Antiviral Activity of TiO2 NPs against Tobacco Mosaic Virus in Chili Pepper (Capsicum annuum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Establishment
2.2. Experimental Design
2.3. Synthesis and Characterization of TiO2 Nanoparticles
2.4. TMV Inoculation in Capsicum annuum L. Plants
2.5. Measurement of Morphological Variables and Evaluation of Symptoms
2.6. Immunoassay DAS-ELISA
2.7. Reactivity Determination
2.8. Hydrogen Peroxide Determination
2.9. Enzymatic Activity
2.9.1. Phenylalanine Ammonia-Lyase (PAL) Activity
2.9.2. Catalase (CAT) Activity Assay
2.9.3. Superoxide Dismutase (SOD) Activity Assay
2.9.4. Peroxidase Activity (POD)
2.10. Antioxidant Activity
2.10.1. DPPH• Radical Scavenging Activity (DRSA)
2.10.2. ABTS •+ Radical Scavenging Activity (ARSA)
2.11. Statistical Analysis
3. Results
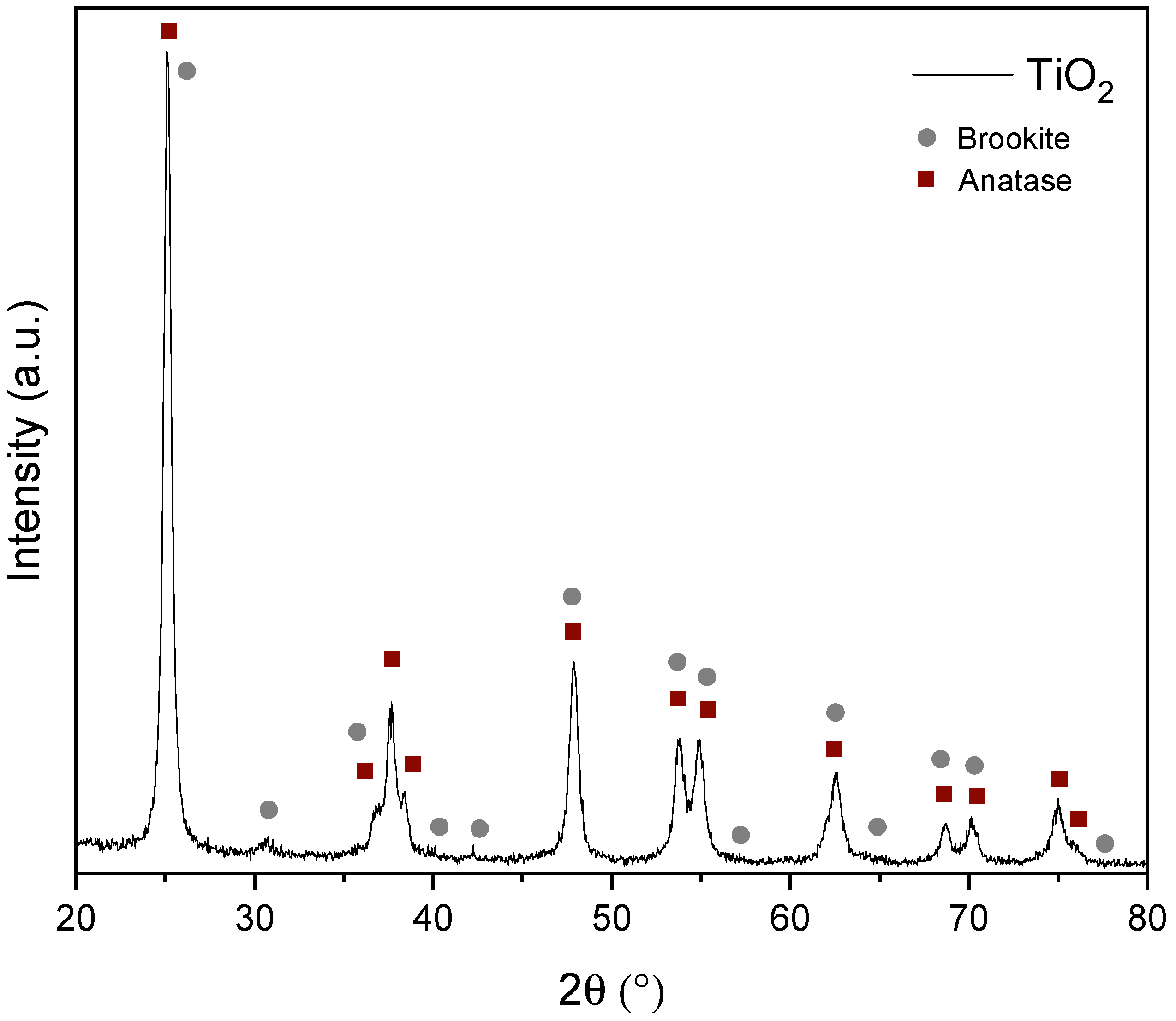
3.1. Characterization of TiO2 Nanoparticles
3.2. Evaluation of the Morphological Characteristics and Symptomatology Scale of the Plants Infected and Treated with TiO2 NPs
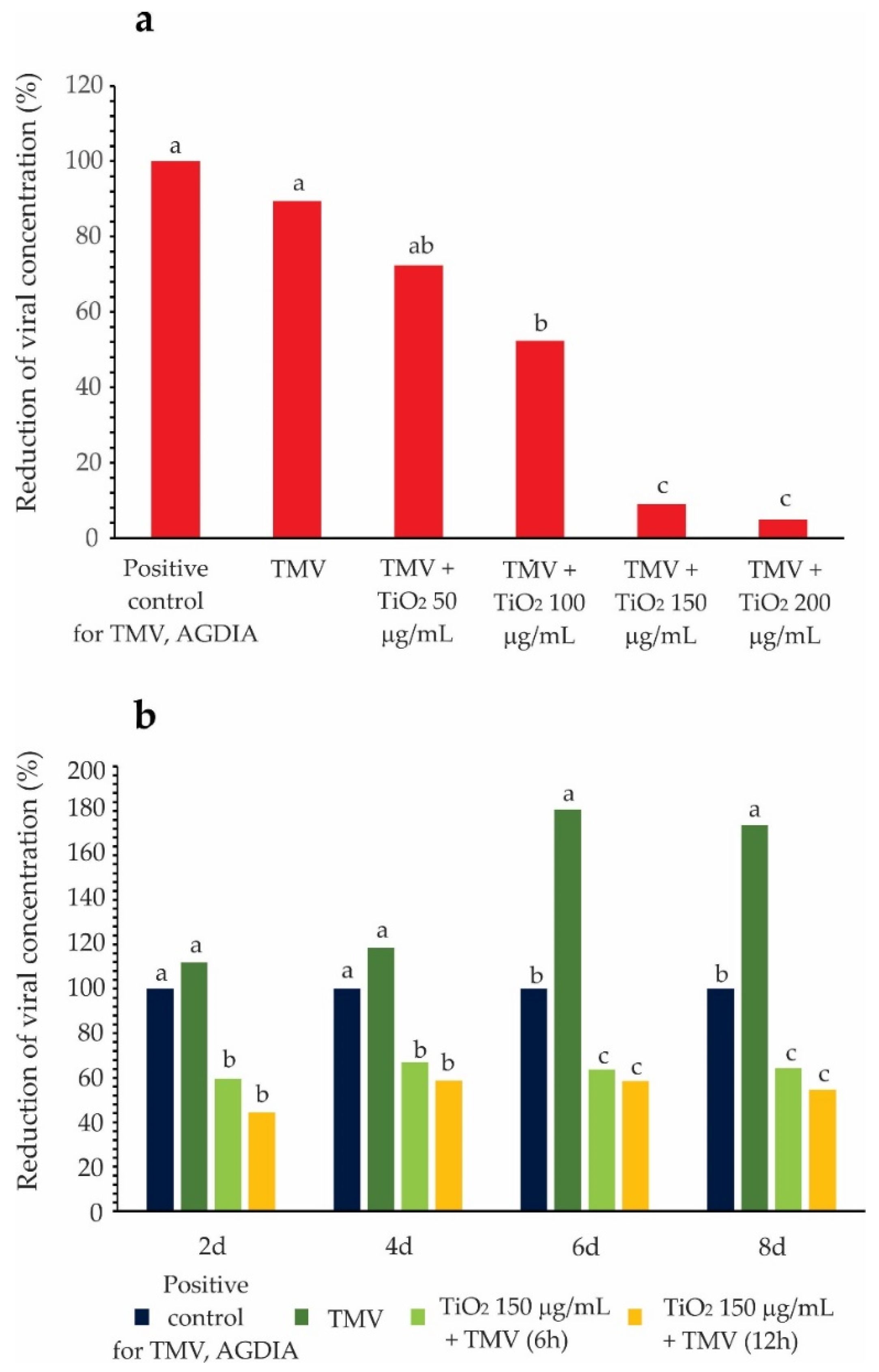
3.3. TMV Detection and Reactivity Test
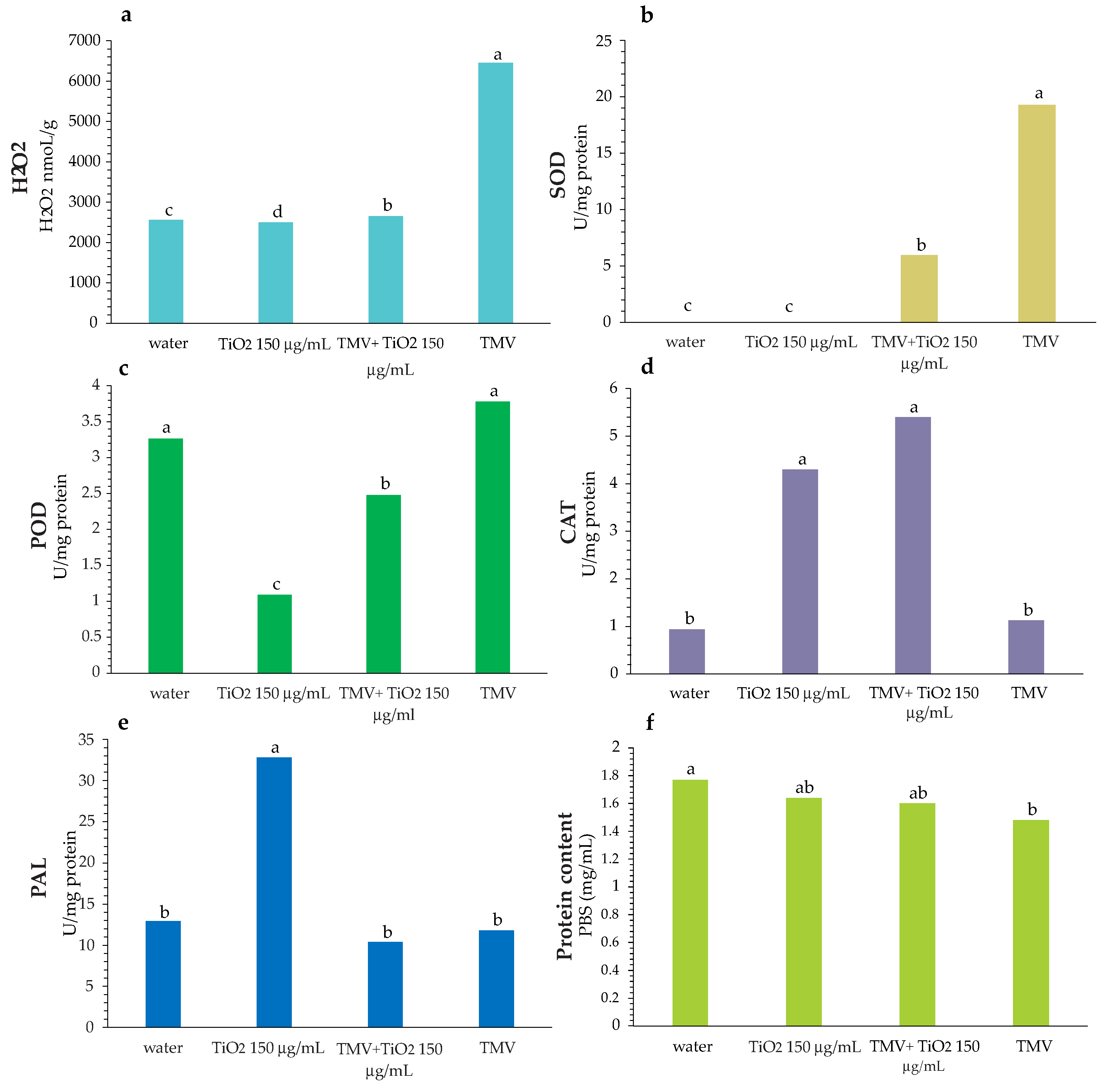
3.4. Enzymatic Activity
3.5. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Avila-Juarez, L.; Torres-Pacheco, I. Nanoparticles as Potential Antivirals in Agriculture. Agriculture 2020, 10, 444. [Google Scholar] [CrossRef]
- Rodrigues, I.C.P.; Campo, K.N.; Arns, C.W.; Gabriel, L.P.; Webster, T.J.; Lopes, É.S.N. From Bulk to Nanoparticles: An Overview of Antiviral Materials, Its Mechanisms, and Applications. Part. Part. Syst. Charact. 2021, 38, 2100044. [Google Scholar] [CrossRef]
