Aqueous Seaweed Extract Alleviates Salinity-Induced Toxicities in Rice Plants (Oryza sativa L.) by Modulating Their Physiology and Biochemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seaweed Collection, Extraction and Extract Preparation
2.2. Rice Seed Collection and Preparation
2.3. Preparation of Salt Solutions
2.4. Experimental Design
2.5. Effect of SWE on Rice Seed Germination under Salinity Stress
- n = number of newly germinated seeds at time T (25 °C)
- T = hours from the beginning of the germination test
- ∑n = final germination.
2.6. Effect of SWE on Rice Salt Alleviation
2.6.1. Physiology
2.6.2. Leaf Mortality
2.6.3. Biochemistry
2.7. Statistical Analysis
3. Results
3.1. Germination Parameters
3.2. Growth Parameters
3.3. Physiological Parameters
3.4. Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S.; et al. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef]
- Kalaivani, K.; Kalaiselvi, M.M.; Senthil-Nathan, S. Effect of Methyl Salicylate (MeSA) induced changes in rice plant (Oryza sativa) that affect growth and development of the rice leaffolder, Cnaphalocrocis medinalis. Physiol. Mol. Plant Pathol. 2018, 101, 116–126. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Yasuor, H.; Yermiyahu, U.; Ben-Gal, A. Consequences of irrigation and fertigation of vegetable crops with variable quality water: Israel as a case study. Agric. Water Manag. 2020, 242, 106362. [Google Scholar] [CrossRef]
- Choudhary, O.P.; Grattan, S.R.; Minhas, P.S. Sustainable crop production using saline and sodic irrigation waters. In Alternative Farming Systems, Biotechnology, Drought Stress and Ecological Fertilisation; Springer: Berlin/Heidelberg, Germany, 2011; pp. 293–318. [Google Scholar]
- Singh, A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Bosch, A.; Rigol-Sanchez, J.P.; Vallejos, A.; Andreu, J.M.; Ceron, J.C.; Molina-Sanchez, L.; Sola, F. Impacts of agricultural irrigation on groundwater salinity. Environ. Earth Sci. 2018, 77, 197. [Google Scholar] [CrossRef] [Green Version]
- FAO. Management of Salt Affected Soils: ‘Soil Management’ under ‘FAO SOILS PORTAL’. In Food and Agriculture Organization’ of the ‘United Nations’. Rome. 2020. Available online: http://www.fao.org/soils-portal/soil-management/management-of-some-problemsoils/salt-affected-soils/more-information-on-salt-affected-soils/en/ (accessed on 15 November 2022).
