z.; Ma, Y.; Ma, J.; Xiao, J.; Liu, Y.; Liu, S.; Khan, A.; Khan, I.M.; Cao, Z. Association of DGAT1 With Cattle, Buffalo, Goat, and Sheep Milk and Meat Production Traits. Front. Vet. Sci. 2021, 8, 712470. [Google Scholar] [CrossRef]
- Morris, C.A.; Cullen, N.G.; Glass, B.C.; Hyndman, D.L.; Manley, T.R.; Hickey, S.M.; McEwan, J.C.; Pitchford, W.S.; Bottema, C.D.; Lee, M.A. Fatty acid synthase effects on bovine adipose fat and milk fat. Mamm. Genome 2007, 18, 64–74. [Google Scholar] [CrossRef]
- Badaoui, B.; Serradilla, J.M.; Tomas, A.; Urrutia, B.; Ares, J.L.; Carrizosa, J.; Sanchez, A.; Jordana, J.; Amills, M. Goat acetyl-coenzyme A carboxylase alpha: Molecular characterization, polymorphism, and association with milk traits. J. Dairy Sci. 2007, 90, 1039–1043. [Google Scholar] [CrossRef]
- Federica, S.; Francesco, N.; Giovanna, D.M.; Carmela, S.M.; Gennaro, C.; Carmela, T.; Bianca, M. Identification of novel single nucleotide polymorphisms in promoter III of the acetyl-CoA carboxylase-{alpha} gene in goats affecting milk production traits. J. Hered. 2009, 100, 386–389. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervas, G.; Suarez-Vega, A.; Arranz, J.J.; Frutos, P. Isolation of RNA from milk somatic cells as an alternative to biopsies of mammary tissue for nutrigenomic studies in dairy ewes. J. Dairy Sci. 2016, 99, 8461–8471. [Google Scholar] [CrossRef]
- Yurchenko, A.A.; Deniskova, T.E.; Yudin, N.S.; Dotsev, A.V.; Khamiruev, T.N.; Selionova, M.I.; Egorov, S.V.; Reyer, H.; Wimmers, K.; Brem, G.; et al. High-density genotyping reveals signatures of selection related to acclimation and economically important traits in 15 local sheep breeds from Russia. BMC Genom. 2019, 20 (Suppl. 3), 294. [Google Scholar] [CrossRef]
- Eaaswarkhanth, M.; dos Santos, A.L.C.; Gokcumen, O.; Al-Mulla, F.; Thanaraj, T.A. Genome-Wide Selection Scan in an Arabian Peninsula Population Identifies a TNKS Haplotype Linked to Metabolic Traits and Hypertension. Genome Biol. Evol. 2020, 12, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.M.; Brown, M.A.; McCarthy, M.I.; Yang, J. Five Years of GWAS Discovery. Am. J. Hum. Genet. 2012, 90, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Nakato, R.; Sakata, T. Methods for ChIP-seq analysis: A practical workflow and advanced applications. Methods 2021, 187, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ayturk, U. RNA-seq in Skeletal Biology. Curr. Osteoporos. Rep. 2019, 17, 178–185. [Google Scholar] [CrossRef]
- Hryhorowicz, M.; Lipianki, D.; Zeyland, J.; Slomski, R. CRISPR/Cas9 Immune System as a Tool for Genome Engineering. Arch. Immunol. Ther. Exp. 2017, 65, 233–240. [Google Scholar] [CrossRef]
