Rice Cultivar Renewal Reduces Methane Emissions by Improving Root Traits and Optimizing Photosynthetic Carbon Allocation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Designs
2.1.1. Experiment 1: Effects of Rice CR on Grain Yield and CH4 Emissions in Paddy Field
2.1.2. Experiment 2: Effects of Panicle Fertilizer on Grain Yield and CH4 Emissions in Paddy Fields
2.2. CH4 Sampling and Measurement Methods
2.3. Rice Root Traits
2.4. Root Exudated Total Organic Carbon (ETOC)
2.5. CH4 Production Potential and CH4 Oxidation Potential
2.6. Grain Yield
2.7. Statistical Analysis
3. Results
3.1. Differences in Experimental Factors
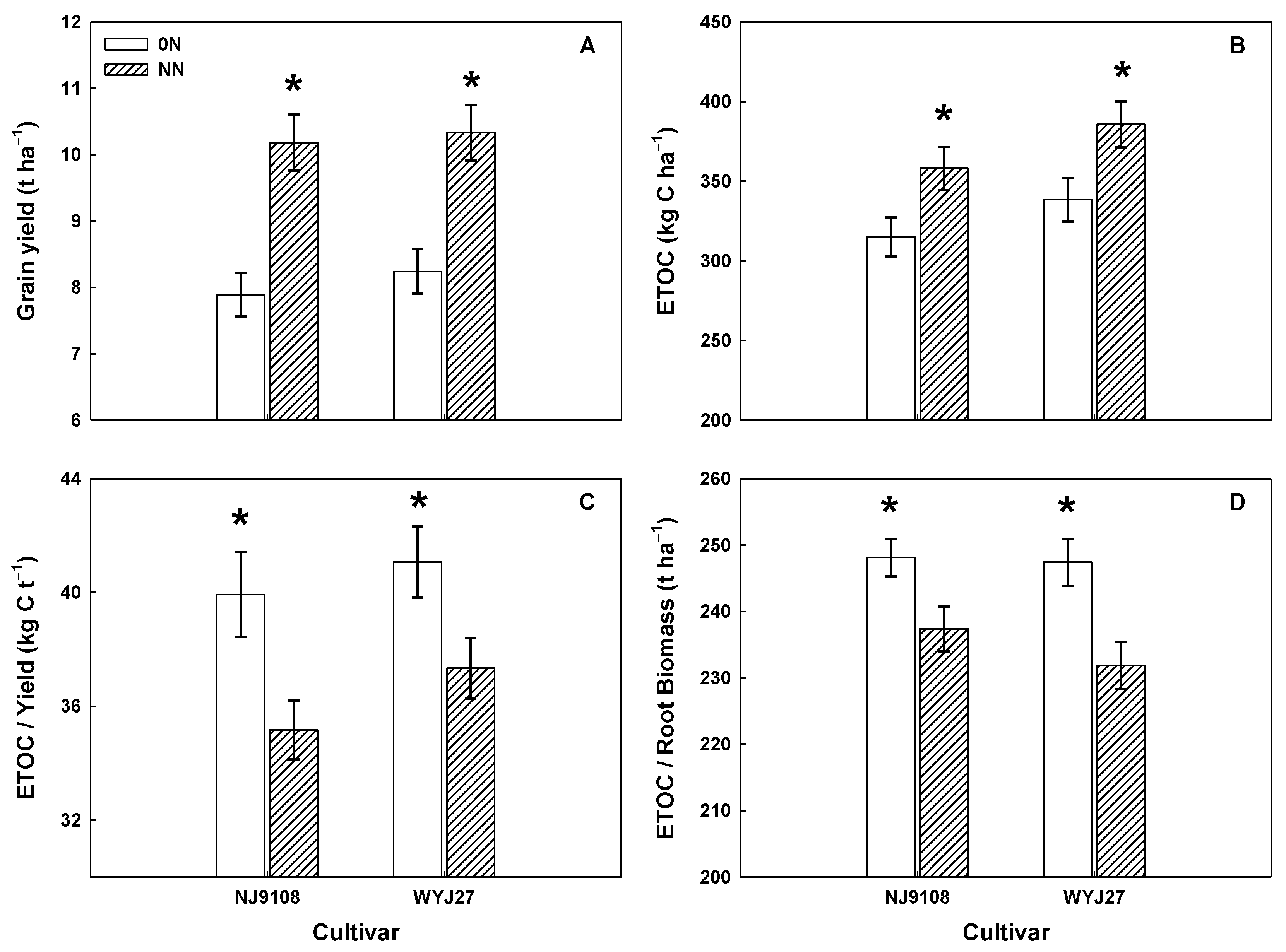
3.2. Grain Yield
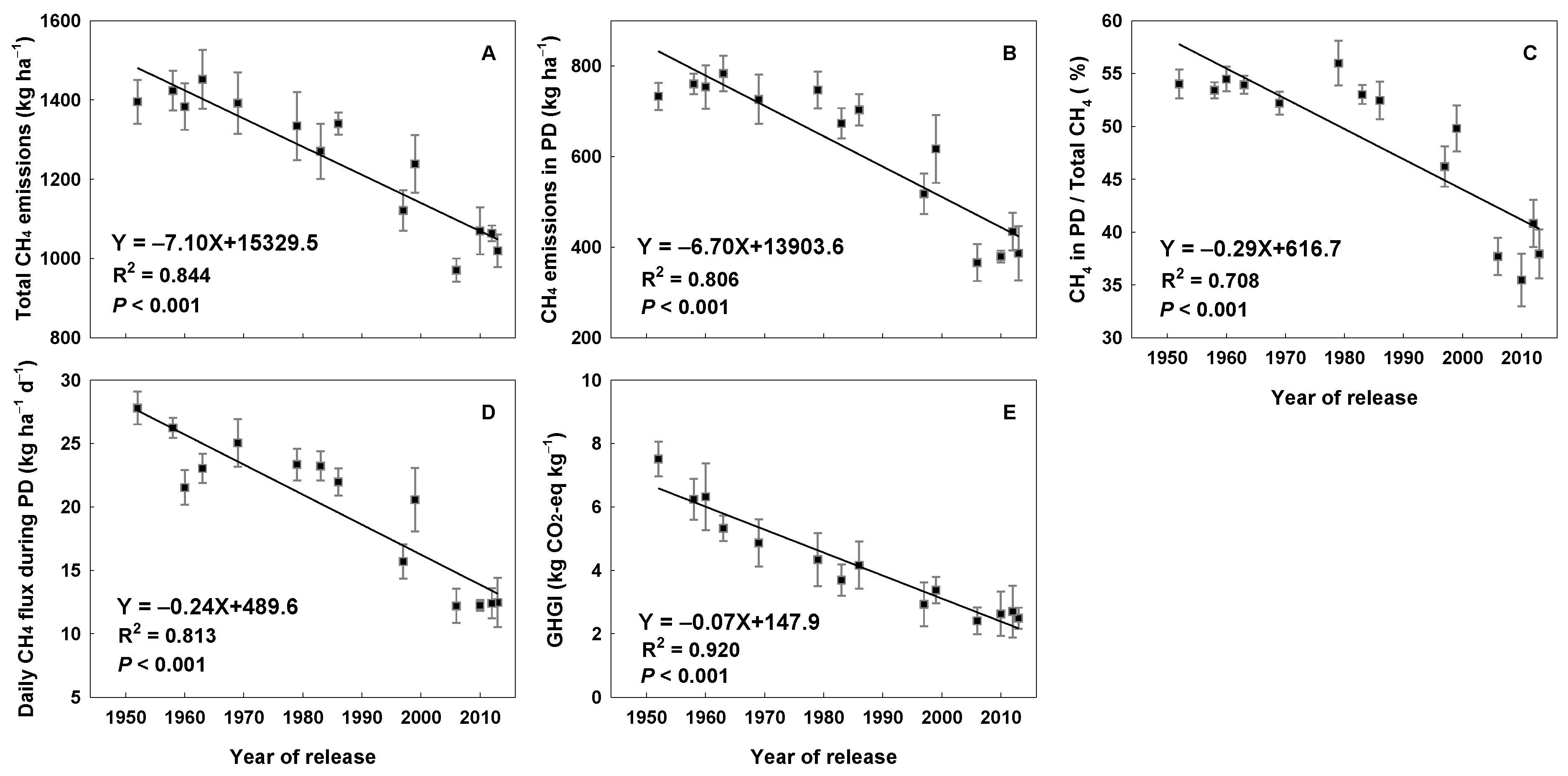
3.3. Methane Emissions
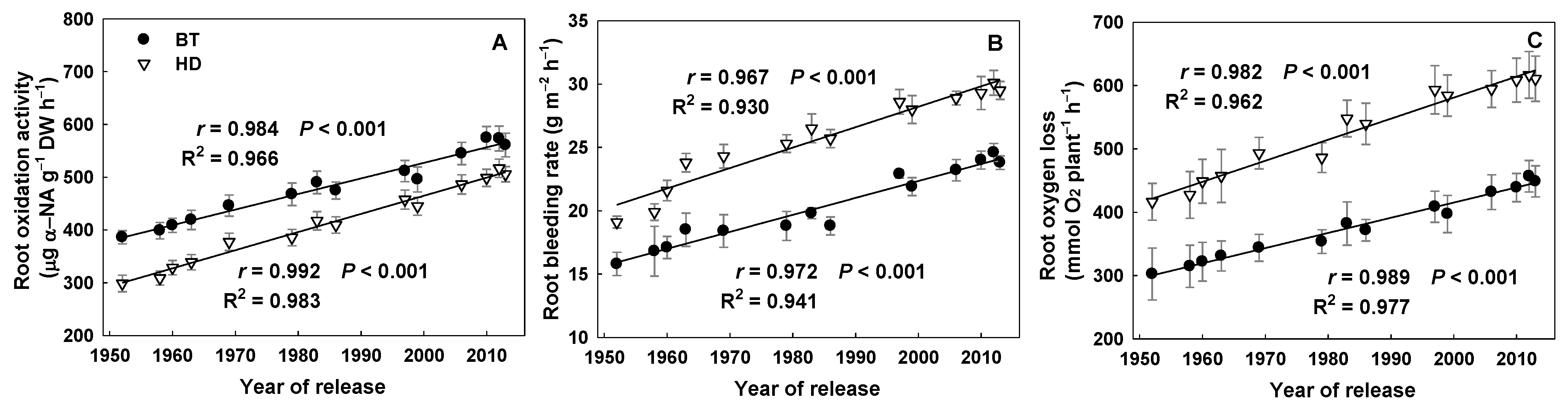
3.4. Shoot Biomass and Root Traits
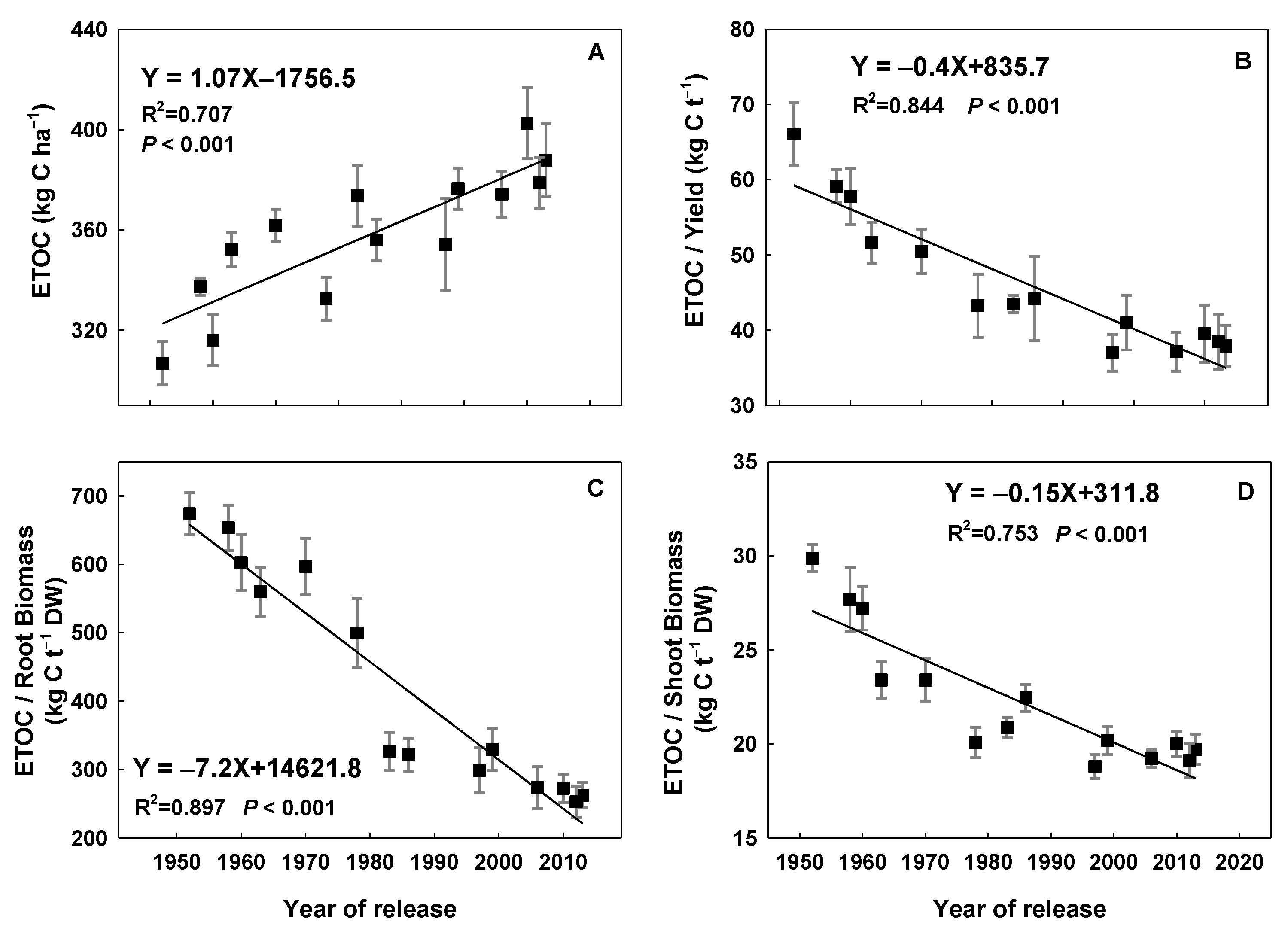
3.5. Root Exudate Organic Carbon
3.6. Methane Production and Oxidation in Rhizosphere Soil
3.7. Relationships between CH4 Emissions and Root Traits and ETOC
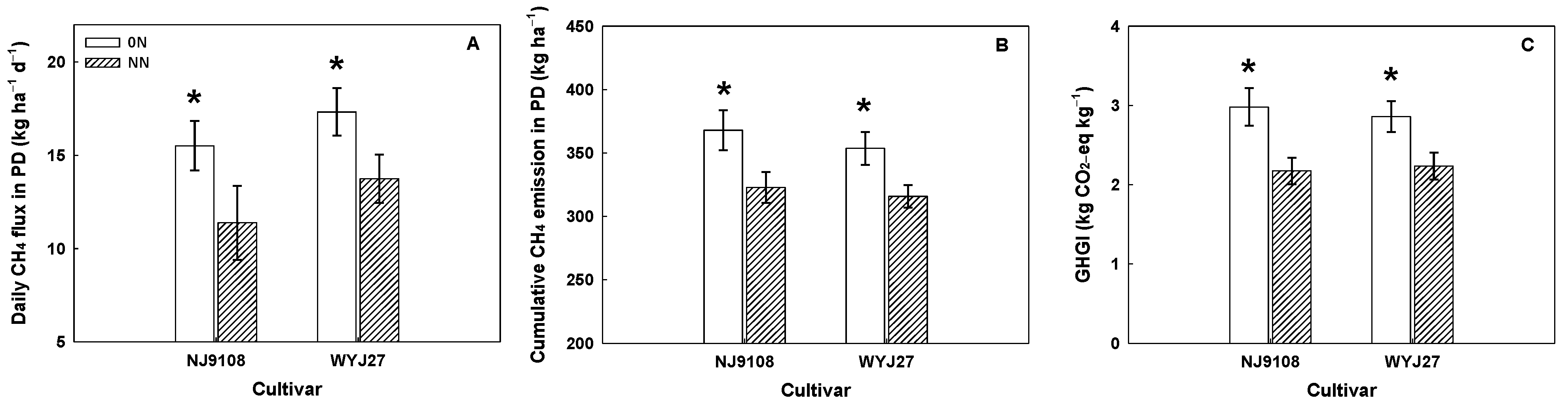
3.8. The Regulation of Panicle Fertilizer on Grain Yield, CH4 Emissions, Root Growth, and Root Exudation
4. Discussion
4.1. The Effect of CR on Rice Yields
4.2. The Effect of CR on CH4 Emissions
4.3. Relationships between Rice Root Traits, ETOC, and CH4 Emissions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; FAO: Rome, Italy, 2012. [Google Scholar]
- Evenson, R.E.; Gollin, D. Assessing the Impact of the Green Revolution, 1960 to 2000. Science 2003, 300, 758–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.; Khush, G.S.; Virk, P.; Tang, Q.; Zou, Y. Progress in Ideotype Breeding to Increase Rice Yield Potential. Field Crops Res. 2008, 108, 32–38. [Google Scholar] [CrossRef]
- Peng, S.; Tang, Q.; Zou, Y. Current Status and Challenges of Rice Production in China. Plant Prod. Sci. 2009, 12, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Chen, J.; Chen, L.; Wang, Z.; Zhang, H.; Yang, J. Grain Quality Changes and Responses to Nitrogen Fertilizer of Japonica Rice Cultivars Released in the Yangtze River Basin from the 1950s to 2000s. Crop J. 2015, 3, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, T.; Liu, L.; Wang, Z.; Yang, J.; Zhang, J. Performance in Grain Yield and Physiological Traits of Rice in the Yangtze River Basin of China During the Last 60 Yr. J. Integr. Agric. 2013, 12, 57–66. [Google Scholar] [CrossRef]