- Hossain, M.A.; Araki, H.; Takahashi, T. Poor grain filling induced by waterlogging is similar to that in abnormal early ripening in wheat in Western Japan. Field Crops Res. 2011, 123, 100–108. [Google Scholar] [CrossRef]
- Luo, X.; Dai, Y.; Zheng, C.; Yang, Y.; Chen, W.; Wang, Q.; Chandrasekaran, U.; Du, J.; Liu, W.; Shu, K. The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol. 2021, 229, 950–962. [Google Scholar] [CrossRef]
- Kalaivani, K.; Maruthi-Kalaiselvi, M.; Senthil-Nathan, S. Seed treatment and foliar application of methyl salicylate (MeSA) as a defense mechanism in rice plants against the pathogenic bacterium, Xanthomonas oryzae pv. oryzae. Pest. Biochem. Physiol. 2021, 171, 104718. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and biochemical responses of Glycine max (L.) Merr. to the use of seaweed extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef]
- Stirk, W.A.; Rengasamy, K.R.; Kulkarni, M.G.; van Staden, J. Plant biostimulants from seaweed: An Overview. Chem. Biol. Plant Biostimulants 2020, 2, 31–55. [Google Scholar]
- Bohra, A.; Jha, U.C.; Jha, R.; Naik, S.J.; Maurya, A.K.; Patil, P.G. Genomic interventions for biofortification of food crops. In Quality Breeding in Field Crops; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–21. [Google Scholar]
- Nakashima, K.; Yanagihara, S.; Muranaka, S.; Oya, T. Development of Sustainable Technologies to Increase Agricultural Productivity and Improve Food Security in Africa. Jpn. Agric. Res. Q. 2022, 56, 7–18. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Abiotic stress effects on performance of horticultural crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.M.; Francies, R.M.; Rasool, S.N.; Reddy, V.R. Breeding for tolerance stress triggered by salinity in rice. Int. J. Appl. Biol. Pharm. Technol. 2014, 5, 167–176. [Google Scholar]
- Rasel, H.M.; Hasan, M.R.; Ahmed, B.; Miah, M.S. Investigation of soil and water salinity, its effect on crop production and adaptation strategy. Int. J. Water Res. Environ. Eng. 2013, 5, 475–481. [Google Scholar]
