Evaluation of Carbonic Maceration Effect as a Pre-Treatment on the Drying Process of Strawberry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Optimization
2.3. Carbonic Maceration Pre-Treatment
2.4. Drying Experiments
2.5. Drying Rate
2.6. Rehydration Rate
2.7. Water Activity
2.8. Acidity and pH Value
2.9. Soluble Dry Matter (ᵒBx)
2.10. Color Measurement
2.11. Total Phenolic Content (TPC)
2.12. Ascorbic Acid Content (AAC)
2.13. Hydroxymethylfurfural Content (HMF)
2.14. Determination of Antioxidant Activity by ABTS Method
2.15. Texture Analysis
2.16. Scanning Electron Microscopy (SEM)
2.17. Energy Utilization
2.18. Statistical Analysis
3. Results
3.1. Changes in Physicochemical and Functional Properties of Strawberries with Drying Process
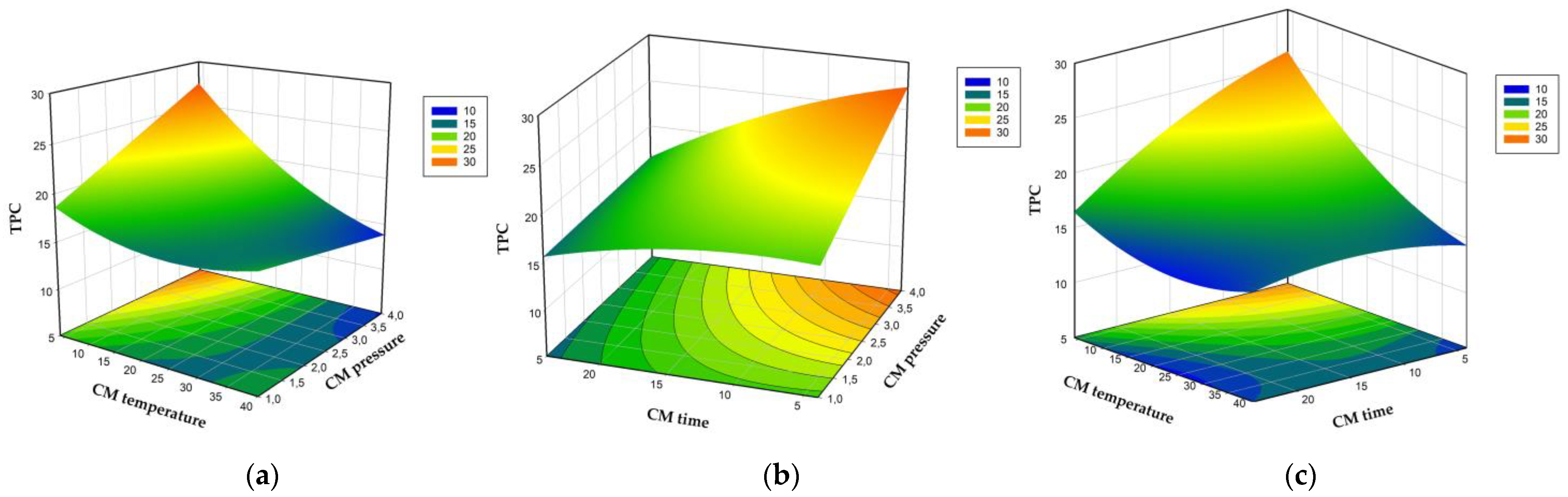
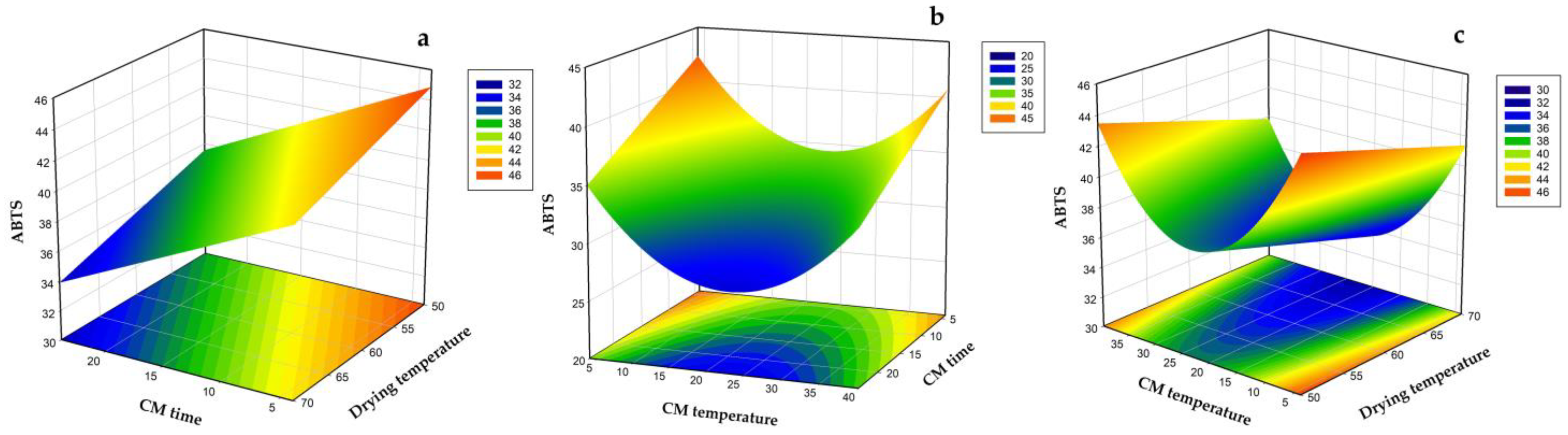
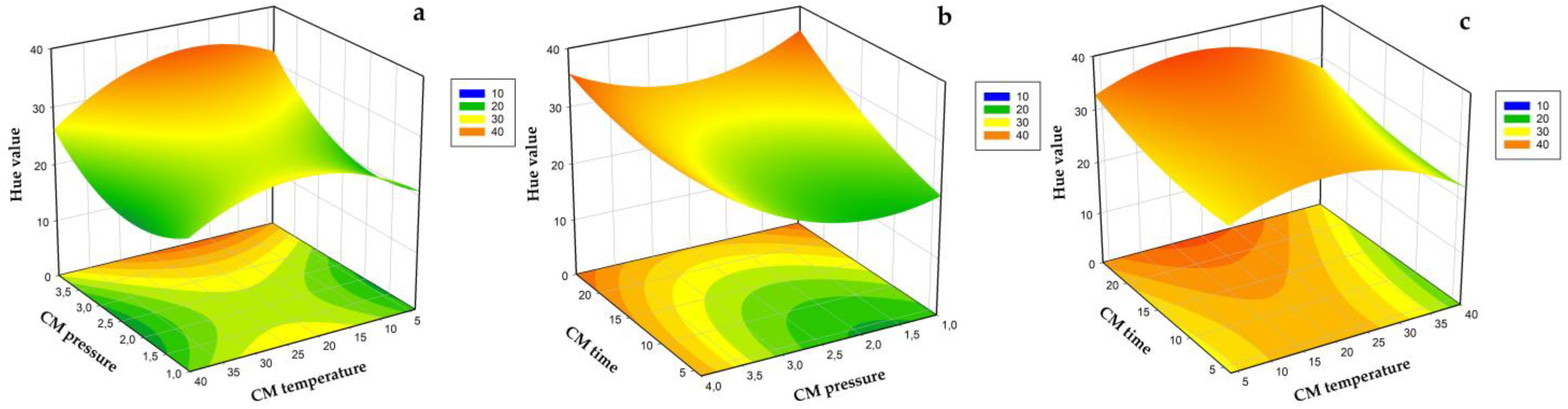
3.2. Modeling of Drying Time, Total Phenolic Content, Antioxidant Capacity, Ascorbic Acid Content, and Hue Angle
3.3. Optimization and Validation of Carbonic Maceration Pre-Treatment Process Conditions and Drying Temperature in Terms of AAC, ABTS, Drying Time, and Hue Value
3.4. Scanning Electron Microscope Images
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anonym. 2021. Available online: https://www.atlasbig.com/tr/ulkelerin-cilek-uretimi, (accessed on 10 September 2022).
