How the Composition of Substrates for Seedling Production Affects Earthworm Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Organism
2.2. Substrates
2.3. Acute Toxicity Test
2.4. Avoidance Tests with Earthworms
2.5. Biomarker Assessment
2.5.1. Chemicals
2.5.2. Sample Preparation
2.5.3. Enzymatic Activities Evaluation
2.6. Data Analysis
3. Results
3.1. Survival Rate
3.2. Behavior Assessment
3.2.1. First Set of Substrates
3.2.2. Second Set of Substrates
3.2.3. Third Set of Substrates
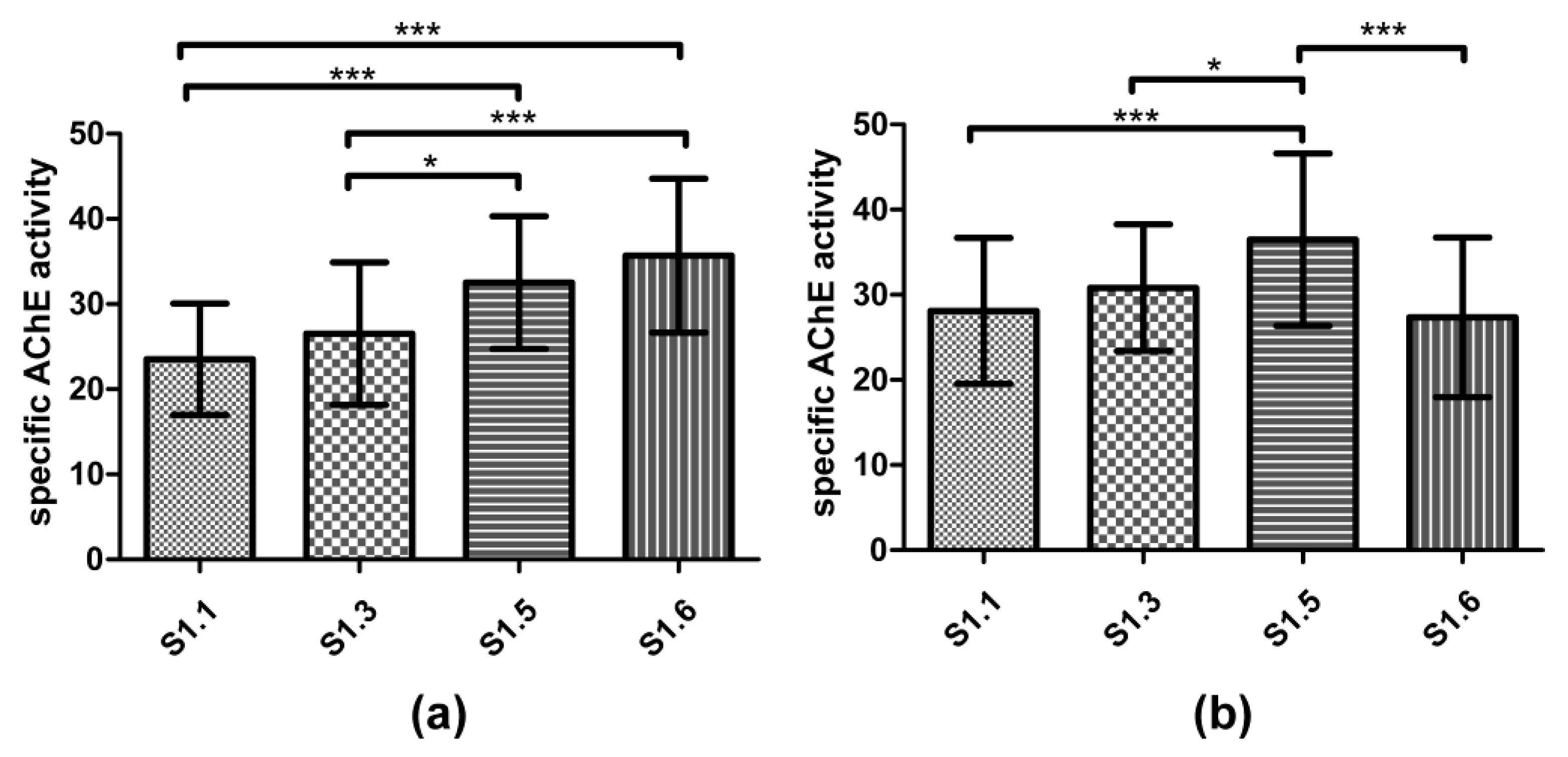
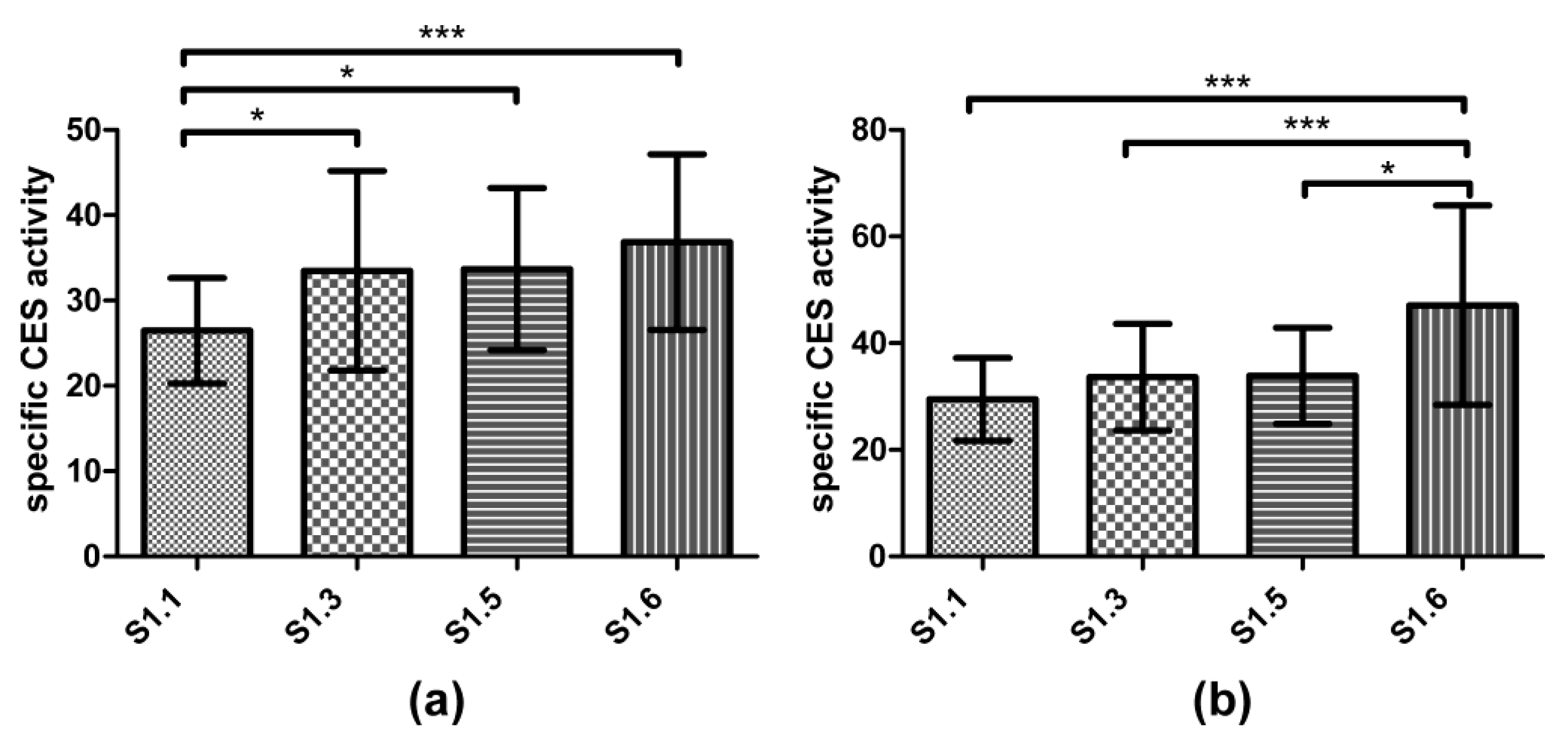
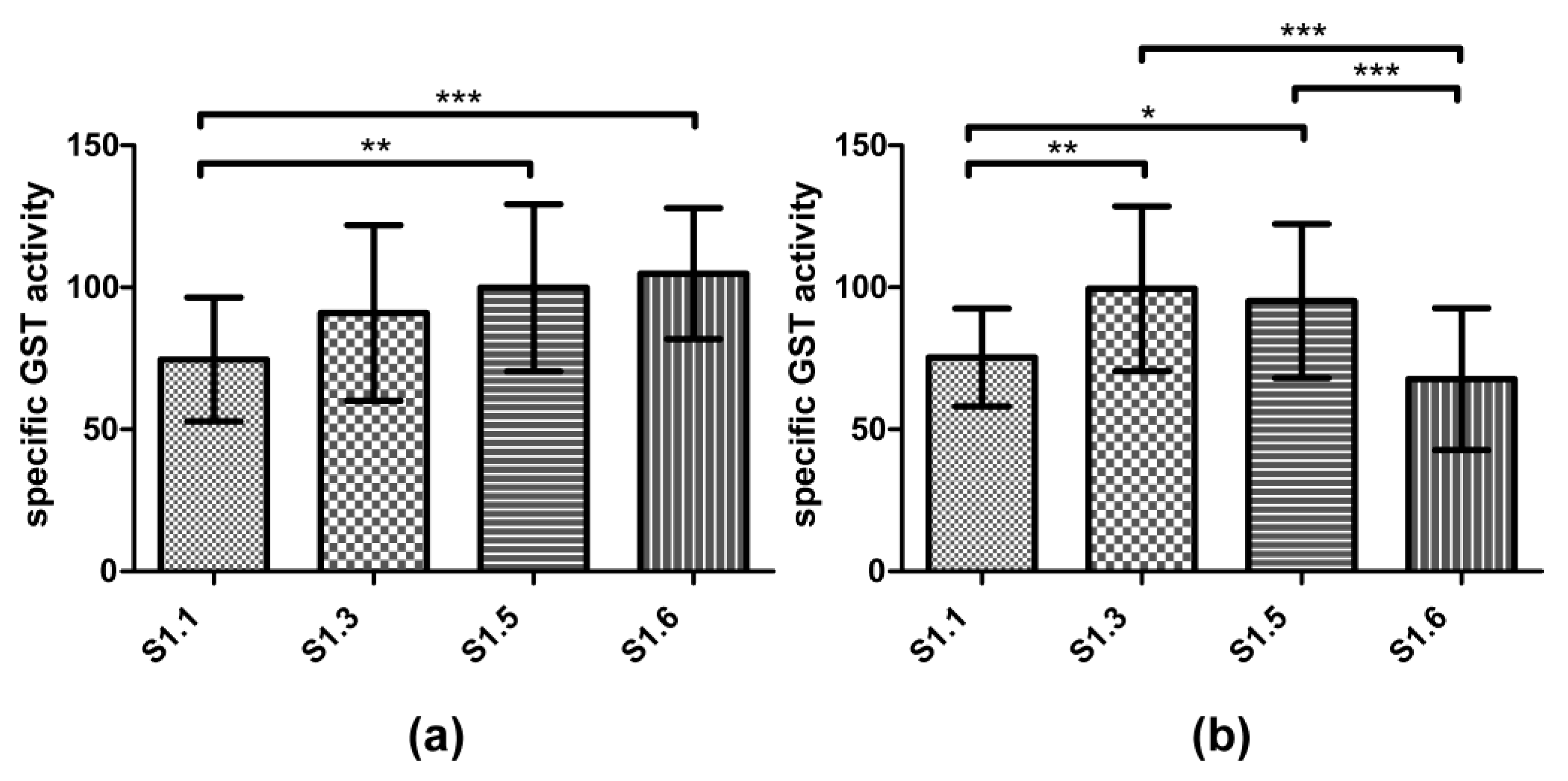
3.3. Biomarker Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ose, A.; Andersone-Ozola, U.; Ievinsh, G. Substrate-dependent effect of vermicompost on yield and physiological indices of container-grown Dracocephalum moldavica Plants. Agriculture 2021, 11, 1231. [Google Scholar] [CrossRef]
- Pierre-Louis, R.C.; Kader, M.A.; Desai, N.M.; John, E.H. Potentiality of vermicomposting in the South Pacific Island countries: A review. Agriculture 2021, 11, 876. [Google Scholar] [CrossRef]
- Nogales, R.; Cifuentes, C.; Benitez, E. Vermicomposting of vinery wastes: A laboratory study. J. Environ. Sci. Health Part B 2005, 40, 659–673. [Google Scholar] [CrossRef]
- Hussain, N.; Singh, A.; Saha, S.; Kumar, M.V.S.; Bhattacharya, P.; Bhattacharya, S.S. Excellent N-fixing and P-solubilizing traits in earthworm gut-izolated bacteria: A vermicompost based assessment with vegetable market waste and rice straw feed mixtures. Bioresour. Technol. 2016, 222, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kouba, A.; Lunda, R.; Hlavac, D.; Kuklina, I.; Hamackova, J.; Randak, T.; Kozak, P.; Koubova, A.; Buric, M. Vermicompostin of sludge from reciculating aquaculture system using Eisenia andrei: Technological feasibility and quality assessment of end-products. J. Clean. Prod. 2018, 177, 665–673. [Google Scholar] [CrossRef]
- Martin, M.; Eudoxie, M.M.G. Feedstock composition influences vermicomposting performance Dichogaster annae relative to Eudrilus eugeniae and Perionyx excavatus. Environ. Sci. Pollut. Res. 2018, 25, 17716–17725. [Google Scholar] [CrossRef]