- Pessarakli, M.; Szabolcs, I. Soil salinity and sodicity as particular plant/crop stress factors. In Handbook of Plant and Crop Stress, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 3–21. [Google Scholar]
- Shi, W.; Yin, X.; Struik, P.C.; Xie, F.; Schmidt, R.C.; Jagadish, K.S. Grain yield and quality responses of tropical hybrid rice to high night-time temperature. Field Crops Res. 2016, 190, 18–25. [Google Scholar] [CrossRef]
- Liu, H.; Hussain, S.; Zheng, M.; Sun, L.; Fahad, S.; Huang, J.; Cui, K.; Nie, L. Progress and constraints of dry direct-seeded rice in China. J. Food Agric. Environ. 2014, 12, 465–472. [Google Scholar]
- Stanley-Raja, V.; Senthil-Nathan, S.; Chanthini, K.M.P.; Sivanesh, H.; Ramasubramanian, R.; Karthi, S.; Shyam-Sundar, N.; Vasantha-Srinivasan, P.; Kalaivani, K. Biological activity of chitosan inducing resistance efficiency of rice (Oryza sativa L.) after treatment with fungal based chitosan. Sci. Rep. 2021, 11, 20488. [Google Scholar] [CrossRef]
- Chanthini, K.M.; Stanley-Raja, V.; Thanigaivel, A.; Karthi, S.; Palanikani, R.; ShyamSundar, N.; Sivanesh, H.; Soranam, R.; Senthil-Nathan, S. Sustainable agronomic strategies for enhancing the yield and nutritional quality of wild tomato, Solanum Lycopersicum (l) var Cerasiforme Mill. Agronomy 2019, 9, 311. [Google Scholar] [CrossRef] [Green Version]
- Jisha, K.C.; Puthur, J.T. Seed priming with BABA (β-amino butyric acid): A cost-effective method of abiotic stress tolerance in Vigna radiata (L.) Wilczek. Protoplasma 2016, 253, 277–289. [Google Scholar] [CrossRef]
- Radanielson, A.M.; Angeles, O.; Li, T.; Ismail, A.M.; Gaydon, D.S. Describing the physiological responses of different rice genotypes to salt stress using sigmoid and piecewise linear functions. Field Crops Res. 2018, 220, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Fujita, M.; Islam, M.N.; Ahamed, K.U.; Nahar, K. Performance of four irrigated rice varieties under different levels of salinity stress. Int. J. Integr. Biol. 2019, 6, 85–90. [Google Scholar]
