Ultrasound-Assisted Extraction of Phenolic Compounds from Different Maturity Stages and Fruit Parts of Cordia dodecandra A. DC.: Quantification and Identification by UPLC-DAD-ESI-MS/MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemical Reagents
2.2. Physicochemical Characteristics
2.3. Ciricote Fruit Pretreatment and Ultrasound-Assisted Extraction (UAE) of Phenolic Compounds
2.4. Determination of Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Antioxidant Activity
2.5. Phenolic Compounds Analysis by UPLC-DAD and Spectral Mass Analysis by ESI-MS/MS
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics in Fresh Ciricote Fruit at Three Stages of Maturity
3.2. TPC, TFC, and Antioxidant Activity of Ciricote Fruit Parts at Different Stages of Maturity
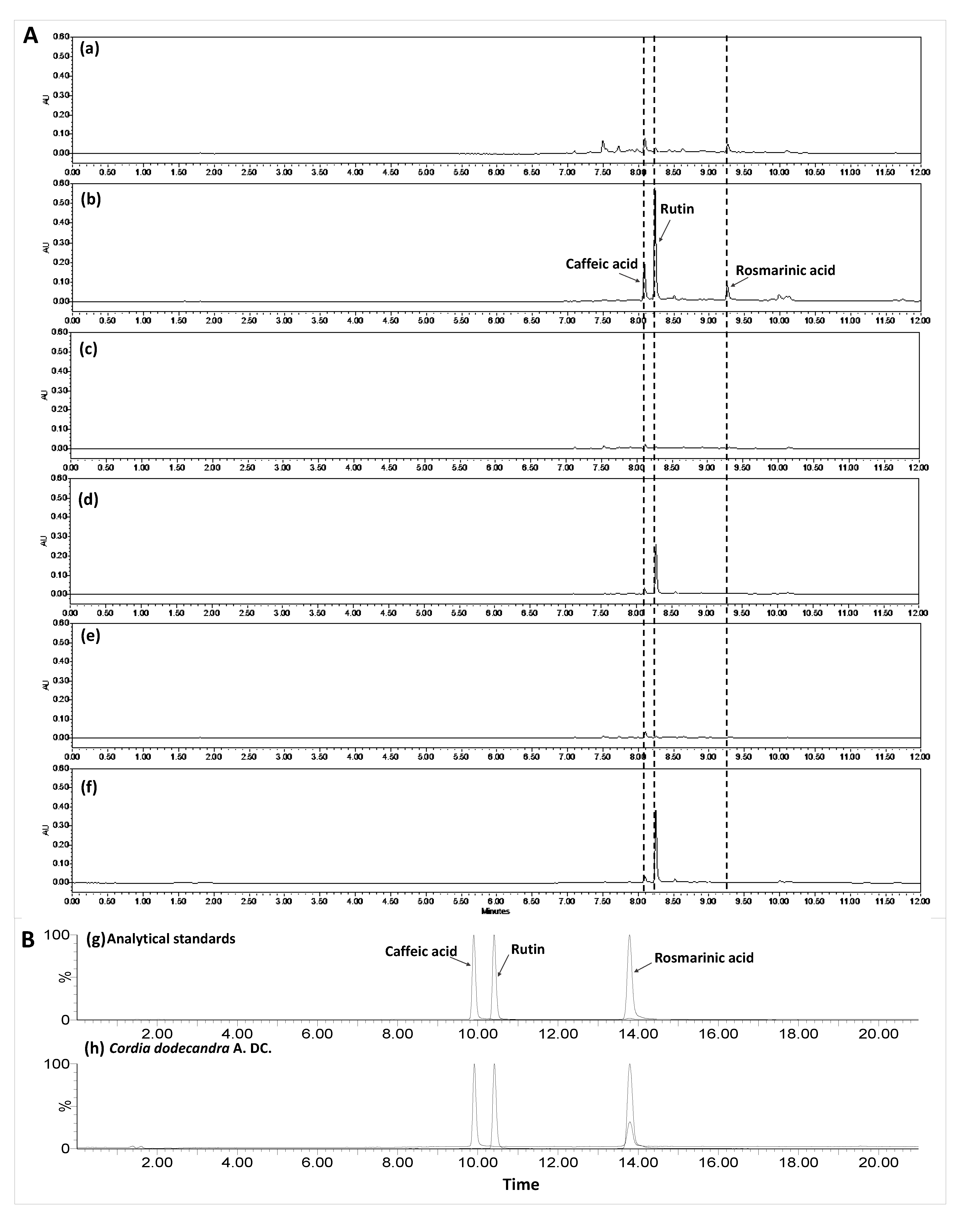
3.3. Phenolic Compound Identification and Quantification by UPLC-PDA-ESI-MS/MS in Extracts of Ciricote Fruit
3.4. Correlation between TPC, TFC, Antioxidant Activity, and Individual Phenolic Compounds
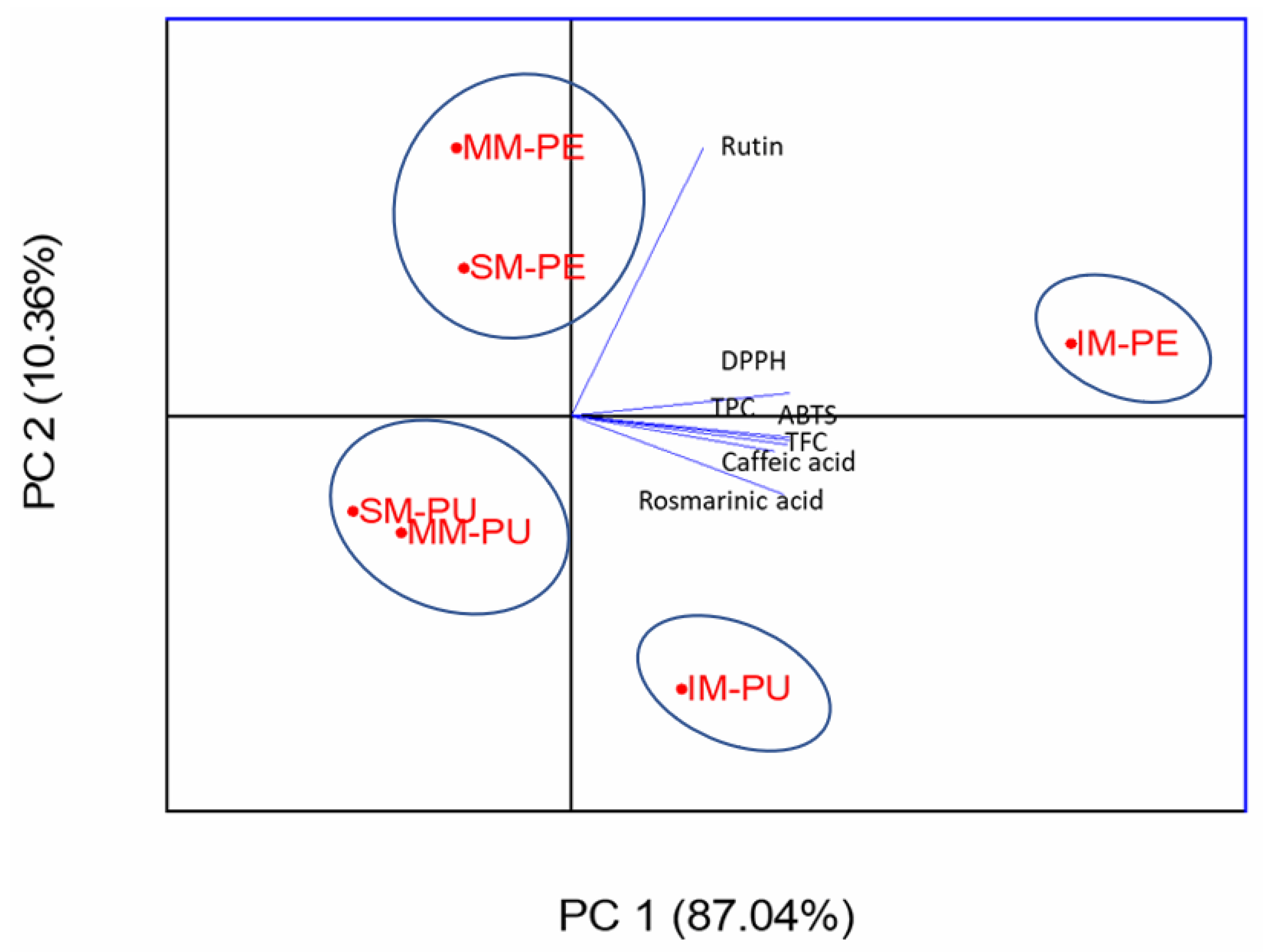
3.5. Principal Components Analysis of Variation of Stages of Maturity and Parts of Ciricote Fruit with TPC, TFC, Antioxidant Activity, and Phenolic Compounds
3.6. Effects of the Diameter of the Ultrasonic Probe in Extracts of Fruit
4. Discussion
4.1. Physicochemical Characteristics in Fresh Ciricote Fruit at Three Stages of Maturity
4.2. TPC, TFC, and Antioxidant Activity of Ciricote Fruit Parts at Different Stages of Maturity
4.3. Phenolic Compounds Identified by UPLC-DAD-ESI-MS/MS in Extracts of Ciricote Fruit
4.4. Correlation between TPC, TFC, Antioxidant Activity, and Individual Phenolic Compounds