- de Jesús Ornelas-Paz, J.; Yahia, E.M.; Ramírez-Bustamante, N.; Pérez-Martínez, J.D.; del Pilar Escalante-Minakata, M.; Ibarra-Junquera, V.; Acosta-Muñiz, C.; Guerrero-Prieto, V.; Ochoa-Reyes, E. Physical attributes and chemical composition of organic strawberry fruit (Fragaria × ananassa Duch, Cv. Albion) at six stages of ripening. Food Chem. 2013, 138, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Cam, I.B.; Basunal Gulmez, H.; Eroglu, E.; Topuz, A. Strawberry drying: Development of a closed-cycle modified atmosphere drying system for food products and the performance evaluation of a case study. Dry. Technol. 2018, 36, 1460–1473. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Delgado, A. Quality of organic compared to conventionally grown strawberries at the retail level. Acta Hortic. 2014, 1049, 723–730. [Google Scholar] [CrossRef]
- Conti, S.; Villari, G.; Faugno, S.; Melchionna, G.; Somma, S.; Caruso, G. Effects of organic vs. conventional farming system on yield and quality of strawberry grown as an annual or biennial crop in southern Italy. Sci. Hortic. 2014, 180, 63–71. [Google Scholar] [CrossRef]
- Yildiz, H.; Ercisli, S.; Hegedus, A.; Akbulut, M.; Topdas, E.F.; Aliman, J. Bioactive content and antioxidant characteristics of wild (Fragaria vesca L.) and cultivated strawberry (Fragaria × ananassa Duch.) fruits from Turkey. J. Appl. Bot. Food Qual. 2014, 87, 274–278. [Google Scholar]
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef]
- Floros, J.D.; Chinnan, M.S. Determining the diffusivity of sodium hydroxide through tomato and capsicum skins. J. Food Eng. 1989, 9, 129–141. [Google Scholar] [CrossRef]
- Baloch, W.A.; Khan, S.; Baloch, A.K. Influence of chemical additives on the stability of dried tomato powder. Int. J. Food Sci. Technol. 1997, 32, 117–120. [Google Scholar] [CrossRef]
- Olorunda, A.O.; Aworh, O.C.; Onuoha, C.N. Upgrading quality of dried tomato: Effects of drying methods, conditions and pre-drying treatments. J. Sci. Food Agric. 1990, 52, 447–454. [Google Scholar] [CrossRef]
- Schlimme, D.V.; Corey, K.A.; Frey, B.C. Evaluation of lye and steam peeling using four processing tomato cultivars. J. Food Sci. 1984, 49, 1415–1418. [Google Scholar] [CrossRef]
- Shi, J.X.; le Maguer, M.; Wang, S.L.; Liptay, A. Application of osmotic treatment in tomato processing—Effect of skin treatments on mass transfer in osmotic dehydration of tomatoes. Food Res. Int. 1997, 30, 669–674. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Le, H.V.; Pomarańska-Łazuka, W. Effect of pre-treatment on convective drying of tomatoes. J. Food Eng. 2002, 54, 141–146. [Google Scholar] [CrossRef]
- Brown, H.E.; Meredith, F.I.; Saldama, G.; Stephens, T.S. Freeze peeling improves quality of tomatoes. J. Food Sci. 1970, 35, 485–488. [Google Scholar] [CrossRef]
- Leonard, S.; Marsh, G.L.; York, G.K.; Heil, J.R.; Wolcott, T. Evaluation of tomato canning practices using flame sterilization. J. Food Process. Preserv. 1977, 1, 313–323. [Google Scholar] [CrossRef]
- Shi, J.; le Maguer, M.; Kakuda, Y.; Liptay, A.; Niekamp, F. Lycopene degradation and isomerization in tomato dehydration. Food Res. Int. 1999, 32, 15–21. [Google Scholar] [CrossRef]
- Flanzy, C.; Flanzy, M.; Benard, P. La Vinification par Macération Carbonique; Editions Quae: Versailles, France, 1987. [Google Scholar]