- Thitisaksakul, M.; Tananuwong, K.; Shoemaker, C.F.; Chun, A.; Tanadul, O.U.; Labavitch, J.M.; Beckles, D.M. Effects of timing and severity of salinity stress on rice (Oryza sativa L.) yield, grain composition, and starch functionality. J. Agric. Food Chem. 2015, 63, 2296–2304. [Google Scholar] [CrossRef] [Green Version]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford assay for determining protein concentration. Cold Spring Harb. Protoc. 2020, 4, 102269. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.S.; Miller, E.E.; Pratt, D.E. Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Manaf, H.H. Beneficial effects of exogenous selenium, glycine betaine and seaweed extract on salt stressed cowpea plant. Ann. Agric. Sci. 2016, 61, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequence of minor changes in conditions. Ann. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Maness, N. Extraction and analysis of soluble carbohydrates. In Plant Stress Tolerance; Humana Press: Totowa, NJ, USA, 2010. [Google Scholar]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Bonomelli, C.; Celis, V.; Lombardi, G.; Mártiz, J. Salt stress effects on avocado (Persea americana Mill.) plants with and without seaweed extract (Ascophyllum nodosum) application. Agronomy 2018, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Kalaivani, K.; Kalaiselvi, M.M.; Senthil-Nathan, S. Effect of methyl salicylate (MeSA), an elicitor on growth, physiology and pathology of resistant and susceptible rice varieties. Sci. Rep. 2016, 6, 34498. [Google Scholar] [CrossRef] [Green Version]
- Seethalakshmi, S.; Umarani, R. Biochemical changes during imbibition stages of seed priming in tomato. Int. J. Chem. Stud. 2018, 6, 454–456. [Google Scholar]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Chanthini, K.M.P.; Senthil-Nathan, S.; Stanley-Raja, V.; Thanigaivel, A.; Karthi, S.; Sivanesh, H.; Sundar, N.S.; Palanikani, R.; Soranam, R. Chaetomorpha antennina (Bory) Kützing derived seaweed liquid fertilizers as prospective bio-stimulant for Lycopersicon esculentum (Mill). Biocatal. Agric. Biotechnol. 2019, 20, 101190. [Google Scholar] [CrossRef]
- Zhumabekova, Z.; Xu, X.; Wang, Y.; Song, C.; Kurmangozhinov, A.; Sarsekova, D. Effects of Sodium Chloride and Sodium Sulfate on Haloxylon ammodendron Seed Germination. Sustainability 2020, 12, 4927. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Begum, M.; Bordoloi, B.C.; Singha, D.D.; Ojha, N.J. Role of seaweed extract on growth, yield and quality of some agricultural crops: A review. Agric. Rev. 2018, 39, 321–326. [Google Scholar] [CrossRef]