- Sharma, K.; Garg, V.K. Vermicomposting of waste: A Zero-Waste Apprroach for Waste Management. In Suistanable Resource Recovery and Zero Waste Approaches, 1st ed.; Taherzadeh, M., Bolton, K., Wong, J., Pandey, A., Eds.; Elsevier: St. Louis, MO, USA, 2019; pp. 133–164. [Google Scholar]
- Sharma, S.; Kumar, A.; Singh, A.P.; Vasudevan, P. Earthworms and Vermitechnology—A Review. Dyn. Soil Dyn. Plant 2009, 3, 1–12. [Google Scholar]
- Bhat, S.A.; Singh, J.; Vig, A.P. Earthworms as organic waste managers and biofertilizer producers. Waste Biomass Valorization 2017, 9, 1073–1086. [Google Scholar] [CrossRef]
- Ramnarain, Y.I.; Ansari, A.A.; Ori, L. Vermicomposting of different organic materials using the epigeic earthworm Eisenia foetida. Int. J. Recycl. Org. Waste Agric. 2019, 8, 23–36. [Google Scholar] [CrossRef]
- Vuković, A.; Velki, M.; Ečimović, S.; Vuković, R.; Čamagajevac, I.Š.; Lončarić, Z. Vermicomposting—Facts, benefits and knowledge gaps. Agonomy 2021, 11, 1952. [Google Scholar] [CrossRef]
- Saranraj, P.; Stella, D. Vermicomposting and its importance in improvement of soil nutrients and agricultural crops. Nov. Nat. Sci. Res. 2012, 1, 14–23. [Google Scholar]
- Manaig, E.M. Vermicomposting efficiency and quality of vermicompost with different bedding materials and worm food sources as substrate. Res. J. Agric. For. Sci. 2016, 4, 6063. [Google Scholar]
- Hussain, N.; Abbasi, S.A. Efficacy of the vermicomposts of different organic wastes as “Clean” fertilizers: State-of-the-Art. Sustainability 2018, 10, 1205. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bohlen, P.J. Biology of Earthworms. In Biology and Ecology of Earthworms, 3rd ed.; Bohlen, P.J., Edwards, C.A., Eds.; Chapman & Hall: New York, NY, USA, 1996; 426p. [Google Scholar]
- Sinha, R.K.; Soni, B.K.; Agarwal, S.; Shankar, B.; Hahn, G. Vermiculture for organic horticulture: Producing chemical-free, nutritive and health protective foods by earthworms. J. Agric. Sci. 2013, 1, 17–44. [Google Scholar] [CrossRef]
- Ečimović, S.; Vrandečić, K.; Kujavec, M.; Žulj, M.; Ćosić, J.; Velki, M. Antifungal activity of earthworm coelomic fluid obtained from Eisenia andrei, Dendrobaena veneta and Allobophora chlorotica on six species of phytopathogenic fungi. Environments 2021, 8, 102. [Google Scholar] [CrossRef]