- Wang, Y.; Tao, H.; Yang, J.; An, K.; Ding, S.; Zhao, D.; Wang, Z. Effect of carbonic maceration on infrared drying kinetics and raisin qualities of Red Globe (Vitis vinifera L.): A new pre-treatment technology before drying. Innov. Food Sci. Emerg. Technol. 2014, 26, 462–468. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhao, D.; An, K.; Ding, S.; Wang, Z. Effect of Carbonic Maceration Pre-treatment on Drying Kinetics of Chilli (Capsicum annuum L.) Flesh and Quality of Dried Product. Food Bioproc. Tech. 2014, 7, 2516–2527. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Zhu, Y.; Ni, Y. Effect of carbonic maceration pre-treatment on the drying behavior and physicochemical compositions of sweet potato dried with intermittent or continuous microwave. Dry. Technol. 2016, 34, 1604–1612. [Google Scholar] [CrossRef]
- Serhat Turgut, S.; Küçüköner, E.; Karacabey, E. Improvements in drying characteristics and quality parameters of tomato by carbonic maceration pretreatment. J. Food Process. Preserv. 2018, 42, e13282. [Google Scholar] [CrossRef]
- An, K.; Wu, J.; Tang, D.; Wen, J.; Fu, M.; Xiao, G.; Xu, Y. Effect of carbonic maceration (CM) on mass transfer characteristics and quality attributes of Sanhua plum (Prunus Salicina Lindl.). LWT 2018, 87, 537–545. [Google Scholar] [CrossRef]
- An, K.; Wei, L.; Fu, M.; Cheng, L.; Peng, J.; Wu, J. Effect of Carbonic Maceration (CM) on the Vacuum Microwave Drying of Chinese Ginger (Zingiber officinale Roscoe) Slices: Drying Characteristic, Moisture Migration, Antioxidant Activity, and Microstructure. Food Bioproc. Tech. 2020, 13, 1661–1674. [Google Scholar] [CrossRef]
- Al-Hilphy, A.R.S.; AlRikabi, A.K.J. Mathematical modeling and experimental study on thin layer halogen dryer of strawberry and study it’s effect on antioxidant activity. Am. J. Agric. Biol. Sci. 2013, 8, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Doymaz, I.; Özdemir, Ö. Effect of air temperature, slice thickness and pretreatment on drying and rehydration of tomato. Int. J. Food Sci. Technol. 2014, 49, 558–564. [Google Scholar] [CrossRef]
- Palou, E.; López-Malo, A.; Barbosa-Cánovas G v Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Effect of semi-drying on the antioxidant components of tomatoes. Food Chem. 2006, 94, 90–97. [Google Scholar] [CrossRef]
- Demiray, E.; Tulek, Y.; Yilmaz, Y. Degradation kinetics of lycopene, β-carotene and ascorbic acid in tomatoes during hot air drying. LWT Food Sci. Technol. 2013, 50, 172–176. [Google Scholar] [CrossRef]
- Guerra-Hernández, E.; García-Villanova, B.; Montilla-Gómez, J. Determination of Hydroxymethylfurfural in Baby Cereals by High Performance Liquid Chromatography. J. Liq. Chromatogr. 1992, 15, 2551–2559. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gunness, P.; Kravchuk, O.; Nottingham, S.M.; D’Arcy, B.R.; Gidley, M.J. Sensory analysis of individual strawberry fruit and comparison with instrumental analysis. Postharvest Biol. Technol. 2009, 52, 164–172. [Google Scholar] [CrossRef]
- Motevali, A.; Minaei, S.; Khoshtagaza, M.H. Evaluation of energy consumption in different drying methods. Energy Convers. Manag. 2011, 52, 1192–1199. [Google Scholar] [CrossRef]