- Amabika, S.; Sujatha, K. Effect of priming with seaweed extracts on germination and vigour under different water holding capacities. Seaweed Res. Utiln. 2015, 37, 37–44. [Google Scholar]
- Sangare, S.K.; Compaore, E.; Buerkert, A.; Vanclooster, M.; Sedogo, M.P.; Bielders, C.L. Field-scale analysis of water and nutrient use efficiency for vegetable production in a West African urban agricultural system. Nutr. Cycl. Agroecosyst. 2012, 92, 207–224. [Google Scholar] [CrossRef]
- Patel, R.V.; Pa, K.Y. Effect of hydropriming and biopriming. Res. J. Agric. For. 2017, 5, 1–14. [Google Scholar]
- Latique, S.; Mohamed Aymen, E.; Halima, C.; Chérif, H.; Mimoun, E.K. Alleviation of salt stress in durum wheat (Triticum durum L.) seedlings through the application of liquid seaweed extracts of Fucus spiralis. Commun. Soil Sci. Plant Anal. 2017, 48, 2582–2593. [Google Scholar] [CrossRef]
- du Jardin, P.; Xu, L.; Geelen, D. Agricultural Functions and Action Mechanisms of Plant Biostimulants (PBs) an Introduction. Chem. Biol. Plant Biostimulants 2020, 1–30. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravinejad, F.; Heydari, R.; Farboodnia, T. Effect of salinity on organic solutes contents in barley. Pak. J. Biol. Sci. 2019, 12, 158–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Filippo-Herrera, D.A.; Hernández-Carmona, G.; Muñoz-Ochoa, M.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E. Monthly variation in the chemical composition and biological activity of Sargassum horridum. Bot. Mar. 2018, 61, 91–102. [Google Scholar] [CrossRef]
- Arioli, T.; Mattner, S.W.; Winberg, P.C. Applications of seaweed extracts in Australian agriculture: Past, present and future. J. Appl. Phycol. 2015, 27, 2007–2015. [Google Scholar] [CrossRef] [Green Version]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Oude Essink, G.H.; Van Baaren, E.S.; De Louw, P.G. Effects of climate change on coastal groundwater systems: A modeling study in the Netherlands. Water Resour. Res. 2010, 46, 1–16. [Google Scholar] [CrossRef]