- Vrandečić, K.; Ćosić, J.; Ilić, J.; Ravnjak, B.; Selmani, A.; Galić, E.; Pem, B.; Barbir, R.; Vinković Vrček, I.; Vinkovićet, T. Antifungal activities of silver and selenium nanoparticles stabilized with different surface coating agents. Pest Manag. Sci. 2020, 76, 2021–2029. [Google Scholar] [CrossRef]
- Rivero-Montejo, S.D.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as Novel Elicitors to Improve Bioactive Compounds in Plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Cai, L.; Liu, C.; Fan, G.; Liu, C.; Sun, X. Preventing viral disease by ZnONPs through directly deactivating TMV and activating the plant immunity in Nicotiana benthamiana. Environ. Sci. Nano. 2019, 6, 3653–3669. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, C.; Xu, C.; Wang, Z.; Zhao, M.; Wang, C.; Liu, L.; Chen, F. Preliminary experiments on nano-silver against tobacco mosaic virus and its mechanism. Tob. Sci. Technol. 2016, 49, 22–30. [Google Scholar]
- Liu, C.; Nelson, R. The cell biology of Tobacco mosaic virus replication and movement. Front. Plant Sci. 2013, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Niu, Y.; Zhang, K.; Liu, Y.; Zhou, X. Virus-derived transgenes expressing hairpin RNA give immunity to Tobacco mosaic virus and Cucumber mosaic virus. Virol. J. 2011, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Lerch, B. On the inhibition of plant virus multiplication by ribavirin. Antivir. Res. 1987, 7, 257–270. [Google Scholar] [CrossRef]