- Edwards, C.A. Historical overview of vermicomposting. Biocycle 1995, 36, 56–58. [Google Scholar]
- Ceritoğlu, M.; Şahin, S.; Erman, M. Effects of vermicompost on plant growth and soil structure. Selcuk J. Agric. Food Sci. 2018, 32, 607–615. [Google Scholar] [CrossRef]
- Dominguez, J.; Edwards, C.A. Biology and ecology of earthworm species used for vermicomposting. In Vermiculture Technology: Earthworms, Organic Wastes, and Environmental Management; Edwards, C.A., Arancon, N.Q., Sherman, R.L., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Singh, J. Role of earthworm in sustainable agriculture. In Sustainable Food Systems from Agriculture to Industry: Improving Production and Processing; Academic Press: Cambridge, MA, USA, 2018; pp. 83–122. [Google Scholar]
- Olee, M. Review: Vermicompost, its importance and benefit in agriculture. Estonian Crop Research Institute. J. Agric. Sci. 2019, 30, 93–98. [Google Scholar]
- Thiruneelakandan, R.; Subbulakshmi, G. Vermicomposting: A superlative for soil, plant and environment. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 930–938. [Google Scholar]
- Arancon, N.Q.; Edwards, C.E.; Atiyeh, R.M.; Metzger, J.D. Effects of vermicompost produced from food waste on the growth and yields of greenhouse peppers. Bioresour. Technol. 2004, 93, 139–144. [Google Scholar] [CrossRef]
- Dominguez, J.; Gómez-Brandón, M. Vermicomposting: Composting with Earthworms to Recycle Organic Wastes. In Management of Organic Waste; Kumar, S., Ed.; Intech Open Science: London, UK, 2012; pp. 29–48. [Google Scholar]
- Organization for Economic Cooperation and Development. OECD Guidelines for Testing of Chemicals, Earthworm, Acute Toxicity Tests: Filter Paper Test and Artificial Soil Test; Organization for Economic Cooperation and Development: Paris, France, 1984; Volume 207, pp. 1–9.
- Bouché, M.B. Lombriciens de France. Ecologie et Systématique. In Lombriciens de France; INRA, Ed.; Institut National des Resherches Agriculturelles: Paris, France, 1972; pp. 72–78. [Google Scholar]