- Crecente-Campo, J.; Nunes-Damaceno, M.; Romero-Rodríguez, M.A.; Vázquez-Odériz, M.L. Color, anthocyanin pigment, ascorbic acid and total phenolic compound determination in organic versus conventional strawberries (Fragaria × ananassa Duch, cv Selva). J. Food Compos. Anal. 2012, 28, 23–30. [Google Scholar] [CrossRef]
- Ersoy, N. Organik ve Konvansiyonel Olarak Yetiştirilen ‘Camarosa’Çilek Çeşidinin Bazı Fizikokimyasal Özellikleri ve Antioksidan Kapasiteleri. Selcuk. J. Agric. Food Sci. 2011, 25, 73–78. [Google Scholar]
- Contreras, C.; Martín-Esparza, M.E.; Martínez-Navarrete, N.; Chiralt, A. Influence of osmotic pre-treatment and microwave application on properties of air dried strawberry related to structural changes. Eur. Food Res. Technol. 2007, 224, 499–504. [Google Scholar] [CrossRef]
- Alibaş, İ. Microwave Drying of Strawberry Slices and the Determination of the Some Quality Parameters. J. Agric. Mach. Sci. 2012, 8, 161–170. [Google Scholar]
- Xu, B.; Chen, J.; Tiliwa, E.S.; Yan, W.; Azam, S.R.; Yuan, J.; Wei, B.; Zhou, C.; Ma, H. Effect of multi-mode dual-frequency ultrasound pretreatment on the vacuum freeze-drying process and quality attributes of the strawberry slices. Ultrason. Sonochem. 2021, 78, 105714. [Google Scholar] [CrossRef]
- Gamboa-Santos, J.; Megías-Pérez, R.; Soria, A.C.; Olano, A.; Montilla, A.; Villamiel, M. Impact of processing conditions on the kinetic of vitamin C degradation and 2-furoylmethyl amino acid formation in dried strawberries. Food Chem. 2014, 153, 164–170. [Google Scholar] [CrossRef]
- Cheng, X.F.; Zhang, M.; Adhikari, B. Effect of Ultrasonically Induced Nucleation on the Drying Kinetics and Physical Properties of Freeze-Dried Strawberry. Dry. Technol. 2014, 32, 1857–1864. [Google Scholar] [CrossRef]
- Shih, C.; Pan, Z.; Mchugh, T.; Wood, D.; Hirschberg, E. Sequential infrared radiation and freeze-drying method for producing crispy strawberries. Trans. ASABE 2008, 51, 205–216. [Google Scholar] [CrossRef]
- El-Beltagy, A.; Gamea, G.R.; Essa, A.H.A. Solar drying characteristics of strawberry. J. Food Eng. 2007, 78, 456–464. [Google Scholar] [CrossRef]
- Alonzo-Macías, M.; Montejano-Gaitán, G.; Allaf, K. Impact of drying processes on strawberry (Fragaria var. Camarosa) texture: Identification of crispy and crunchy features by instrumental measurement. J. Texture Stud. 2014, 45, 246–259. [Google Scholar] [CrossRef]
- Doymaz, I. Convective drying kinetics of strawberry. Chem. Eng. Process. Process Intensif. 2008, 47, 914–919. [Google Scholar] [CrossRef]
- Lammerskitten, A.; Wiktor, A.; Mykhailyk, V.; Samborska, K.; Gondek, E.; Witrowa-Rajchert, D.; Toepfl, S.; Parniakov, O. Pulsed electric field pre-treatment improves microstructure and crunchiness of freeze-dried plant materials: Case of strawberry. LWT 2020, 134, 110266. [Google Scholar] [CrossRef]