- Luvisi, A.; Panattoni, A.; Triolo, E. Eradication trials of tobacco mosaic virus using chemical drugs. Acta Virol. 2012, 56, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, J.; Hu, Z.; Li, S.; Su, X.; Zhang, J.; Yin, Y.; Xu, T.; Zhang, Z.; Chen, H. Anti-TMV activity and functional mechanisms of two sesquiterpenoids isolated from Tithonia diversifolia. Pestic. Biochem. Physiol. 2017, 140, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Yuan, W.; Ma, C.; White, J.; Zhang, Z.; Adeel, M.; Zhou, T.; Rui, Y.; Xing, B. Engineered nanomaterials suppress Turnip mosaic virus infection in tobacco (Nicotiana benthamiana). Environ. Sci. Nano. 2018, 5, 1685–1693. [Google Scholar] [CrossRef]
- Elsharkawy, M.; Derbalah, A. Antiviral activity of titanium dioxide nanostructures as a control strategy for broad bean strain virus in faba bean Pest Manag. Sci. 2018, 75, 828–834. [Google Scholar]
- Peza-Ledesma, C.L.; Escamilla-Perea, L.; Nava, R.; Pawelec, B.; Fierro, J.L.G. Supported gold catalysts in SBA-15 modified with TiO2 for oxidation of carbon monoxide. Appl. Catal. A Gen. 2010, 375, 37–48. [Google Scholar] [CrossRef]
- Torres-Pacheco, I.; Garzon-Tiznado, J.A.; Brown, J.; Becerra-Flora, A.; Rivera-Bustamante, R. Detection and distribution of geminiviruses in Mexico and the southern United States. Phytopathology 1996, 86, 1186–1192. [Google Scholar] [CrossRef]
- Hernández-Santiago, R.; Vargas-Hernández, M.; Zamora-Macorra, E.J. Evaluation of TMV resistance inducers in tomato. Rev. Mex. Cienc. Agric. 2020, 11, 377–390. [Google Scholar]