- ISO 17512-1:2008; Soil Quality—Avoidance Test for Determining the Quality of Soils and Effects of Chemicals on Behavior—Part 1: Test with Earthworms (Eisenia fetida and Eisenia andrei). ISO: Geneva, Switzerland, 2008.
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Satoh, T. Measurement of carboxylesterase (CES) activities. Curr. Protoc. Toxicol. 2001, 10, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione-S-transferase: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Haimi, J.; Hunta, V. Capacity of various organic residues to support adequate earthworm biomass for vermicomposting. Biol. Fertil. Soils 1986, 2, 23–27. [Google Scholar] [CrossRef]
- Hund-Rinke, K.; Achazi, R.; Römbke, J.; Warnecke, D. Avoidance test with Eisenia fetida as indicator for the habitat function of soils: Results of a laboratory comparison test. J. Soils Sediments 2003, 3, 7–12. [Google Scholar] [CrossRef]
- Lowe, C.; Butt, K.R.; Chenier, K.Y.-M. Assesment of avoidance behavior by earthworms (Lumbricus rubellus and Octolasion cyaneum) in linear pollution gradients. Ecotoxicol. Environ. Saf. 2016, 124, 324–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomez–Brandon, M.; Lores, M.; Martinez–Cordeiro, H.; Dominguez, J. Effectiveness of vermicomposting for bioconversion of grape marc derived from red winemaking into a value-added product. Environ. Sci. Pollut. Res. 2019, 27, 33438–33445. [Google Scholar] [CrossRef]
- Dominguez, J.; Martinez–Cordeiro, H.; Alvarez–Cases, M.; Lores, M. Vermicomposting grape marc yields high quality organic biofertiliser and bioactive polyphenols. Waste Manag. Res. 2014, 32, 1235–1240. [Google Scholar] [CrossRef]
- Bussel, W.T.; Mckennie, S. Rockwool in horticulture, and its importance and sustainable use in New Zealand. N. Z. J. Crop Hortic. Sci. 2004, 32, 29–37. [Google Scholar] [CrossRef]
- Hansen, M.; Gronborg, H.; Starkey, N.; Hansen, L. Alternative substrates for potted plants. Acta Hortic. 1993, 342, 191–196. [Google Scholar] [CrossRef]
- Ellyard, R.K.; Ollerenshaw, P.J.; Hadobas, H.L. Growool as a cutting medium for Australian native plants. Aust. Hortic. 1985, 83, 26–27. [Google Scholar]