- Shahi, C.; Bargali, K.; Bargali, S.S. Assessment of salt stress tolerance in three varieties of rice (Oryza sativa L.). Progress. Agric. 2015, 6, 50–56. [Google Scholar]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, Z.K.; Khan, M.A.; Flowers, T.J. Causes of sterility in seed set of rice under salinity stress. J. Agron. Crop Sci. 2001, 187, 25–32. [Google Scholar] [CrossRef]
- Zou, P.; Lu, X.; Zhao, H.; Yuan, Y.; Meng, L.; Zhang, C.; Li, Y. Polysaccharides derived from the brown algae Lessonia nigrescens enhance salt stress tolerance to wheat seedlings by enhancing the antioxidant system and modulating intracellular ion concentration. Front. Plant Sci. 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, F.; Deyab, M.; El-katony, T. Biochemical Composition and Bioactivity of Dictyota from Egypt; LAP LAMBERT Academic Publishing: Sunnyvale, CA, USA, 2017. [Google Scholar]
- Irakoze, W.; Prodjinoto, H.; Nijimbere, S.; Rufyikiri, G.; Lutts, S. NaCl and Na2SO4 salinities have different impact on photosynthesis and yield-related parameters in rice (Oryza sativa L.). Agronomy 2020, 10, 864. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, A.; Safdar, L.B.; Zafar, M.M.; Rui, Y.; Shakeel, A.; Shaukat, A.; Ashraf, M.; Gong, W.; Yuan, Y. Salt stress induces physiochemical alterations in rice grain composition and quality. J. Food Sci. 2020, 85, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Calingacion, M.; Laborte, A.; Nelson, A.; Resurreccion, A.; Concepcion, J.C.; Daygon, V.D.; Mumm, R.; Reinke, R.; Dipti, S.; Bassinello, P.Z.; et al. Diversity of global rice markets and the science required for consumer-targeted rice breeding. PLoS ONE 2014, 9, e85106. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.S.; Lee, J.O.; Ge, L. Comparison of terrestrial laser scanner with digital aerial photogrammetry for extracting ridges in the rice paddies. Surv. Rev. 2009, 41, 253–267. [Google Scholar] [CrossRef]
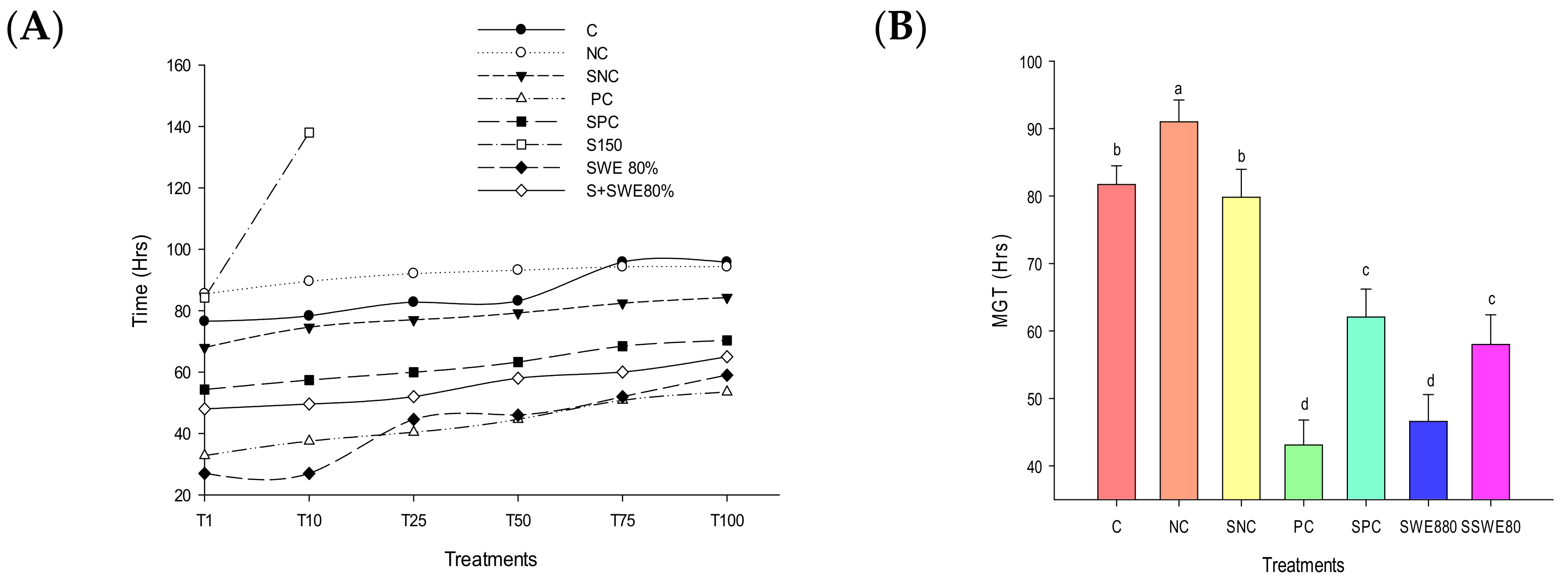
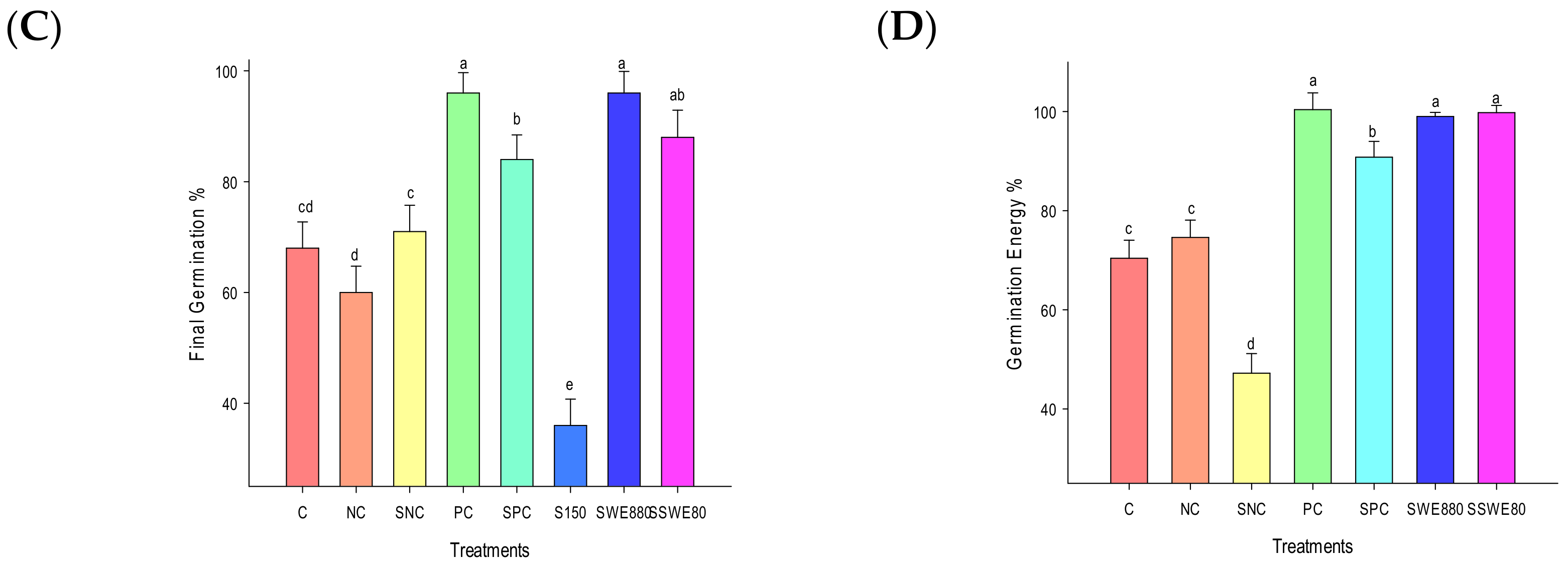
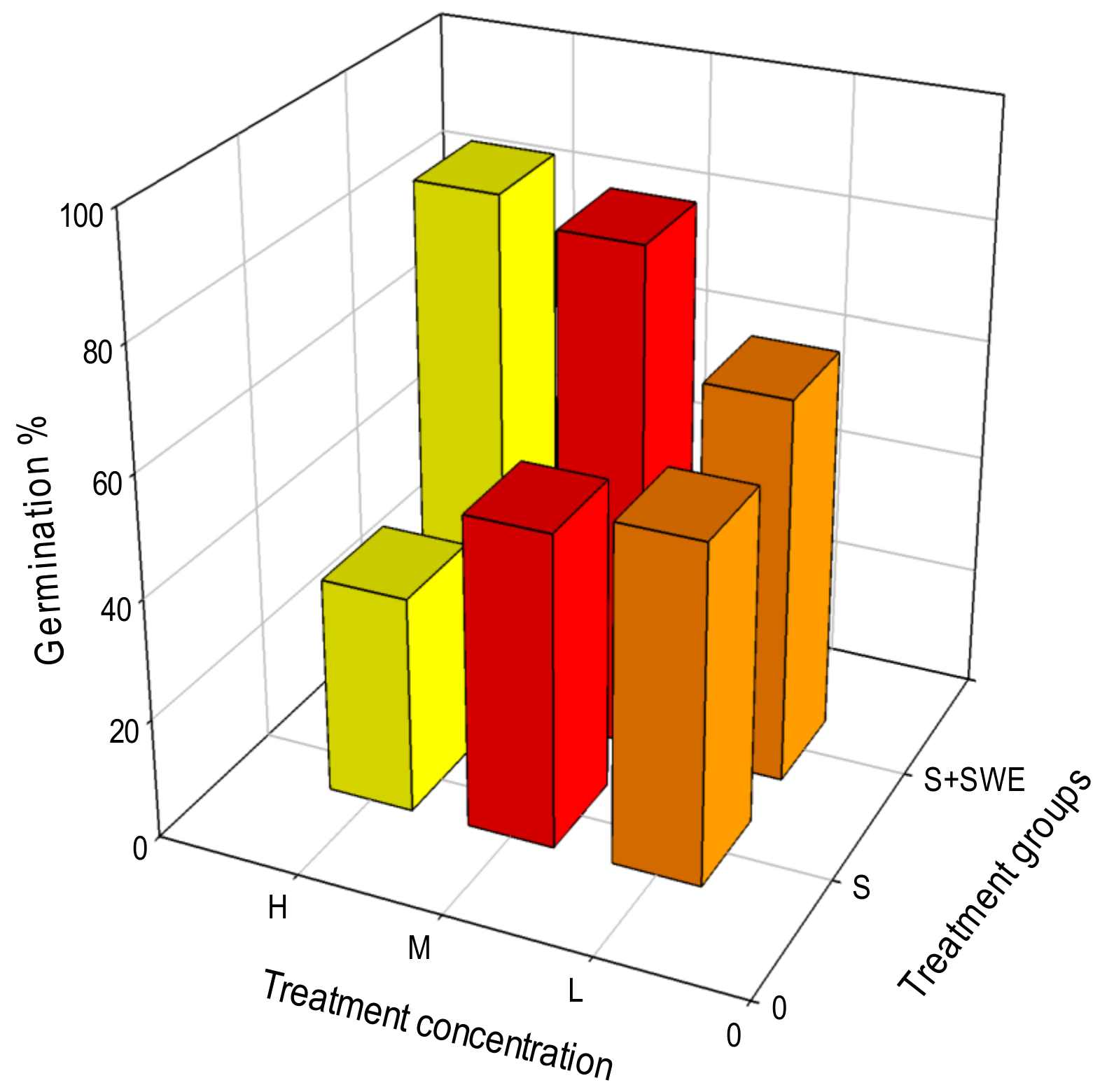
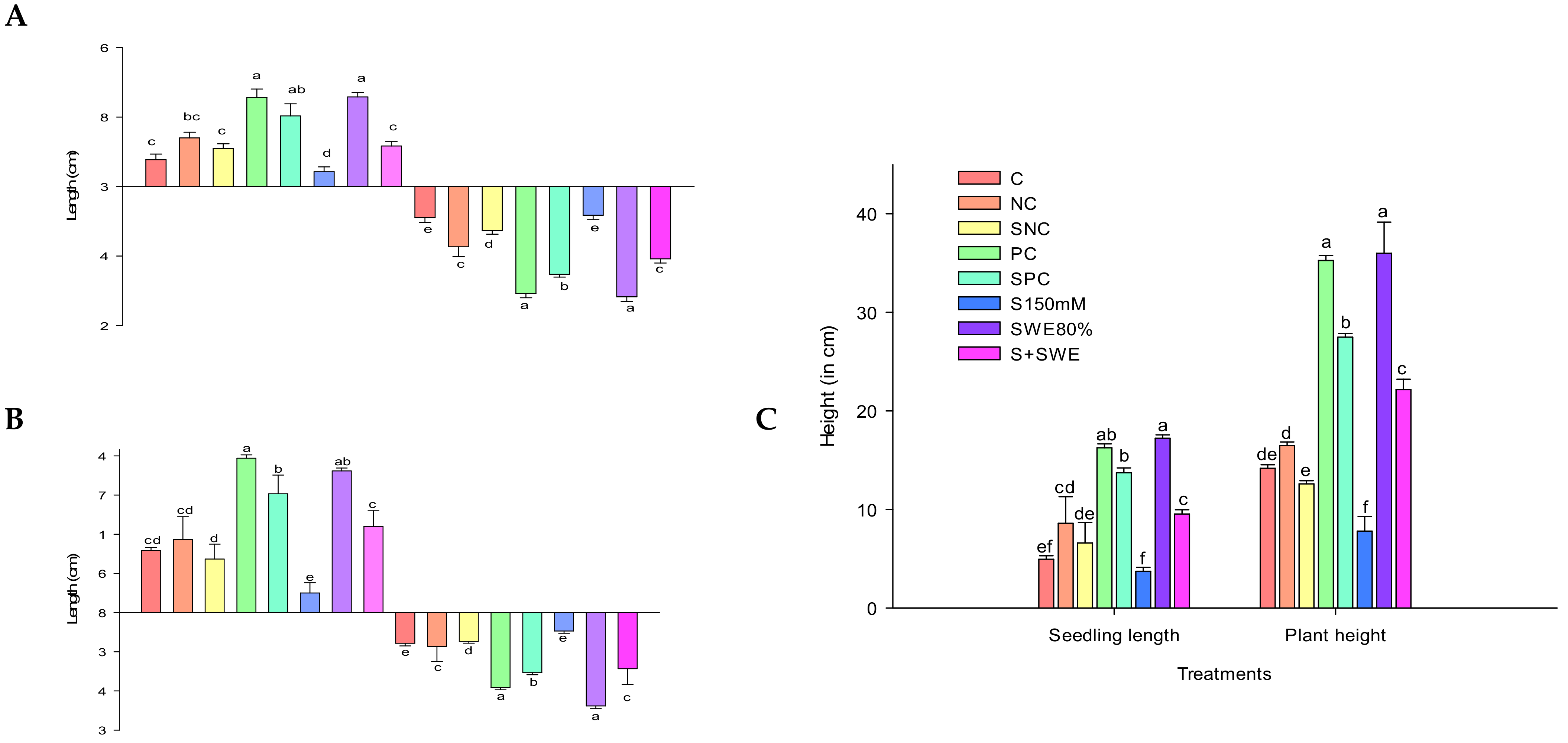


| C | NC | SNC | PC | SPC | S150 mM | SWE80 | S + SWE |
|---|---|---|---|---|---|---|---|