- Malyan, S.K.; Bhatia, A.; Kumar, A.; Gupta, D.K.; Singh, R.; Kumar, S.S.; Tomer, R.; Kumar, O.; Jain, N. Methane Production, Oxidation and Mitigation: A Mechanistic Understanding and Comprehensive Evaluation of Influencing Factors. Sci. Total Environ. 2016, 572, 874–896. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Yu, F.; Zhang, Y.; Liu, K.; Zhuo, X.; Qiu, Y.; Zhang, H.; Gu, J.; Wang, W.; et al. Reducing Methane Emission by Promoting Its Oxidation in Rhizosphere through Nitrogen-Induced Root Growth in Paddy Fields. Plant Soil 2022, 474, 541–560. [Google Scholar] [CrossRef]
- Carlson, K.M.; Gerber, J.S.; Mueller, N.D.; Herrero, M.; MacDonald, G.K.; Brauman, K.A.; Havlik, P.; O’Connell, C.S.; Johnson, J.A.; Saatchi, S.; et al. Greenhouse Gas Emissions Intensity of Global Croplands. Nat. Clim. Chang. 2017, 7, 63–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Tai, A.P.K.; Feng, J.; Li, Z.; Zhu, X.; Chen, J.; Zhang, J.; Song, Z.; Deng, A.; et al. Contribution of Rice Variety Renewal and Agronomic Innovations to Yield Improvement and Greenhouse Gas Mitigation in China. Environ. Res. Lett. 2019, 14. [Google Scholar] [CrossRef]
- Cai, Z.; Mosier, A.R. Effect of NH4Cl Addition on Methane Oxidation by Paddy Soils. Soil Biol. Biochem. 2000, 32, 1537–1545. [Google Scholar] [CrossRef]
- Serrano-Silva, N.; Sarria-Guzmán, Y.; Dendooven, L.; Luna-Guido, M. Methanogenesis and Methanotrophy in Soil: A Review. Pedosphere 2014, 24, 291–307. [Google Scholar] [CrossRef]
- Bodegom, P.; Stams, F.; Mollema, L.; Boeke, S.; Leffelaar, P. Methane Oxidation and the Competition for Oxygen in the Rice Rhizosphere. Appl. Environ. Microbiol. 2001, 67, 3586–3597. [Google Scholar] [CrossRef] [Green Version]
- Theint, E.E.; Suzuki, S.; Ono, E.; Bellingrath-Kimura, S.D. Influence of Different Rates of Gypsum Application on Methane Emission from Saline Soil Related with Rice Growth and Rhizosphere Exudation. Catena 2015, 133, 467–473. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, D.; Beebout, S.S.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Effect of Irrigation Regime on Grain Yield, Water Productivity, and Methane Emissions in Dry Direct-Seeded Rice Grown in Raised Beds with Wheat Straw Incorporation. Crop J. 2018, 6, 495–508. [Google Scholar] [CrossRef]