- Hutchison, M.L.; Walters, L.D.; Avery, S.M.; Munro, F.; Moore, A. Analyses of livestock production, waste storage, and pathogen levels and prevalences in farm manures. Appl. Environ. Microbiol. 2005, 71, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, M.; Taušnerova, H.; Hanč, A.; Tlustoš, P. Stabilization of different starting materials through vermicomposting in a continuous-feeding system: Changes in chemical and biological parameters. Waste Manag. 2017, 62, 33–42. [Google Scholar] [CrossRef]
- De la Vega, A. Vermicomposting: The Future of Sustainable Agriculture and Organic Waste Management; Lessons from the USA and Cuba; Royal Horticultural Society: London, UK, 2016; pp. 1–48. [Google Scholar]
- Lončarić, Z.; Parađiković, N.; Popović, B.; Lončarić, R.; Kanisek, J. Vegetables Fertilization, Organic Fertilizers and Composting; Faculty of Agriculture, Josip Juraj Strossmayer University: Osijek, Croatia, 2015. [Google Scholar]
- Lončarić, Z. Plodnost tala i gospodarenje organskim gnojivima. In Proceedings of the 11th Consultation of Cattle Breeders in the Republic of Croatia, Ivanić Grad, Croatia, 28–29 January 2016. [Google Scholar]
- Kumar, S.; Sharma, V.; Bhoyar, R.V.; Bhattacharyya, J.K.; Chakrabarti, T. Effect of heavy metals on earthworm activities during vermicomposting municipal solid waste. Water Environ. Res. 2008, 80, 154–161. [Google Scholar] [CrossRef]
- Velki, M.; Hackenberger, B.K. Different sensitivities of biomarker responses in two epigeic earthworm species after exposure to pyrethroid and organophosphate insecticides. Arch. Environ. Contam. Toxicol. 2013, 65, 498–509. [Google Scholar] [CrossRef]
- Velki, M.; Ečimović, S. Important Issues in Ecotoxicological Investigations using Earthworms. In Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2016; pp. 157–184. [Google Scholar]
| Substrate Label | Substrate Composition | Composition Abbreviations | Ratio (v/v) | Number of Days in the Thermophilic Phase | pH |
|---|---|---|---|---|---|
| S1.1 | Leaves and horse manure | a + b | 1:1 (a:b) | 8 | 9.11 |
| S1.2 | Leaves, horse manure, microorganisms, and urea | a + b + c * + d ** | 1:1 (a:b) | 30 | 8.73 |
| S1.3 | Leaves, horse manure, microorganisms, wood chips, and phosphorite | a + b + c * + e + f *** | 2.5:2.5:1 (a:b:e) | 11 | 9.25 |