| Control | Negative Control | Salt 150 mM + NC | Positive control (Commercial biostimulant) | Salt 150 mM + PC | Salt treatment at 150 mM | 80% SWE concentration | Salt 150 mM + SWE 80 |
| Treatments | SVI |
|---|---|
| C | 333.083 ± 1.378 g |
| NC | 541.5 ± 4.23 e |
| SNC | 498.67± 3.98 f |
| PC | 1555.65 ± 1.37 b |
| SPC | 1160.17 ± 2.95 d |
| S150 | 133.267 ± 1.938 h |
| SWE80 | 1651.37 ± 3.14 a |
| SSWE80 | 1407.82 ± 3.74 c |
| Treatments | RWC (%) | Panicle Length (cm) | Tiller Number/Hill | 100 Grain wt (g) | Grain Yield (kg/Hectare) |
|---|---|---|---|---|---|
| C | 70.4 ± 4.28 c | 23 ± 4.12 bc | 44.8 ± 3.83 cd | 2.299 ± 0.359 b | 5668.8 ± 25.2 e |
| NC | 75.2 ± 4.44 bc | 26.146 ± 1.452 ab | 52 ± 3.16 bcd | 2.849 ± 0.353 ab | 6867.9 ± 32.9 d |
| SNC | 46 ± 23.1 d | 17.81 ± 3.03 cd | 38.02 ± 3.16 de | 2.155 ± 0.243 bc | 4842.2 ± 37.9 f |
| PC | 95.2 ± 3.7 a | 30.18 ± 3.19 a | 65.63 ± 3.35 ab | 3.391 ± 0.445 a | 8253.6 ± 39.9 a |
| SPC | 85.4 ± 3.65 abc | 27.58 ± 4.61 ab | 59.38 ± 4.16 abc | 3.261 ± 0.397 a | 7467.1 ± 51 c |
| S150 | 40.6 ± 3.97 d | 13.4 ± 3.05 d | 25.2 ± 3.96 e | 1.455 ± 0.398 c | 3230.8 ± 37.7 g |
| SWE80 | 93 ± 4.12 ab | 30.71 ± 3.03 a | 66.96 ± 2.05 a | 3.502 ± 0.412 a | 8174.4 ± 46.9 a |
| SSWE | 82.6 ± 3.97 abc | 28.39 ± 3.5 ab | 56.37 ± 2.87 abc | 3.345 ± 0.357 a | 7743.7 ± 42.1 b |
| Treatments | Leaf Mortality (%) | TSP (mg/gFW) | TPC (GAE mg/g DW) | SOD (U/mg Protein) |
|---|---|---|---|---|
| C | 2.52 ± 0.396 c | 1.786 ± 0.0397 d | 16.16 ± 1.85 b | 1.76 ± 0.428 ab |
| NC | 2.44 ± 0.365 c | 1.344 ± 0.0355e f | 26.08 ± 2.008 a | 2.3 ± 0.346 ab |
| SNC | 5.2 ± 2.68 b | 1.3 ± 0.0346d e | 11.96 ± 2.24 bc | 1.52 ± 0.396 b |
| PC | 1.048 ± 0.0013 e | 2.192 ± 0.0317 a | 28.04 ± 3.16 a | 2.454 ± 0.415 ab |
| SPC | 1.23 ± 0.3 d | 1.738 ± 0.0445b c | 25.89 ± 3.09 a | 2.192 ± 0.259 ab |
| S150 | 8.4 ± 1.14 a | 1.21 ± 0.0351 ef | 10.86 ± 2.144 c | 1.3 ± 0.346 b |
| SWE80 | 1.118 ± 0.0604 d | 1.93 ± 0.0383 b | 27.66 ± 3.35 a | 2.45 ± 0.415 ab |
| SSWE | 1.248 ± 0.233 de | 1.656 ± 0.0304 c | 25.07 ± 2.3 a | 3 ± 1.414 a |
| Treatments | Carbohydrate (mg/g DW) | Protein (mg/g DW) | Protein: Starch | |
|---|---|---|---|---|
| TSS | Starch | |||
| C | 138.2 ± 2.21 e | 588 ± 3.8 ef | 53.1 ± 1.8 d | 0.090 ± 0.001 c |
| NC | 157 ± 2. 1c | 662.5 ± 4.03 d | 62.4 ± 2.03 b | 0.0941 ± 0.0001 b |