4.5. Principal Components Analysis of Variation of Stages of Maturity and Parts of Ciricote Fruit with TPC, TFC, Antioxidant Activity, and Phenolic Compounds
4.6. Effects of the Diameter of the Ultrasonic Probe in Extracts of Fruit
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Janick, J.; Paull, R.E. The Encyclopedia of Fruits and Nuts; CABI: Wallingford, UK, 2013; ISBN 9788578110796. [Google Scholar]
- Chivenge, P.; Mabhaudhi, T.; Modi, A.T.; Mafongoya, P. The Potential Role of Neglected and Underutilised Crop Species as Future Crops under Water Scarce Conditions in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2015, 12, 5685–5711. [Google Scholar] [CrossRef] [PubMed]
- Can-Cauich, C.A.; Sauri-Duch, E.; Betancur-Ancona, D.; Chel-Guerrero, L.; González-Aguilar, G.A.; Cuevas-Glory, L.F.; Pérez-Pacheco, E.; Moo-Huchin, V.M. Tropical Fruit Peel Powders as Functional Ingredients: Evaluation of Their Bioactive Compounds and Antioxidant Activity. J. Funct. Foods 2017, 37, 501–506. [Google Scholar] [CrossRef]
- Pacheco, N.; Méndez-Campos, G.K.; Herrera-Pool, I.E.; Alvarado-López, C.J.; Ramos-Díaz, A.; Ayora-Talavera, T.; Talcott, S.U.; Cuevas-Bernardino, J.C. Physicochemical Composition, Phytochemical Analysis and Biological Activity of Ciricote (Cordia dodecandra A. D.C.) Fruit from Yucatán. Nat. Prod. Res. 2022, 36, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Kewlani, P.; Singh, L.; Belwal, T.; Bhatt, I.D. Optimization of Ultrasonic-Assisted Extraction for Bioactive Compounds in Rubus ellipticus Fruits: An Important Source for Nutraceutical and Functional Foods. Sustain. Chem. Pharm. 2022, 25, 100603. [Google Scholar] [CrossRef]
- Yahia, E.M.; Carrillo-Lopez, A. Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Cambridgeshire, UK, 2018; 476p. [Google Scholar]