- Fan, D.; Liu, T.; Sheng, F.; Li, S.; Cao, C.; Li, C. Nitrogen Deep Placement Mitigates Methane Emissions by Regulating Methanogens and Methanotrophs in No-Tillage Paddy Fields. Biol. Fertil. Soils 2020, 56, 711–727. [Google Scholar] [CrossRef]
- Jiang, Y.; Carrijo, D.; Huang, S.; Chen, J.; Balaine, N.; Zhang, W.; van Groenigen, K.J.; Linquist, B. Water Management to Mitigate the Global Warming Potential of Rice Systems: A Global Meta-Analysis. Field Crops Res. 2019, 234, 47–54. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Li, S.; Liu, K.; Li, G.; Zhang, D.; Lv, B.; Gu, J.; Zhang, H.; Yang, J.; et al. OsRGA1 Optimizes Photosynthate Allocation for Roots to Reduce Methane Emissions and Improve Yield in Paddy Ecosystems. Soil Biol. Biochem. 2021, 160, 108344. [Google Scholar] [CrossRef]
- Tokida, T.; Adachi, M.; Cheng, W.; Nakajima, Y.; Fumoto, T.; Matsushima, M.; Nakamura, H.; Okada, M.; Sameshima, R.; Hasegawa, T. Methane and Soil CO2 Production from Current-Season Photosynthates in a Rice Paddy Exposed to Elevated CO2 Concentration and Soil Temperature. Glob. Chang. Biol. 2011, 17, 3327–3337. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Gavrichkova, O. Time Lag between Photosynthesis and Carbon Dioxide Efflux from Soil: A Review of Mechanisms and Controls. Glob. Chang. Biol. 2010, 16, 3386–3406. [Google Scholar] [CrossRef]
- Kaštovská, E.; Šantrůčková, H. Fate and Dynamics of Recently Fixed C in Pasture Plant-Soil System under Field Conditions. Plant Soil 2007, 300, 61–69. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, H.; Wang, L.; Feng, J.; Huang, S.; Hungate, B.A.; van Kessel, C.; Horwath, W.R.; Zhang, X.; Qin, X.; et al. Limited Potential of Harvest Index Improvement to Reduce Methane Emissions from Rice Paddies. Glob. Chang. Biol. 2019, 25, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, S.; Zhang, Y.; Li, T.; Ge, H.; Xia, S.; Gu, J.; Zhang, H.; Lü, B.; Wu, X.; et al. Rice Root Morphological and Physiological Traits Interaction with Rhizosphere Soil and Its Effect on Methane Emissions in Paddy Fields. Soil Biol. Biochem. 2019, 129, 191–200. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Wassmann, R.; Bueno, C.; Rennenberg, H. Impact of Root Exudates of Different Cultivars and Plant Development Stages of Rice (Oryza sativa L.) on Methane Production in a Paddy Soil. Plant Soil 2001, 230, 77–86. [Google Scholar] [CrossRef]