- Junglee, S.; Urban, L.; Huguette, S.; Lopez, F. Optimized Assay for Hydrogen Peroxide Determination in Plant Tissue Using Potassium Iodide. Am. J. Anal. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Leonardi, C.; Romano, D. PAL activities in asparagus spears during storage after ammonium sulfate treatments. Postharvest Biol. Technol. 2018, 140, 34–41. [Google Scholar] [CrossRef]
- Afiyanti, M.; Chen, H.J. Catalase activity is modulated by calcium and calmodulin in detached mature leaves of sweet potato. J. Plant Physiol. 2014, 171, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Ahmad, H.; Ali, M.; Ren, K.; Cheng, Z. Aqueous garlic extract stimulates growth and antioxidant enzymes activity of tomato (Solanum lycopersicum). Sci. Hortic. 2018, 240, 139–146. [Google Scholar] [CrossRef]
- Chen, N.; Yang, H.; Sun, Y.; Niu, J.; Liu, S. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 2012, 38, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Akbar, S.; Wei, Y.; Yuan, Y.; Khan, M.T.; Qin, L.; Powell, A.; Chen, B.; Zhang, M. Gene expression profiling of reactive oxygen species (ROS) and antioxidant defense system following Sugarcane mosaic virus (SCMV) infection. BMC Plant Biol. 2020, 20, 532. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Kapoor, H.C.; Lodha, M.L. Modification of Antioxidant Status of Host Cell in Response to Bougainvillea Antiviral Proteins. J. Plant Biochem. Biotechnol. 2004, 13, 113–118. [Google Scholar] [CrossRef]
- Yu, F.; Huaxia, Y.; Lu, W.; Wu, C.A.; Cao, X.; Guo, X. GhWRKY15, a member of the WRKY transcription factor family identified from cotton (Gossypium hirsutum L.), is involved in disease resistance and plant development. BMC Plant Biol. 2012, 12, 144. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.Y.; Yu, S.H.; Choi, D. Involvement of hydrogen peroxide in repression of catalase in TMV-infected resistant tobacco. Mol. Cells 2003, 15, 364–369. [Google Scholar]