- Medina-Torres, N.; Espinosa-Andrews, H.; Trombotto, S.; Ayora-Talavera, T.; Patrón-Vázquez, J.; González-Flores, T.; Sánchez-Contreras, Á.; Cuevas-Bernardino, J.C.; Pacheco, N. Ultrasound-Assisted Extraction Optimization of Phenolic Compounds from Citrus latifolia Waste for Chitosan Bioactive Nanoparticles Development. Molecules 2019, 24, 3541. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Zhao, W.H.; Shi, Y.P. Comprehensive Analysis of Phenolic Compounds in Four Varieties of Goji Berries at Different Ripening Stages by UPLC–MS/MS. J. Food Compos. Anal. 2022, 106, 104279. [Google Scholar] [CrossRef]
- Herrera-Pool, E.; Ramos-Díaz, A.L.; Lizardi-Jiménez, M.A.; Pech-Cohuo, S.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; García-Cruz, U.; Pacheco, N. Effect of Solvent Polarity on the Ultrasound Assisted Extraction and Antioxidant Activity of Phenolic Compounds from Habanero Pepper Leaves (Capsicum chinense) and Its Identification by UPLC-PDA-ESI-MS/MS. Ultrason. Sonochem. 2021, 76, 105658. [Google Scholar] [CrossRef]
- Sánchez-Recillas, A.; Rivero-Medina, L.; Ortiz-Andrade, R.; Araujo-León, J.A.; Flores-Guido, J.S. Airway Smooth Muscle Relaxant Activity of Cordia dodecandra A. DC. Mainly by CAMP Increase and Calcium Channel Blockade. J. Ethnopharmacol. 2019, 229, 280–287. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Moo-Huchin, M.I.; Estrada-León, R.J.; Cuevas-Glory, L.; Estrada-Mota, I.A.; Ortiz-Vázquez, E.; Betancur-Ancona, D.; Sauri-Duch, E. Antioxidant Compounds, Antioxidant Activity and Phenolic Content in Peel from Three Tropical Fruits from Yucatan, Mexico. Food Chem. 2015, 166, 17–22. [Google Scholar] [CrossRef]