- Prosapio, V.; Norton, I. Influence of osmotic dehydration pre-treatment on oven drying and freeze drying performance. LWT 2017, 80, 401–408. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Qiao, Y.; Liao, L.; Shi, D.; Wang, J.; Shi, L. Effects of ultrasound and ultra-high pressure pretreatments on volatile and taste compounds of vacuum-freeze dried strawberry slice. LWT 2022, 160, 113232. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, L.; Qiao, Y.; Wang, C.; Shi, D.; An, K.; Hu, J. Effects of ultrahigh pressure and ultrasound pretreatments on properties of strawberry chips prepared by vacuum-freeze drying. Food Chem. 2020, 303, 125386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qiao, Y.; Wang, C.; Liao, L.; Shi, D.; An, K.; Hu, J. Impact of ultrasound combined with ultrahigh pressure pretreatments on color, moisture characteristic, tissue structure, and sensory quality of freeze-dried strawberry slices. J. Food Process. Preserv. 2021, 45, e15200. [Google Scholar] [CrossRef]
- Turgut, S.S.; Küçüköner, E.; Karacabey, E. Influence of Carbonic Maceration Pre-treatment on Functional Quality of Dried Tomato Quarters. Food Bioproc. Tech. 2018, 11, 1818–1827. [Google Scholar] [CrossRef]
- Ertekin, C.; Gozlekci, S.; Heybeli, N.; Gencer, A.; Adak, N.; Sengul, B. Drying of Strawberries with Infrared Dryer. In Proceedings of the International Conference of Agricultural Engineering, Zurich, Switzerland, 6–10 July 2014. [Google Scholar]
- Böhm, V.; Kühnert, S.; Rohm, H.; Scholze, G. Improving the nutritional quality of microwave-vacuum dried strawberries: A preliminary study. Food Sci. Technol. Int. 2006, 12, 67–75. [Google Scholar] [CrossRef]
- Cochrane, S. The Munsell Color System: A scientific compromise from the world of art. Stud. Hist. Philos. Sci. Part A 2014, 47, 26–41. [Google Scholar] [CrossRef]
- Abers, J.E.; Wrolstad, R.E. Causative factors of color deterioration in strawberry preserves during processing and storage. J. Food Sci. 1979, 44, 75–81. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT Food Sci. Technol. 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Putra, R.N.; Ajiwiguna, T.A. Influence of Air Temperature and Velocity for Drying Process. Procedia Eng. 2017, 170, 516–519. Available online: https://www.sciencedirect.com/science/article/pii/S1877705817311980 (accessed on 10 September 2022). [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.Y.O.; Lima, G.P.P. Phenolic compounds: Functional properties, impact of processing and bioavailability. In Phenolic Compounds—Biological Activity; IntechOpen: London, UK, 2017; pp. 1–24. [Google Scholar]
- Zhong, J.; Duan, X.W.; Qu, H.X.; Yang, B.; Chen, Y.L.; Ruenroengklin, N.; Jiang, Y.M. Effects of Various Extraction Conditions on Phenolic Contents and Their Antioxidant Activities of Litchi Fruit Pericarp. Acta Hortic. 2008, 804, 327–332. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Kowalska, J.; Kowalska, H.; Marzec, A.; Brzeziński, T.; Samborska, K.; Lenart, A. Dried strawberries as a high nutritional value fruit snack. Food Sci. Biotechnol. 2018, 27, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Ruenroengklin, N.; Zhong, J.; Duan, X.; Yang, B.; Li, J.; Jiang, Y. Effects of various temperatures and pH values on the extraction yield of phenolics from litchi fruit pericarp tissue and the antioxidant activity of the extracted anthocyanins. Int. J. Mol. Sci. 2008, 9, 1333–1341. [Google Scholar] [CrossRef]
- Cantrell, K.; Erenas, M.M.; de Orbe-Payá, I.; Capitán-Vallvey, L.F. Use of the hue parameter of the hue, saturation, value color space as a quantitative analytical parameter for bitonal optical sensors. Anal Chem. 2010, 82, 531–542. [Google Scholar] [CrossRef]