- Liu, L.; Xiong, Y.; Bian, J.; Zhang, H.; Gu, J.; Wang, Z.; Yang, J. Effect of Genetic Improvement of Grain Yield and Nitrogen Efficiency of Mid-Season Indica Rice Cultivars. J. Plant Nutr. Soil Sci. 2015, 178, 297–305. [Google Scholar] [CrossRef]
- Liu, K.; Li, T.; Chen, Y.; Huang, J.; Qiu, Y.; Li, S.; Wang, H.; Zhu, A.; Zhuo, X.; Yu, F.; et al. Effects of Root Morphology and Physiology on the Formation and Regulation of Large Panicles in Rice. Field Crops Res. 2020, 258, 107946. [Google Scholar] [CrossRef]
- Krüger, M.; Potthast, K.; Michalzik, B.; Tischer, A.; Küsel, K.; Deckner, F.F.K.; Herrmann, M. Drought and Rewetting Events Enhance Nitrate Leaching and Seepage-Mediated Translocation of Microbes from Beech Forest Soils. Soil Biol. Biochem. 2021, 154, 108153. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Y.; Zhang, W. Changes in Rice Yields in China since 1980 Associated with Cultivar Improvement, Climate and Crop Management. Field Crops Res. 2012, 136, 65–75. [Google Scholar] [CrossRef]
- Song, Y.; Wang, C.; Ren, G.; Zhao, Y.; Linderholm, H.W. The Relative Contribution of Climate and Cultivar Renewal to Shaping Rice Yields in China since 1981. Theor. Appl. Climatol. 2015, 120, 1–9. [Google Scholar] [CrossRef]
- Xin, L.; Li, X.; Tan, M. Temporal and Regional Variations of China’s Fertilizer Consumption by Crops during 1998-2008. J. Geogr. Sci. 2012, 22, 643–652. [Google Scholar] [CrossRef]
- Sun, W.; Huang, Y. Synthetic Fertilizer Management for China’s Cereal Crops Has Reduced N2O Emissions since the Early 2000s. Environ. Pollut. 2012, 160, 24–27. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Yang, L. Response of Rice Yield Traits to Elevated Atmospheric CO2 Concentration and Its Interaction with Cultivar, Nitrogen Application Rate and Temperature: A Meta-Analysis of 20 Years FACE Studies. Sci. Total Environ. 2021, 764, 142797. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Du, Y.; Wu, C.; Liu, L.; Wang, Z.; Zhu, Q. Growth and Development Characteristics of Super-High-Yielding Mid-Season Japonica Rice. Front. Agric. China. 2007, 1, 166–174. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Y.; Wang, Z.; Yang, J.; Zhang, J. Morphological and Physiological Traits of Roots and Their Relationships with Shoot Growth in ‘Super’ Rice. Field Crops Res. 2009, 113, 31–40. [Google Scholar] [CrossRef]