- Hunter, D.; Borelli, T.; Beltrame, D.M.O.; Oliveira, C.N.S.; Coradin, L.; Wasike, V.W.; Wasilwa, L.; Mwai, J.; Manjella, A.; Samarasinghe, G.W.L.; et al. The Potential of Neglected and Underutilized Species for Improving Diets and Nutrition. Planta 2019, 250, 709–729. [Google Scholar] [CrossRef] [PubMed]
- Monroy Cárdenas, D.M.; Cardona, W.A.; García Muñoz, M.C.; Bolaños Benavides, M.M. Relationship between Variable Doses of N, P, K and Ca and the Physicochemical and Proximal Characteristics of Andean Blackberry (Rubus glaucus Benth.). Sci. Hortic. 2019, 256, 108528. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis; Horwitz, W., Latimer, G., Eds.; AOAC International: Rockville, Maryland, 2005. [Google Scholar]
- Xu, X.; Lu, X.; Tang, Z.; Zhang, X.; Lei, F.; Hou, L.; Li, M. Combined Analysis of Carotenoid Metabolites and the Transcriptome to Reveal the Molecular Mechanism Underlying Fruit Colouration in Zucchini (Cucurbita pepo L.). Food Chem. Mol. Sci. 2021, 2, 100021. [Google Scholar] [CrossRef]
- Manera, F.J.; Legua, P.; Melgarejo, P.; Brotons, J.M.; Hernández, F.C.A.; Martínez, J.J. Determination of a Colour Index for Fruit of Pomegranate Varietal Group “Mollar de Elche”. Sci. Hortic. 2013, 150, 360–364. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Cuevas-Bernardino, J.C.; Ayora-Talavera, T.; Patrón-Vázquez, J.A.; Rodríguez-Buenfil, I.; Pacheco, N. Changes in the Physicochemical, Rheological, Biological, and Sensorial Properties of Habanero Chili Pastes Affected by Ripening Stage, Natural Preservative and Thermal Processing. Rev. Mex. Ing. Química 2021, 20, 195–212. [Google Scholar] [CrossRef]
- Color-hex. 2021. Available online: https://color-hex.org (accessed on 2 September 2021).