| S1.4 | Leaves, microorganisms, urea, and wood chips | a + c * + d ** + e | 2.5:1 (a:e) | 14 | 8.83 |
| S1.5 | Leaves and wood chips | a + e | 2.5:1 (a:e) | 12 | 8.83 |
| S1.6 | Leaves, urea, and wood chips | a + d ** + e | 2.5:1 (a:e) | 10+5 | 9.12 |
| Substrate Label | Substrate Composition | Composition Abbreviations | Ratio (w/w) | Number of Days in the Thermophilic Phase | pH |
|---|---|---|---|---|---|
| S2.1 | Grape pomace | g | - | 7.33 | |
| S2.2 | Fresh horse manure | h | - | 7.71 | |
| S2.3 | Grape pomace and fresh horse manure | g + h | 1:1 | - | 7.14 |
| S2.4 | Grape pomace and rock wool | g + j | 4:1 | - | 7.33 |
| S2.5 | Fresh horse manure and rock wool | h + j | 4:1 | - | 8.23 |
| S2.6 | Grape pomace, fresh horse manure, and rock wool | g + h + j | 2:2:1 | - | 7.78 |
| Substrate Label | Substrate Composition | Composition Abbreviations | Ratio (w/w) | Number of Days in the Thermophilic Phase | pH |
|---|---|---|---|---|---|
| S3.1 | Fresh horse manure and sawdust | h + i | 4:1 | - | 7.59 |
| S3.2 | Grape pomace and sawdust | g + i | 4:1 | - | 6.42 |
| S3.3 | Grape pomace, sawdust, and rock wool | g + i + j | 3:1:1 | - | 7.24 |
| S3.4 | Grape pomace, fresh horse manure, rock wool, and sawdust | g + h + i + j | 1.5:1.5:1:1 | - | 7.59 |
| S3.5 | Grape pomace, fresh horse manure, and sawdust | g + h + i | 2:2:1 | - | 6.69 |
| S3.6 | Fresh horse manure, sawdust, and rock wool | h + i + j | 3:1:1 | - | 7.31 |
| Substrate | Survival Rate after 48 h | Survival Rate after 14 Days |
|---|---|---|
| S1.1 | 100% | 100% |
| S1.2 | 0% | 0% |
| S1.3 | 100% | 100% |
| S1.4 | 0% | 0% |
| S1.5 | 100% | 100% |
| S1.6 | 100% | 100% |
| S2.1 | 100% | 95% |
| S2.2 | 30% | 25% |
| S2.3 | 95% | 85% |
| S2.4 | 90% | 80% |
| S2.5 | 100% | 90% |
| S2.6 | 95% | 90% |
| S3.1 | 73% | 50% |
| S3.2 | 100% | 100% |
| S3.3 | 90% | 90% |
| S3.4 | 53% | 43% |
| S3.5 | 63% | 53% |
| S3.6 | 57% | 47% |
| Distribution | Significance | Result | |
|---|---|---|---|
| S1.1 | S1.3 | *** | preference of substrate S1.1 |
| 90% | 10% | ||
| S1.1 | S1.5 | ** | preference of substrate S1.1 |
| 80% | 20% | ||
| S1.1 | S1.6 | ** | preference of substrate S1.1 |
| 67% | 33% | ||