- Mazurkova, N.A.; Spitsyna, Y.; Shikina, N.; Ismagilov, Z.; Zagrebelny, S.; Ryabchikova, E. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnol. Russ. 2010, 5, 417–420. [Google Scholar] [CrossRef]
- Qi, M.; Liu, Y.; Li, T. Nano-TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol. Trace Elem. Res. 2013, 156, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šebesta, M.; Kolenčík, M.; Sunil, B.R.; Illa, R.; Mosnáček, J.; Ingle, A.P.; Urík, M. Field Application of ZnO and TiO2 Nanoparticles on Agricultural Plants. Agronomy 2021, 11, 2281. [Google Scholar] [CrossRef]



| Symptom Description Level | Category | Percentage in Leaf (%) |
|---|---|---|
| A few apical wrinkles Small yellow spots were approximately 1 mm in diameter, only visible with light exposure | 1 | 0–10 |
| Light yellow spots on apical leaves were clearly visible | 2 | 10–20 |
| These spots appeared to form networks primarily at the base of the apical leaves | 3 | 20–30 |
| Evident network and little protuberances | 4 | 30–40 |
| The protuberance rolled up the leaves and necrosis | 5 | 40–50 |
| Treatment | High (mm) | Diameter (mm) | Disease Symptoms |
|---|---|---|---|
| Water | 21.0 b | 4.3 a | 0.88 d |
| TiO2 150 μg/mL | 20.2 b | 3.7 b | 1.00 d |
| TMV | 13.7 c | 3.02 c | 3.65 a |
| TMV + TiO2 50 μg/mL | 22.2 b | 4.1 ab | 3.50 ab |
| TMV + TiO2 100 μg/mL | 22.6 b | 4.0 ab | 3.58 a |
| TMV + TiO2 150 μg/mL | 30.4 a | 4.5 a | 2.10 c |