- Sonics & Materials, Inc. Stepped Microtips and Probes. Optional Accesories; Sonics & Materials, Inc.: Newtown, CT, USA, 2021. [Google Scholar]
- Medina-Torres, N.; Covarrubias-Cárdenas, A.; Herrera-Pool, E.; Espinosa-Andrews, H.; Trombotto, S.; Ayora-Talavera, T.; Pacheco-López, N. Evaluation of the Formation Conditions and Physicochemical Characterization of Chitosan Nanoparticles. J. Bioeng. Biomed. Res. 2018, 2, 9–16. [Google Scholar]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-Chemical and Bioactive Properties of Different Floral Origin Honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Ana, C.C.; Jesús, P.V.; Hugo, E.A.; Teresa, A.T.; Ulises, G.C.; Neith, P. Antioxidant Capacity and UPLC–PDA ESI–MS Polyphenolic Profile of Citrus aurantium Extracts Obtained by Ultrasound Assisted Extraction. J. Food Sci. Technol. 2018, 55, 5106–5114. [Google Scholar] [CrossRef]
- Alonso-Carrillo, N.; Aguilar-Santamaría, M.d.L.Á.; Vernon-Carter, E.J.; Jiménez-Alvarado, R.; Cruz-Sosa, F.; Román-Guerrero, A. Extraction of Phenolic Compounds from Satureja macrostema Using Microwave-Ultrasound Assisted and Reflux Methods and Evaluation of Their Antioxidant Activity and Cytotoxicity. Ind. Crop. Prod. 2017, 103, 213–221. [Google Scholar] [CrossRef]
- Pati, S.; Losito, I.; Palmisano, F.; Zambonin, P.G. Characterization of Caffeic Acid Enzymatic Oxidation By-Products by Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry. J. Chromatogr. A 2006, 1102, 184–192. [Google Scholar] [CrossRef]
- Kicel, A.; Owczarek, A.; Michel, P.; Skalicka-Woźniak, K.; Kiss, A.K.; Olszewska, M.A. Application of HPCCC, UHPLC-PDA-ESI-MS3 and HPLC-PDA Methods for Rapid, One-Step Preparative Separation and Quantification of Rutin in Forsythia Flowers. Ind. Crop. Prod. 2015, 76, 86–94. [Google Scholar] [CrossRef]
- Mariappan, G.; Sundaraganesan, N.; Manoharan, S. Experimental and Theoretical Spectroscopic Studies of Anticancer Drug Rosmarinic Acid Using HF and Density Functional Theory. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, I.; Gokbulut, I.; Bilenler, T.; Sislioglu, K.; Ozdemir, I.S.; Bahar, B.; Çelik, B.; Seyhan, F. Effect of Fruit Maturity Level on Quality, Sensory Properties and Volatile Composition of Two Common Apricot (Prunus armeniaca L.) Varieties. J. Food Sci. Technol. 2018, 55, 2671–2678. [Google Scholar] [CrossRef]
- Ribeiro, B.S.; de Freitas, S.T. Maturity Stage at Harvest and Storage Temperature to Maintain Postharvest Quality of Acerola Fruit. Sci. Hortic. 2020, 260, 108901. [Google Scholar] [CrossRef]
- Gull, J.; Sultana, B.; Anwar, F.; Naseer, R.; Ashraf, M.; Ashrafuzzaman, M. Variation in Antioxidant Attributes at Three Ripening Stages of Guava (Psidium guajava L.) Fruit from Different Geographical Regions of Pakistan. Molecules 2012, 17, 3165–3180. [Google Scholar] [CrossRef] [PubMed]