- Li, G.; Hu, Q.; Shi, Y.; Cui, K.; Nie, L.; Huang, J.; Peng, S. Low Nitrogen Application Enhances Starch-Metabolizing Enzyme Activity and Improves Accumulation and Translocation of Non-Structural Carbohydrates in Rice Stems. Front. Plant Sci. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Van De Gon, H.D. Changes in CH4 Emission from Rice Fields from 1960 to 1990s 1. Impacts of Modern Rice Technology. Glob. Biogeochem. Cycles 2000, 14, 61–72. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, L.; Yan, X.; Tian, Y.; Deng, A.; Zhang, W. Super Rice Cropping Will Enhance Rice Yield and Reduce CH4 Emission: A Case Study in Nanjing, China. Rice Sci. 2013, 20, 427–433. [Google Scholar] [CrossRef]
- Liao, P.; Sun, Y.; Jiang, Y.; Zeng, Y.; Wu, Z.; Huang, S. Hybrid Rice Produces a Higher Yield and Emits Less Methane. Plant Soil Environ. 2019, 65, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Qiu, Q.; Lu, Y. Microbial Mechanism for Rice Variety Control on Methane Emission from Rice Field Soil. Glob. Chang. Biol. 2010, 16, 3085–3095. [Google Scholar] [CrossRef]
- Pump, J.; Pratscher, J.; Conrad, R. Colonization of Rice Roots with Methanogenic Archaea Controls Photosynthesis-Derived Methane Emission. Environ. Microbiol. 2015, 17, 2254–2260. [Google Scholar] [CrossRef]
- Maurer, D.; Kiese, R.; Kreuzwieser, J.; Rennenberg, H. Processes That Determine the Interplay of Root Exudation, Methane Emission and Yield in Rice Agriculture. Plant Biol. 2018, 20, 951–955. [Google Scholar] [CrossRef]
- Xiao, M.; Zang, H.; Ge, T.; Chen, A.; Zhu, Z.; Zhou, P.; Atere, C.T.; Wu, J.; Su, Y.; Kuzyakov, Y. Effect of Nitrogen Fertilizer on Rice Photosynthate Allocation and Carbon Input in Paddy Soil. Eur. J. Soil Sci. 2019, 70, 786–795. [Google Scholar] [CrossRef]
- Wang, B.; Neue, H.; Samonte, H. Effect of Cultivar Difference (‘IR72’, ‘IR65598’ and ‘Dular’) on Methane Emission. Agric. Ecosyst. Environ. 1997, 62, 31–40. [Google Scholar] [CrossRef]
