Abstract
Around the world, salinity a critical limiting factor in agricultural productivity. Plant growth is affected by salt stress at all stages of development. The contemporary investigation focused on Chaetomorpha antennina aqueous extracts (SWEs) to decrease the effects of salt strain on rice germination, growth, yield, and the production of key biological and biochemical characters of the rice, Oryza sativa L. (Poaceae). SWE improved the germination capacities of rice seedlings by promoting their emergence 36.27 h prior to those that had been exposed to saline stress. The creation of 79.647% longer radicles by SWE treatment on salt-stressed seeds which boosted the establishment effectiveness of seeds produced under salt stress longer radicles resulted in plants that were 64.8% taller. SWE treatment was effective in revoking the levels of protein (26.9%), phenol (35.54%), and SOD (41.3%) enzyme levels that were previously constrained by salinity stress. Additionally, SWE were also efficient in retaining 82.6% of leaf water content and enhancing the production of photosynthetic pigments affected by salt exposure earlier. The improvement in plant functionality was evident from the display of increase in tiller numbers/hill (62.36%), grain yield (58.278%), and weight (56.502%). The outcome of our research shows that SWEs protected the plants from the debarring effects of salinity by enhancing the plant functionality and yield by mechanistically enriching their physiological (germination and vegetative growth) and biochemical attributes (leaf RWC, photosynthetic pigments, protein, phenol, and SOD). Despite the increase in TSS and starch levels in rice grain exposed to salinity stress, SWE improved the grain protein content thus cumulatively enhancing rice nutrition and marketability. The current investigation reveals that the extracts of C. antennina can help alleviate rice plants from salt stress in an efficient, eco-friendly, as well as economical way.
1. Introduction
Agriculture is confronting multiple issues that are escalating. Increasing food production to feed an expanding population is a major challenge. This can be attained by either expanding farmland for increased food production or by improving existing yields by applying fertilizers or using revolutionary technologies such as precision farming systems, cutting-edge irrigation, and ecologically feasible crop revolutions [1]. Abiotic stressors are major environmental restrictions reducing crop productivity globally. These abiotic stressors induce an osmotic action, specific ion effect, creation of nutritional imbalance, and oxidative damage to biomolecules and membranes [2].
Coastal areas provide ideal soil and climate conditions for agriculture, which has been practiced from time immemorial and is vital to the coastal economy. It is important to focus on coastal agriculture, making it more fruitful and attractive, and integrating it into the coastline, plans to address forthcoming encounters of food besides nutritive security for an ever-increasing human population, as well as climate change [3]. Using water resources that are low in quality such as wells and brackish surface waters causes secondary salinization [4]. Soil flooding in arid as well as humid regions causes soluble salt accumulation at the soil surface due to increased evaporation. These constraints severely limit the production of arable land, particularly in emerging nations [5]. Soil salinity is also caused by human activities such as fertilising crops. Due to the potassium in fertilisers, which can naturally create salt sylvite. Salts are a naturally occurring substance in water and soil. The ions Na+, K+, Ca2+, Mg2+, and Cl are in charge of salination. Normal soil pH ranges from 2.2 to 9.7, and anything beyond that causes salt content degradation in the soil. The long-term viability of irrigated farming is seriously threatened by the salinization of the soil. A major problem in agricultural sustainability is the possibility of salinization and waterlogging caused by inadequate irrigation. Globally, more than 3% of soil resources are now damaged by salt [6]. Moreover, this number is steadily increasing at a pace of two Mha annually. Numerous earlier experiments indicated that waterlogging and salinization reduce yields in a variety of crops [7]. Between 18 and 43% of agricultural productivity is lost due to salinization in arid and semiarid regions of the world. Saline soils range from salt contents of 9–18 and >18 millimhos/cm designated as moderate to highly saline soils [8]. According to Hossain et al. [9], root-zone salinization and water logging significantly reduce field crop output. According to reports, the combined impact of root-zone salinization and water logging is worse than each factor acting alone.
Plant salt sensitivity varies with developmental stage. Seed germination determines plant establishment in saltwater environments. Salinity can impair germination rates, resulting in uneven crop development and lower yields [10]. However, the plant retort to salinity at germination can be altered and sought out as a quick and reliable indication of plant establishment in salt-affected settings. To boost plant performance and protect plants from biotic and abiotic stresses, several amendments were utilized. They can be provided to germinating seeds or to plants during vegetative growth [11]. In this milieu, marine macroalgal extracts show promising effects in reducing the influence of abiotic strain on plant performances. Algae are well-known sources of plant macro- and micronutrients, as well as bioactive chemicals [12]. SWEs can be sprayed on leaves, added to hydroponic systems, or treated directly to the soil. Plants respond to their application in a variety of positive ways. SWEs have been shown to include larger bioactive compounds, including oligosaccharides and phlorotannins. Activating molecular and metabolic pathways, bioactive chemicals from SWE have been demonstrated to function as elicitor agents, promoting plant development and inducing stress responses. They are rich in growth-promoting phytohormones along with inorganic elements indispensable for plant development. The application of seaweed extracts (SWEs) as natural regulators increased crop development and yield to endure tough environmental influences [13].