- Moo-Huchin, V.M.; Estrada-Mota, I.; Estrada-León, R.; Cuevas-Glory, L.; Ortiz-Vázquez, E.; De Lourdes Vargas Y Vargas, M.; Betancur-Ancona, D.; Sauri-Duch, E. Determination of Some Physicochemical Characteristics, Bioactive Compounds and Antioxidant Activity of Tropical Fruits from Yucatan, Mexico. Food Chem. 2014, 152, 508–515. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; de Jesus Filho, M.; da Silva, L.C.; da Silva Constant, L.; Filho, J.T.; Wagner, R.; Godoy, H.T. Chlorogenic and Caffeic Acids in 64 Fruits Consumed in Brazil. Food Chem. 2019, 286, 51–63. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Zhou, D.; Rakariyatham, N.; Shahidi, F. Sapindaceae (Dimocarpus longan and Nephelium lappaceum) Seed and Peel by-Products: Potential Sources for Phenolic Compounds and Use as Functional Ingredients in Food and Health Applications. J. Funct. Foods 2020, 67, 103846. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of Phenolic Compounds vs. Variety, Part of Apple and Cultivation Model, Extraction of Phenolic Compounds, Biological Properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Multari, S.; Licciardello, C.; Caruso, M.; Martens, S. Monitoring the Changes in Phenolic Compounds and Carotenoids Occurring during Fruit Development in the Tissues of Four Citrus Fruits. Food Res. Int. 2020, 134, 109228. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Gaonkar, A.A.; Payamalle, S. Evaluation of Chemical Composition and Antioxidant Activity of Cordia myxa Fruit Pulp. J. Herbs Spices Med. Plants 2019, 25, 192–201. [Google Scholar] [CrossRef]
- Marini, G.; Graikou, K.; Zengin, G.; Karikas, G.A.; Gupta, M.P.; Chinou, I. Phytochemical Analysis and Biological Evaluation of Three Selected Cordia Species from Panama. Ind. Crop. Prod. 2018, 120, 84–89. [Google Scholar] [CrossRef]
- Viveros-Valdez, E.; Jaramillo-Mora, C.; Oranday-Cárdenas, A.; Morán-Martínez, J.; Carranza-Rosales, P. Antioxidant, Cytotoxic and Alpha-Glucosidase Inhibition Activities from the Mexican Berry “Anacahuita” (Cordia Boissieri). Arch. Latinoam. Nutr. 2016, 656, 211–218. [Google Scholar]
- Wang, B.; Huang, Q.; Venkitasamy, C.; Chai, H.; Gao, H.; Cheng, N.; Cao, W.; Lv, X.; Pan, Z. Changes in Phenolic Compounds and Their Antioxidant Capacities in Jujube (Ziziphus jujuba Miller) during Three Edible Maturity Stages. LWT 2016, 66, 56–62. [Google Scholar] [CrossRef]