- Jiang, Y.; van Groenigen, K.J.; Huang, S.; Hungate, B.A.; van Kessel, C.; Hu, S.; Zhang, J.; Wu, L.; Yan, X.; Wang, L.; et al. Higher Yields and Lower Methane Emissions with New Rice Cultivars. Glob. Chang. Biol. 2017, 23, 4728–4738. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R. Microbial Ecology of Methanogens and Methanotrophs. Adv. Agron. 2007, 96, 1–63. [Google Scholar]
- Zhang, H.; Liu, H.; Hou, D.; Zhou, Y.; Liu, M.; Wang, Z.; Liu, L.; Gu, J.; Yang, J. The Effect of Integrative Crop Management on Root Growth and Methane Emission of Paddy Rice. Crop J. 2019, 7, 444–457. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X. Root-Induced Changes in Radial Oxygen Loss, Rhizosphere Oxygen Profile, and Nitrification of Two Rice Cultivars in Chinese Red Soil Regions. Plant Soil 2013, 365, 115–126. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.; Huang, J.; Qiu, Y.; Li, S.; Zhuo, X.; Yu, F.; Gao, J.; Li, G.; Zhang, W.; et al. Spikelet Differentiation and Degeneration in Rice Varieties with Different Panicle Sizes. Food Energy Secur. 2021, 1–18. [Google Scholar] [CrossRef]
- Qi, D.; Hu, T.; Song, X.; Zhang, M. Effect of Nitrogen Supply Method on Root Growth and Grain Yield of Maize under Alternate Partial Root-Zone Irrigation. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]


| Cultivar Type | Cultivar | Release Year | Panicle Differentiation Stage (Days) | Growth Duration (Days) | Proportion of PD in GD (%) |
|---|---|---|---|---|---|
| 1950’s | Huangkezao | 1952 | 33 | 113 | 29.2 |
| Guihuaqiu | 1958 | 29 | 120 | 24.2 | |
| Average | 31.0 b | 116.5 bc | 26.7 b | ||
| 1960’s | Jinnanfeng | 1960 | 35 | 118 | 29.7 |
| Guihuahuang | 1963 | 34 | 118 | 28.8 | |
| Average | 34.5 a | 118 bc | 29.2 a | ||
| 1970’s | Liming | 1970 | 29 | 115 | 25.2 |
| Xudao2 | 1979 | 32 | 114 | 28.1 | |
| Average | 30.5 b | 114.5 c | 26.6 b | ||
| 1980’s | Yanjing2 | 1983 | 29 | 116 | 25.0 |
| Sidao8 | 1986 | 32 | 115 | 27.8 | |
| Average | 30.5 b | 115.5 c | 26.4 b | ||
| 1990’s | Zhendao88 | 1997 | 33 | 118 | 28.0 |
| Huaidao5 | 1999 | 30 | 125 | 24.0 | |
| Average | 31.5 b | 121.5 ab | 26.0 bc | ||
| 2000’s | Huaidao9 | 2006 | 30 | 122 | 24.6 |
| Lianjing7 | 2010 | 31 | 125 | 24.8 | |
| Average | 30.5 b | 123.5 a | 24.7 c | ||
| 2010’s | Wuyunjing27 | 2012 | 33 | 126 | 26.2 |
| Nanjing9108 | 2013 | 31 | 123 | 25.2 | |