- Gupta, D.; Bhattacharjee, O.; Mandal, D.; Sen, M.K.; Dey, D.; Dasgupta, A.; Kazi, T.A.; Gupta, R.; Sinharoy, S.; Acharya, K.; et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci. 2019, 232, 116636. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, Y.J.; Bi, Y.L.; Tang, B.B.; Wang, M.; Zhang, C.; Zhang, W.H.; Jin, J.; Li, T.T.; Zhao, R.F.; et al. CRISPR/Cas9-mediated sheep MSTN gene knockout and promote sSMSCs differentiation. J. Cell Biochem. 2019, 120, 1794–1806. [Google Scholar] [CrossRef]
| Breed | Total Milk Yield/Lactation (kg) | Lactation (Day) | Average Milk Yield per Day (kg) | Average Protein Percent (%) | Average Fat Percent (%) | Reference |
|---|---|---|---|---|---|---|
| East Friesian | 700 | 300 | 2.29 | 5.35 | 6.50 | Thomas, D.L. et al., 2014 |
| Lacanue | 454 | 200 | 2.50 | 5.00 | 5.93 | Alba, D.F. et al., 2019 |
| Sarda | 376 | 150 | 2.50 | 5.32 | 6.35 | Bittante, G. et al., 2017 |
| Latxa | 446 | 147 | 3.03 | 5.75 | 6.09 | Juste, R.A. et al., 2020 |
| Awassi | 460 | 214 | 3.71 | 5.15 | 6.86 | Nudda, A. et al., 2020 |
| Assaf | 506 | 173 | 2.90 | 6.00 | 6.50 | Pollott, G.E. et al., 2004 |
| Content | Unit | Cow Milk | Goat Milk | Sheep Milk | Reference 2 |
|---|---|---|---|---|---|
| Fat | g/kg | 36 | 38 | 79 * | Pietrzak-Fiećko, R. et al., 2020 |
| Protein | g/kg | 33 | 35 | 62 * | Roy, D. et al., 2020 |
| Lactose | g/kg | 46 | 41 | 49 | Roy, D. et al., 2020 |
| Ash | g/kg | 7 | 8 | 9 | Pietrzak-Fiećko, R. et al., 2020 |
| Non-fat solid | g/kg | 79 | 76 | 111 * | Pulina, G. et al., 2018 |
| Iodine | mg/100 g | 0.5 | 0.41 | 0.47 | Slovenian, P. et al., 2014 |
| Phosphorus | mg/100 g | 96 | 87 | 160 * | Park, Y.W. et al., 2000 |
| Cuprum | mg/100 g | n/a 1 | 0.013 | 0.019 | Stocco, G. et al., 2018 |
| Calcium | mg/dL | 120 | 130 | 180 * | Pulina, G. et al., 2018 |
| Zinc | mg/dL | 0.62 | 0.69 | 0.58 | Claumarchirant, L. et al., 2015 |
| Sodium | mg/dL | 49.3 | 35.9 | 52.1 | Singh, M. et al., 2019 |
| Magnesium | mg/dL | 12 | 16 | 21 | Khan, I.T. et al., 2019 |
| Potassium | mg/dL | 148 | 184 | 179 | Khan, I.T. et al., 2019 |
| VitaminA | mg/100 g | 0.04 | 0.05 | 0.08 | Burrow, K. et al., 2019 |
| Vitamin B6 | mg/100 g | 1.09 | 1.01 | 1.75 * | Ochoa-Flores, A.A. et al., 2021 |
| Vitamin C | mg/100 g | 0.09 | 1.29 | 4.16 | Caboni, P. et al., 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Ma, Y.; Jiang, L. Review: Research Progress of Dairy Sheep Milk Genes. Agriculture 2022, 12, 169. https://doi.org/10.3390/agriculture12020169
Li R, Ma Y, Jiang L. Review: Research Progress of Dairy Sheep Milk Genes. Agriculture. 2022; 12(2):169. https://doi.org/10.3390/agriculture12020169
Chicago/Turabian StyleLi, Ruonan, Yuehui Ma, and Lin Jiang. 2022. "Review: Research Progress of Dairy Sheep Milk Genes" Agriculture 12, no. 2: 169. https://doi.org/10.3390/agriculture12020169
APA StyleLi, R., Ma, Y., & Jiang, L. (2022). Review: Research Progress of Dairy Sheep Milk Genes. Agriculture, 12(2), 169. https://doi.org/10.3390/agriculture12020169