- Diamante, L.; Durand, M.; Savage, G.; Vanhanen, L. Effect of temperature on the drying characteristics, colour and ascorbic acid content of green and gold kiwifruits. Int. Food Res. J. 2010, 17, 441–451. [Google Scholar]
- Cojocaru, C.; Khayet, M.; Zakrzewska-Trznadel, G.; Jaworska, A. Modeling and multi-response optimization of pervaporation of organic aqueous solutions using desirability function approach. J. Hazard. Mater. 2009, 167, 52–63. [Google Scholar] [CrossRef]
- Cernîşev, S. Effects of conventional and multistage drying processing on non-enzymatic browning in tomato. J. Food Eng. 2010, 96, 114–118. [Google Scholar] [CrossRef]
- Abano, E.E.; Ma, H.; Qu, W. Influence of air temperature on the drying kinetics and quality of tomato slices. J. Food Process. Technol. 2011, 2, 2–9. [Google Scholar]
- Liu, M.; Guo, Y.; Zhao, C.; Wang, Z. Drying and fermentation process optimization of grapes pre-fermented with carbon dioxide. Trans. Chin. Soc. Agric. Eng. 2012, 28, 269–272. [Google Scholar]
- Piotrowski, D.; Kostyra, E.; Grzegory, P.; Janiszewska-Turak, E. Influence of drying methods on the structure, mechanical and sensory properties of strawberries. Eur. Food Res. Technol. 2021, 247, 1859–1867. [Google Scholar] [CrossRef]




| Dependent Variable | Minimum Value | Maximum Value |
|---|---|---|
| X1: Drying temperature (°C) | 50 | 70 |
| X2: CM pressure (bar) | 1 | 4 |
| X3: CM temperature (°C) | 4 | 40 |
| X4: CM time (hour) | 4 | 24 |
| Runs | Maceration | Drying Temperature (°C) | ||
|---|---|---|---|---|
| Temperature (°C) | Time (h) | Pressure (bar) | ||
| 1 | 4 | 14 | 2.50 | 60 |
| 2 | 22 | 24 | 2.50 | 60 |
| 3 | 22 | 14 | 2.50 | 50 |
| 4 | 40 | 14 | 2.50 | 60 |
| 5 | 22 | 4 | 2.50 | 60 |
| 6 | 22 | 14 | 2.50 | 70 |
| 7 | 22 | 14 | 1.00 | 60 |
| 8 | 22 | 14 | 2.50 | 60 |
| 9 | 22 | 14 | 4.00 | 60 |
| 10 | 22 | 14 | 2.50 | 60 |
| 11 | 31 | 19 | 3.25 | 65 |
| 12 | 13 | 9 | 3.25 | 65 |
| 13 | 22 | 14 | 2.50 | 60 |
| 14 | 13 | 19 | 3.25 | 55 |
| 15 | 31 | 9 | 1.75 | 65 |
| 16 | 13 | 9 | 1.75 | 55 |
| 17 | 13 | 19 | 1.75 | 65 |
| 18 | 31 | 9 | 3.25 | 55 |
| 19 | 22 | 14 | 2.50 | 60 |
| 20 | 31 | 19 | 1.75 | 55 |
| 21 | 13 | 19 | 1.75 | 55 |
| 22 | 22 | 14 | 2.50 | 60 |
| 23 | 31 | 19 | 1.75 | 65 |
| 24 | 31 | 9 | 3.25 | 65 |
| 25 | 13 | 19 | 3.25 | 65 |
| 26 | 31 | 19 | 3.25 | 55 |
| 27 | 31 | 9 | 1.75 | 55 |
| 28 | 13 | 9 | 1.75 | 65 |
| 29 | 22 | 14 | 2.50 | 60 |
| 30 | 13 | 9 | 3.25 | 55 |
| Run a | A b | B | C | D | E |
|---|---|---|---|---|---|
| 1 | 270 | 21.29 | 40.75 | 401.41 | 23.51 |
| 2 | 225 | 16.85 | 27.60 | 121.87 | 32.42 |
| 3 | 500 | 19.16 | 34.24 | 218.98 | 29.74 |
| 4 | 195 | 19.74 | 38.10 | 64.61 | 22.19 |
| 5 | 290 | 17.91 | 34.53 | 390.88 | 29.07 |
| 6 | 225 | 15.64 | 30.23 | 162.42 | 29.64 |
| 7 | 247 | 18.41 | 33.51 | 386.68 | 33.51 |
| 8 | 320 | 18.50 | 31.74 | 357.11 | 28.96 |
| 9 | 265 | 19.92 | 36.19 | 101.14 | 35.61 |
| 10 | 260 | 19.55 | 34.24 | 213.21 | 29.25 |
| 11 | 175 | 16.20 | 24.74 | 112.80 | 29.76 |
| 12 | 195 | 21.08 | 27.28 | 367.87 | 29.44 |
| 13 | 250 | 17.62 | 24.59 | 308.86 | 28.59 |
| 14 | 255 | 20.13 | 27.84 | 231.48 | 28.46 |
| 15 | 210 | 17.90 | 25.71 | 233.07 | 25.27 |
| 16 | 330 | 18.73 | 27.65 | 698.53 | 28.56 |
| 17 | 205 | 18.08 | 24.24 | 302.13 | 31.29 |