| Average | 32.0 ab | 124.5 a | 25.7 bc |
| Cultivar Type | Cultivar | Panicle Number (×104 ha−1) | Spikelets per Panicle | Total Numbers of Spikelets (×106 ha−1) | 1000-Grain Weight (g) | Filled Grains (%) | Yield (t ha−1) |
|---|---|---|---|---|---|---|---|
| 1950’s | Huangkezao | 288.3 | 116.5 | 335.9 | 25.6 | 54.0 | 4.6 |
| Guihuaqiu | 254.3 | 118.5 | 301.3 | 26.0 | 72.8 | 5.7 | |
| Average | 271.3 b | 117.5 de | 318.6 f | 25.8 a | 63.4 f | 5.2 f | |
| 1960’s | Jinnanfeng | 286.9 | 117.8 | 338.0 | 24.6 | 65.8 | 5.5 |
| Guihuahuang | 273.6 | 122.6 | 335.4 | 25.0 | 81.3 | 6.8 | |
| Average | 280.3 ab | 120.2 d | 336.7 d | 24.8 bc | 73.6 e | 6.1 e | |
| 1970’s | Liming | 304.3 | 122.5 | 372.8 | 25.5 | 80.9 | 7.7 |
| Xudao2 | 268.7 | 121.2 | 325.7 | 25.5 | 86.2 | 7.2 | |
| Average | 286.5 ab | 121.9 cd | 349.2 e | 25.5 ab | 83.5 cd | 7.4 d | |
| 1980’s | Yanjing2 | 302.4 | 123.4 | 373.2 | 25.6 | 84.3 | 8.1 |
| Sidao8 | 315.8 | 128.8 | 406.8 | 24.4 | 86.6 | 8.6 | |
| Average | 309.1 a | 126.1 c | 389.9 c | 25.0 bc | 85.5 b | 8.3 c | |
| 1990’s | Zhendao88 | 287.9 | 141.8 | 408.1 | 25.7 | 91.2 | 9.6 |
| Huaidao5 | 274.5 | 148.3 | 407.1 | 25.2 | 89.5 | 9.2 | |
| Average | 281.2 ab | 145.0 b | 407.6 b | 25.5 ab | 90.4 a | 9.4 ab | |
| 2000’s | Huaidao9 | 276.4 | 169.6 | 468.8 | 25.2 | 85.3 | 10.1 |
| Lianjing7 | 284.6 | 165.6 | 471.3 | 25.6 | 84.4 | 10.2 | |
| Average | 280.5 ab | 167.6 a | 470.0 a | 25.4 ab | 84.9 bc | 10.1 a | |
| 2010’s | Wuyunjing27 | 290.3 | 160.7 | 466.6 | 25.1 | 87.3 | 10.2 |
| Nanjing9108 | 262.1 | 162.0 | 425.0 | 26.2 | 88.4 | 9.8 | |
| Average | 276.2 ab | 161.4 a | 445.6 a | 25.7 a | 87.9 ab | 10.0 a |
| Cultivar Type | Cultivar | Shoot Biomass (g m−2) | Root Biomass (g m−2) | Root/Shoot Ratio | Harvest Index | |||
|---|---|---|---|---|---|---|---|---|
| Booting | Heading | Booting | Heading | Booting | Heading | |||
| 1950’s | Huangkezao | 303.5 | 545.5 | 45.5 | 49.6 | 0.15 | 0.09 | 0.45 |
| Guihuaqiu | 353.7 | 575.8 | 51.6 | 57.0 | 0.15 | 0.10 | 0.47 | |
| Average | 328.2 e | 561.3 d | 48.6 d | 53.3 d | 0.15 c | 0.10 d | 0.46 c | |
| 1960’s | Jinnanfeng | 337.5 | 613.8 | 52.4 | 57.7 | 0.16 | 0.09 | 0.47 |
| Guihuahuang | 406.7 | 684.9 | 62.9 | 69.9 | 0.15 | 0.10 | 0.45 | |
| Average | 372.0 d | 650.8 c | 57.7 c | 63.8 c | 0.16 c | 0.10 d | 0.46 c | |