| SNC | 164.2 ± 2.03 b | 684 ± 3.85 c | 54 ± 1.7 d | 0.0789 ± 0.001 f |
| PC | 142.1 ± 1.98 d | 567 ± 3.81 g | 67.36 ± 1.89 ab | 0.118 ± 0.002 a |
| SPC | 148.34 ±2.07 d | 713 ± 4.02 b | 59 ± 2.01 bc | 0.082 ± 00.01 e |
| S150 | 168.3 ± 2.31 b | 719 ± 4.36 ab | 49.4 ± 2.02 e | 0.0687 ± 0.001 g |
| SWE80 | 145 ± 1.9 d | 581.23 ± 4.2 e | 70.2 ± 1.9 a | 0.1207 ± 0.002 a |
| SSWE | 179 ± 2.13 a | 725 ± 3.95 a | 64.26 ± 2 b | 0.0886 ± 0.001 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanthini, K.M.-P.; Senthil-Nathan, S.; Pavithra, G.-S.; Malarvizhi, P.; Murugan, P.; Deva-Andrews, A.; Janaki, M.; Sivanesh, H.; Ramasubramanian, R.; Stanley-Raja, V.; et al. Aqueous Seaweed Extract Alleviates Salinity-Induced Toxicities in Rice Plants (Oryza sativa L.) by Modulating Their Physiology and Biochemistry. Agriculture 2022, 12, 2049. https://doi.org/10.3390/agriculture12122049
Chanthini KM-P, Senthil-Nathan S, Pavithra G-S, Malarvizhi P, Murugan P, Deva-Andrews A, Janaki M, Sivanesh H, Ramasubramanian R, Stanley-Raja V, et al. Aqueous Seaweed Extract Alleviates Salinity-Induced Toxicities in Rice Plants (Oryza sativa L.) by Modulating Their Physiology and Biochemistry. Agriculture. 2022; 12(12):2049. https://doi.org/10.3390/agriculture12122049
Chicago/Turabian StyleChanthini, Kanagaraj Muthu-Pandian, Sengottayan Senthil-Nathan, Ganesh-Subbaraja Pavithra, Pauldurai Malarvizhi, Ponnusamy Murugan, Arulsoosairaj Deva-Andrews, Muthusamy Janaki, Haridoss Sivanesh, Ramakrishnan Ramasubramanian, Vethamonickam Stanley-Raja, and et al. 2022. "Aqueous Seaweed Extract Alleviates Salinity-Induced Toxicities in Rice Plants (Oryza sativa L.) by Modulating Their Physiology and Biochemistry" Agriculture 12, no. 12: 2049. https://doi.org/10.3390/agriculture12122049
APA StyleChanthini, K. M.-P., Senthil-Nathan, S., Pavithra, G.-S., Malarvizhi, P., Murugan, P., Deva-Andrews, A., Janaki, M., Sivanesh, H., Ramasubramanian, R., Stanley-Raja, V., Ghaith, A., Abdel-Megeed, A., & Krutmuang, P. (2022). Aqueous Seaweed Extract Alleviates Salinity-Induced Toxicities in Rice Plants (Oryza sativa L.) by Modulating Their Physiology and Biochemistry. Agriculture, 12(12), 2049. https://doi.org/10.3390/agriculture12122049