| 18 | 340 | 18.88 | 26.30 | 227.14 | 29.18 |
| 19 | 250 | 17.41 | 21.15 | 313.88 | 27.65 |
| 20 | 295 | 18.38 | 27.06 | 423.34 | 28.18 |
| 21 | 325 | 17.42 | 36.57 | 431.45 | 28.64 |
| 22 | 210 | 18.76 | 36.66 | 297.54 | 28.45 |
| 23 | 200 | 18.10 | 36.08 | 292.01 | 32.48 |
| 24 | 210 | 16.02 | 41.68 | 305.90 | 26.03 |
| 25 | 185 | 16.58 | 33.61 | 410.59 | 32.55 |
| 26 | 260 | 18.85 | 38.94 | 80.67 | 27.68 |
| 27 | 310 | 17.81 | 41.52 | 387.71 | 26.91 |
| 28 | 190 | 17.86 | 43.89 | 495.10 | 25.81 |
| 29 | 235 | 18.65 | 38.06 | 394.80 | 28.15 |
| 30 | 285 | 24.03 | 45.19 | 388.91 | 31.07 |
| Mean | 257.06 ± 64.71 | 18.53 ± 1.82 | 33.00 ± 6.40 | 304.07 ± 138.63 | 28.94 ± 2.83 |
| Variables a | A b | B | C | D | E |
|---|---|---|---|---|---|
| β0 | 4761 *** | −47.9 *** | 3348 *** | 59.34 *** | 69.77 *** |
| β1 (X1) | −138.1 *** | 1.748 *** | −40.8 ns | −0.1821 * | −0.7490 ns |
| β2 (X2) | - | 16.50 ** | −983 *** | - | −7.51 ** |
| β3 (X3) | - | −0.256 *** | −8.97 *** | −1.02 ns | 0.9515 ** |
| β4 (X4) | −2.583 * | 0.260 ** | −11.31 *** | −0.3667 *** | −3.155 *** |
| β11 (X1X1) | 1.054 *** | 0.01188 * | - | - | - |
| β22 (X2X2) | - | - | - | - | 2.455 *** |
| β33 (X3X3) | - | 0.00596 ** | - | 0.02221 *** | −0.01910 *** |
| β44 (X4X4) | - | 0.01203 * | - | - | 0.01706 ** |
| β12 (X1X2) | - | −0.1938 ** | 14.79 ** | - | - |
| β13 (X1X3) | - | - | - | - | - |
| β14 (X1X4) | - | - | - | - | - |
| β23 (X2X3) | - | −0.1110 *** | - | - | −0.0685 ** |
| β24 (X2X4) | - | −0.1320 * | - | - | −0.1883 *** |
| β34 (X3X4) | - | 0.01446 ** | - | - | 0.05573 *** |
| Model | *** | *** | *** | *** | *** |
| R2 | 89.64 | 89.65 | 85.16 | 92.39 | 97.04 |
| Pred. R2 | 80.67 | 72.69 | 70.87 | 87.56 | 89.73 |
| Lack of fit | 0.109 | 0.213 | 0.731 | 0.492 | 0.235 |
| Result | With Optimum CM Pre-Treated | Without CM Pre-Treatment |
|---|---|---|
| DT | 195 ± 5 a | 285 ± 10 b |
| DR | 0.0456 ± 0.01 a | 0.0313 ± 0.01 b |
| pH | 3.85 ± 0.02 a | 3.85 ± 0.01 a |
| Moisture % | 9.86 ± 0.30 a | 8.90 ± 0.25 b |
| aw | 0.378 ± 0.03 a | 0.374 ± 0.04 a |
| h° | 27.61 ± 2.41 a | 27.57 ± 4.39 a |
| C* | 29.39 ± 6.75 a | 29.59 ± 7.51 a |
| BI | 95.86 ± 14.48 a | 110.1 ± 13.15 a |
| TPC | 45.93 ± 2.19 a | 36.50 ± 2.11 b |
| AAC | 521.70 ± 4.24 a | 481.96 ± 6.71 b |
| ABTS | 47.53 ± 0.38 a | 38.95 ± 0.35 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozcelik, M.M.; Ozkan, G.; Karacabey, E. Evaluation of Carbonic Maceration Effect as a Pre-Treatment on the Drying Process of Strawberry. Agriculture 2022, 12, 2113. https://doi.org/10.3390/agriculture12122113
Ozcelik MM, Ozkan G, Karacabey E. Evaluation of Carbonic Maceration Effect as a Pre-Treatment on the Drying Process of Strawberry. Agriculture. 2022; 12(12):2113. https://doi.org/10.3390/agriculture12122113
Chicago/Turabian StyleOzcelik, Muhammed Mustafa, Gulcan Ozkan, and Erkan Karacabey. 2022. "Evaluation of Carbonic Maceration Effect as a Pre-Treatment on the Drying Process of Strawberry" Agriculture 12, no. 12: 2113. https://doi.org/10.3390/agriculture12122113
APA StyleOzcelik, M. M., Ozkan, G., & Karacabey, E. (2022). Evaluation of Carbonic Maceration Effect as a Pre-Treatment on the Drying Process of Strawberry. Agriculture, 12(12), 2113. https://doi.org/10.3390/agriculture12122113