| S1.3 | S1.5 | NS | - |
| 53% | 47% | ||
| S1.3 | S1.6 | NS | - |
| 48% | 52% | ||
| S1.5 | S1.6 | NS | - |
| 43% | 57% | ||
| Distribution | Significance | Result | |
|---|---|---|---|
| S2.1 | S2.3 | *** | preference of substrate S2.1 |
| 93% | 7% | ||
| S2.1 | S2.4 | NS | - |
| 33% | 67% | ||
| S2.1 | S2.5 | *** | preference of substrate S2.1 |
| 100% | 0% | ||
| S2.1 | S2.6 | ** | preference of substrate S2.1 |
| 77% | 23% | ||
| S2.3 | S2.4 | *** | preference of substrate S2.4 |
| 10% | 90% | ||
| S2.3 | S2.5 | NS | - |
| 50% | 50% | ||
| S2.3 | S2.6 | preference of substrate S2.6 | |
| 3% | 97% | ||
| S2.4 | S2.5 | preference of substrate S2.4 | |
| 97% | 3% | ||
| S2.4 | S2.6 | preference of substrate S2.4 | |
| 97% | 3% | ||
| S2.5 | S2.6 | preference of substrate S2.6 | |
| 27% | 73% | ||
| Distribution | Significance | Result | |
|---|---|---|---|
| S3.2 | S3.3 | *** | preference of substrate S3.3 |
| 23% | 77% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ečimović, S.; Velki, M.; Mikuška, A.; Bažon, J.; Kovačić, L.S.; Kristek, S.; Jović, J.; Nemet, F.; Perić, K.; Lončarić, Z. How the Composition of Substrates for Seedling Production Affects Earthworm Behavior. Agriculture 2022, 12, 2128. https://doi.org/10.3390/agriculture12122128
Ečimović S, Velki M, Mikuška A, Bažon J, Kovačić LS, Kristek S, Jović J, Nemet F, Perić K, Lončarić Z. How the Composition of Substrates for Seedling Production Affects Earthworm Behavior. Agriculture. 2022; 12(12):2128. https://doi.org/10.3390/agriculture12122128
Chicago/Turabian StyleEčimović, Sandra, Mirna Velki, Alma Mikuška, Jelena Bažon, Lucija Sara Kovačić, Suzana Kristek, Jurica Jović, Franjo Nemet, Katarina Perić, and Zdenko Lončarić. 2022. "How the Composition of Substrates for Seedling Production Affects Earthworm Behavior" Agriculture 12, no. 12: 2128. https://doi.org/10.3390/agriculture12122128
APA StyleEčimović, S., Velki, M., Mikuška, A., Bažon, J., Kovačić, L. S., Kristek, S., Jović, J., Nemet, F., Perić, K., & Lončarić, Z. (2022). How the Composition of Substrates for Seedling Production Affects Earthworm Behavior. Agriculture, 12(12), 2128. https://doi.org/10.3390/agriculture12122128