- Yingyuen, P.; Sukrong, S.; Phisalaphong, M. Isolation, Separation and Purification of Rutin from Banana Leaves (Musa balbisiana). Ind. Crop. Prod. 2020, 149, 112307. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Jediyi, H.; Naamani, K.; Ait Elkoch, A.; Dihazi, A.; El Alaoui El Fels, A.; Arkize, W. First Study on Technological Maturity and Phenols Composition during the Ripeness of Five Vitis vinifera L Grape Varieties in Morocco. Sci. Hortic. 2019, 246, 390–397. [Google Scholar] [CrossRef]
- Silva, E.K.; Saldaña, M.D.A. High-Intensity Ultrasound-Assisted Recovery of Cinnamyl Alcohol Glycosides from Rhodiola rosea Roots: Effect of Probe Diameter on the Ultrasound Energy Performance for the Extraction of Bioactive Compounds. Food Bioprod. Process. 2020, 122, 245–253. [Google Scholar] [CrossRef]
| IM | SM | MM | |
|---|---|---|---|
| Moisture (%) | |||
| Pulp (PU) | 87.47 ± 0.59 a | 82.21 ± 1.40 b | 83.17 ± 0.65 b |
| Peel (PE) | 86.01 ± 0.47 a | 82.76 ± 0.47 b | 82.93 ± 0.98 b |
| Weight (g) | 34.50 ± 5.49 b | 41.14 ± 7.22 a | 40.33 ± 3.49 a |
| Color | |||
| L* | 45.03 ± 2.84 a | 52.30 ± 6.76 b | 62.33 ± 4.24 b |
| a* | −9.18 ± 0.64 a | 3.58 ± 1.62 b | 11.78 ± 2.20 c |
| b* | 26.73 ± 4.18 a | 42.48 ± 3.61 b | 52.05 ± 2.25 c |
| C* (Chroma) | 26.44 ± 1.87 c | 43.53 ± 3.98 b | 53.04 ± 3.12 a |
| (Hue) Angle | 110.47 ± 1.26 a | 84.70 ± 1.78 b | 77.48 ± 2.24 c |
| CCI | −7.81 ± 1.49 a | 1.60 ± 0.68 b | 3.61 ± 0.46 c |
| RGB |  |  |  |
| TSS (°Brix) | 8.00 ± 1.00 c | 11.00 ± 1.00 b | 15.00 ± 1.00 a |
| pH | 5.73 ± 0.02 a | 5.70 ± 0.03 a | 5.63 ± 0.02 a |
| Firmness (N) | 50.43 ± 11.47 a | 13.24 ± 1.47 b | 8.15 ± 0.61 b |
| TA (as % citric acid) | 0.13 ± 0.01 a | 0.09 ± 0.01 b | 0.11 ± 0.01 b |
| Maturity index | 60.18 ± 9.90 c | 115.70 ± 3.89 b | 138.34 ± 8.87 a |
| PF | TPC (mg GAE g−1 dw) | TFC (mg RE g−1 dw) | DPPH (µM TE g−1 dw) | ABTS (µM TE g−1 dw) | ||
|---|---|---|---|---|---|---|
| IM | PU | 10.66 ± 0.67 b,B | 9.82 ± 0.28 b | 54.48 ± 3.088 b,B | 55.04 ± 8.40 b,B | |
| PE | 15.62 ± 1.77 a,A | 14.23 ± 2.01 a | 82.39 ± 10.00 a,A | 86.94 ± 4.20 a,A | ||
| SM | PU | 5.32 ± 0.43 c,C | 2.47 ± 0.41 d | 32.69 ± 0.80 c,D | 19.22 ± 2.95 c,CD | |
| UAE | PE | 6.61 ± 0.71 c,C | 5.56 ± 0.72 c | 44.30 ± 1.26 bc,BC | 27.97 ± 1.03 c,C | |
| MM | PU | 5.76 ± 0.93 c,C | 3.82 ± 0.30 cd | 36.12 ± 2.64 c,CD | 18.93 ± 0.70 c,CD | |
| PE | 6.54 ± 0.68 c,C | 4.29 ± 2.20 cd | 41.23 ± 1.23 c,CD | 25.42 ± 0.84 c,CD | ||
| MAC | IM | PU | 5.79 ± 0.28 b,C | NA | 14.11 ± 1.16 b,E | 16.82 ± 1.25 b,D |
| PE | 1.42 ± 0.07 a,D | NA | 3.46 ± 0.28 a,E | 4.13 ± 0.31 a,E |
| CN | RT | PDA UV(λmax) | [M-H]- | (m/z) | TI | Concentrations (µmol g−1 dw) | |||
|---|---|---|---|---|---|---|---|---|---|
| PF | IM | SM | MM | ||||||
| 1 | 9.83 | 217 323 | 179 | 135 | Caffeic acid | PU | 1.24 ± 0.03 b | 0.65 ± 0.00 c | 1.18 ± 0.03 b |
| PE | 3.03 ± 0.037 a | 0.49 ± 0.00 c | 0.56 ± 0.04 c | ||||||