| 1970’s | Liming | 406.5 | 684.0 | 66.5 | 72.5 | 0.16 | 0.11 | 0.46 |
| Xudao2 | 374.8 | 596.5 | 60.6 | 68.0 | 0.16 | 0.11 | 0.46 | |
| Average | 390.7 d | 638.6 c | 63.6 c | 70.3 c | 0.16 c | 0.11 c | 0.46 c | |
| 1980’s | Yanjing2 | 593.2 | 859.8 | 110.5 | 119.5 | 0.19 | 0.14 | 0.51 |
| Sidao8 | 607.4 | 925.6 | 114.4 | 121.2 | 0.19 | 0.13 | 0.48 | |
| Average | 600.4 bc | 891.7 b | 112.5 b | 120.4 b | 0.19 b | 0.14 b | 0.49 b | |
| 1990’s | Zhendao88 | 596.1 | 923.9 | 118.4 | 127.5 | 0.20 | 0.14 | 0.51 |
| Huaidao5 | 569.3 | 849.7 | 114.2 | 124.1 | 0.20 | 0.15 | 0.49 | |
| Average | 582.6 c | 885.8 b | 116.3 b | 125.8 b | 0.20 b | 0.14 ab | 0.50 ab | |
| 2000’s | Huaidao9 | 604.9 | 1005.4 | 136.9 | 148.8 | 0.23 | 0.15 | 0.52 |
| Lianjing7 | 635.1 | 1029.4 | 147.6 | 158.5 | 0.23 | 0.15 | 0.51 | |
| Average | 620.2 ab | 1017.6 a | 142.2 a | 153.7 a | 0.23 a | 0.15 a | 0.51 ab | |
| 2010’s | Wuyunjing27 | 657.1 | 1100.6 | 149.8 | 162.1 | 0.23 | 0.15 | 0.52 |
| Nanjing9108 | 638.1 | 1002.7 | 147.6 | 159.1 | 0.23 | 0.16 | 0.52 | |
| Average | 647.5 a | 1049.7 a | 148.7 a | 160.6 a | 0.23 a | 0.15 a | 0.52 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Chen, L.; Han, X.; Yang, K.; Liu, K.; Wang, J.; Chen, Y.; Liu, L. Rice Cultivar Renewal Reduces Methane Emissions by Improving Root Traits and Optimizing Photosynthetic Carbon Allocation. Agriculture 2022, 12, 2134. https://doi.org/10.3390/agriculture12122134
Li S, Chen L, Han X, Yang K, Liu K, Wang J, Chen Y, Liu L. Rice Cultivar Renewal Reduces Methane Emissions by Improving Root Traits and Optimizing Photosynthetic Carbon Allocation. Agriculture. 2022; 12(12):2134. https://doi.org/10.3390/agriculture12122134
Chicago/Turabian StyleLi, Siyu, Lu Chen, Xian Han, Kai Yang, Kun Liu, Jun Wang, Yun Chen, and Lijun Liu. 2022. "Rice Cultivar Renewal Reduces Methane Emissions by Improving Root Traits and Optimizing Photosynthetic Carbon Allocation" Agriculture 12, no. 12: 2134. https://doi.org/10.3390/agriculture12122134
APA StyleLi, S., Chen, L., Han, X., Yang, K., Liu, K., Wang, J., Chen, Y., & Liu, L. (2022). Rice Cultivar Renewal Reduces Methane Emissions by Improving Root Traits and Optimizing Photosynthetic Carbon Allocation. Agriculture, 12(12), 2134. https://doi.org/10.3390/agriculture12122134