| TMV + TiO2 200 μg/mL | 21.4 b | 3.7 b | 2.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acuña-Fuentes, N.L.; Vargas-Hernandez, M.; Rivero-Montejo, S.d.J.; Rivas-Ramirez, L.K.; Macias-Bobadilla, I.; Palos-Barba, V.; Rivera-Muñoz, E.M.; Guevara-Gonzalez, R.G.; Torres-Pacheco, I. Antiviral Activity of TiO2 NPs against Tobacco Mosaic Virus in Chili Pepper (Capsicum annuum L.). Agriculture 2022, 12, 2101. https://doi.org/10.3390/agriculture12122101
Acuña-Fuentes NL, Vargas-Hernandez M, Rivero-Montejo SdJ, Rivas-Ramirez LK, Macias-Bobadilla I, Palos-Barba V, Rivera-Muñoz EM, Guevara-Gonzalez RG, Torres-Pacheco I. Antiviral Activity of TiO2 NPs against Tobacco Mosaic Virus in Chili Pepper (Capsicum annuum L.). Agriculture. 2022; 12(12):2101. https://doi.org/10.3390/agriculture12122101
Chicago/Turabian StyleAcuña-Fuentes, Noemi L., Marcela Vargas-Hernandez, Samantha de Jesus Rivero-Montejo, Luisa K. Rivas-Ramirez, Israel Macias-Bobadilla, Viviana Palos-Barba, Eric M. Rivera-Muñoz, Ramon G. Guevara-Gonzalez, and Irineo Torres-Pacheco. 2022. "Antiviral Activity of TiO2 NPs against Tobacco Mosaic Virus in Chili Pepper (Capsicum annuum L.)" Agriculture 12, no. 12: 2101. https://doi.org/10.3390/agriculture12122101
APA StyleAcuña-Fuentes, N. L., Vargas-Hernandez, M., Rivero-Montejo, S. d. J., Rivas-Ramirez, L. K., Macias-Bobadilla, I., Palos-Barba, V., Rivera-Muñoz, E. M., Guevara-Gonzalez, R. G., & Torres-Pacheco, I. (2022). Antiviral Activity of TiO2 NPs against Tobacco Mosaic Virus in Chili Pepper (Capsicum annuum L.). Agriculture, 12(12), 2101. https://doi.org/10.3390/agriculture12122101