| 2 | 10.96 | 209 255 353 | 609 | 300 301 271 254 243 | Rutin | PU | NQ | NQ | NQ |
| PE | 3.35 ± 0.04 a | 1.73 ± 0.02 c | 2.52 ± 0.01 b | ||||||
| 3 | 15.97 | 219 318 | 359 | 197 179 161 132 135 | Rosmarinic acid | PU | 0.59 ± 0.11 b | NQ | NQ |
| PE | 0.93 ± 0.06 a | NQ | NQ | ||||||
| Compound | Molecular Weight (uma) | Retention Time (min) | Transition | Parent (m/z) | Daughter (m/z) | Dwell (secs) | Cone Voltaje (eV) | Collision Energy |
|---|---|---|---|---|---|---|---|---|
| Caffeic acid | 180 | 9.91 | Qualification | 179 | 135 | 0.045 | 14 | 18 |
| Caffeic acid | 180 | 9.91 | Quantification | 179 | 79 | 0.045 | 14 | 30 |
| Rutin | 610 | 10.41 | Qualification | 609 | 300 | 0.045 | 100 | 54 |
| Rutin | 610 | 10.41 | Quantification | 609 | 271 | 0.045 | 100 | 76 |
| Rosmarinic acid | 359 | 13.79 | Qualification | 359 | 197 | 0.045 | 50 | 22 |
| Rosmarinic acid | 359 | 13.79 | Quantification | 359 | 161 | 0.045 | 50 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Morales, K.; Castañeda-Pérez, E.; Herrera-Pool, E.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; García-Cruz, U.; Pech-Cohuo, S.C.; Pacheco, N. Ultrasound-Assisted Extraction of Phenolic Compounds from Different Maturity Stages and Fruit Parts of Cordia dodecandra A. DC.: Quantification and Identification by UPLC-DAD-ESI-MS/MS. Agriculture 2022, 12, 2127. https://doi.org/10.3390/agriculture12122127
Jiménez-Morales K, Castañeda-Pérez E, Herrera-Pool E, Ayora-Talavera T, Cuevas-Bernardino JC, García-Cruz U, Pech-Cohuo SC, Pacheco N. Ultrasound-Assisted Extraction of Phenolic Compounds from Different Maturity Stages and Fruit Parts of Cordia dodecandra A. DC.: Quantification and Identification by UPLC-DAD-ESI-MS/MS. Agriculture. 2022; 12(12):2127. https://doi.org/10.3390/agriculture12122127
Chicago/Turabian StyleJiménez-Morales, Karina, Eduardo Castañeda-Pérez, Emanuel Herrera-Pool, Teresa Ayora-Talavera, Juan Carlos Cuevas-Bernardino, Ulises García-Cruz, Soledad Cecilia Pech-Cohuo, and Neith Pacheco. 2022. "Ultrasound-Assisted Extraction of Phenolic Compounds from Different Maturity Stages and Fruit Parts of Cordia dodecandra A. DC.: Quantification and Identification by UPLC-DAD-ESI-MS/MS" Agriculture 12, no. 12: 2127. https://doi.org/10.3390/agriculture12122127
APA StyleJiménez-Morales, K., Castañeda-Pérez, E., Herrera-Pool, E., Ayora-Talavera, T., Cuevas-Bernardino, J. C., García-Cruz, U., Pech-Cohuo, S. C., & Pacheco, N. (2022). Ultrasound-Assisted Extraction of Phenolic Compounds from Different Maturity Stages and Fruit Parts of Cordia dodecandra A. DC.: Quantification and Identification by UPLC-DAD-ESI-MS/MS. Agriculture, 12(12), 2127. https://doi.org/10.3390/agriculture12122127