O. sativa is an important grain and indispensable food for preponderance of people globally [14]. With rising rice consumption, farmers and agriculturalists are under pressure to meet demand while safeguarding the crop from diseases and pests [15]. Produce damages of up to 50–70% are stressors. As common crop, rice is cultured comprehensively in coastal areas frequently flooded by saline sea water at high tides [16]. Response of rice to salt varies greatly within species, allowing for genetic improvement for the progress of salinity stress resistant crops [17]. Salinity particularly affects the physical elements affecting rice productivity. Salinity is subsequently the most common soil concern in rice-growing countries, after drought, and it is limiting global rice output. Coastal soils are discreetly saline on the exterior, but severely saline in sub surface strata besides substrata due to a variety of environmental causes [18]. Soluble salts, particularly sodium chloride, are abundant in saline soil [19].
Poorly germinating seeds are not only a yield constrainer, but also serve as a potential host for various diseases [20]. Seeds with decreased vigour also have more difficulty responding to field conditions, causing stress [21]. Numerous seed priming approaches are used to boost seed quality and reduce yield loss [22]. Primed seeds have early emergence, a higher seed vigour index, and increased biomass along with yield [23]. Aside from enhancing disease resistance, the treated seeds may also endure abiotic stress [24]. Root-zone salinity has a noteworthy influence on yield components relevant to final grain yield. Salinity reduces the number of main branches/panicle, their dimension, and other yield-related caryopsis features. Around panicle initiation, the biggest salt effects on yield are noted, while plants recover pre-eminent from strain at the seedling stage [25]. In a harsh environment, seed priming is a meek and cost-effective technique to promote kernel germination, prompt seedling development, and yield. Seed priming with various inorganic and organic substances increased wheat salt tolerance.
The damaging effects of abiotic stress and the positive effects of organic amendments on rice have both been studied independently. However, little is known about the underlying processes that might link the capabilities of SWE in protecting the crop from salinity-induced toxicities and driving these stressed plants towards their active functioning. Henceforth, by analysing the effect of salt stress and the ameliorating effect of SWE through investigating the plant’s biochemical patterns and antioxidant enzymes, this study sought to identify the effects of liquid SWE of green alga C. antennina on rice physiology and biochemistry exposed to salinity strain.
2. Materials and Methods
2.1. Seaweed Collection, Extraction and Extract Preparation
The seaweed, C. antennina was collected (Colachel beach, Kanyakumari, Tamil Nadu, India; 8°14′5168″ N and 77°14′35.209″ E; December 2020), washed, and carried forward for extraction with boiling hot water (100 g/L; 1 h) (Figure 1). The concoction was clarified and the filtrated, SWE was stored until use (4 °C). The test solutions of SWE were devised by diluting the extract with distilled water of 60, 40, and 20 mL to obtain treatment concentration of SWE 40, 60, and 80%, respectively.
Figure 1.
Sample collection site, seaweed C. antennina. (A) Sample collection site (B) C. antennina attached to rocks (C) liquid SWE.
2.2. Rice Seed Collection and Preparation
During the growing season, rice seeds (TN1) were collected from farming areas. Seeds of uniform size and colour were preferred for the investigation, surface sterilized with 0.1% mercuric chloride, rinsed three times in sterile distilled water, and the trial was accomplished at the biopesticide and environmental toxicology lab, Environmental Science research centre (SPKCEES) of Manonmaniam Sundaranar University, Alwarkurichi.
2.3. Preparation of Salt Solutions
For the preparation of salt treatment solutions, 58.44 g of NaCl was dissolved in 1 L of distilled water to form the stock solution, from which respective quantities (0.8, 1.0 and 1.50) were made up to 10 mL using distilled water, viz., 9.2, 1, and 8.5 mL of distilled water to obtain treatment concentrations 80, 100, and 150 mM, respectively. Since treatment concentration of 150 mM was highly significant in causing salinity stress and the SWE was able to exert a positive influence on the aftereffects of salinity stress, only S150 mM was carried forward for future experiments (treatments with salt treatments <150 mM did not produce any statistically significant results).
2.4. Experimental Design
The experimentation consisted of different culture groups categorized by NaCl as well as SWE treatment concentrations. Starter fertilizer solution (SFS) was used as a negative control (NC) [23]. Commercial seaweed biostimulant solution (10%) was used as a positive control (PC). Both NC and PC treatments included exposure to salt (150 mM) designated as SNC and SPC. A treatment test involving a combination of SWE 80% with 150 mM of salt concentration was designated as S + SWE was also used (Table 1).

Table 1.
Treatments involved in experimental design.
The experiment to analyse the physiology and biochemistry of plants propagated in pots with sterile soil was brought out in randomized block design at the centre’s greenhouse. The pots (15 cm diameter, 750 mL volume, and sterilized sandy soil) containing one seedling were irrigated with the appropriate treatment solutions mentioned earlier. The pots were arranged in a complete randomized design and maintained under 22–28 °C with a relative humidity of 30% and a 16 h/8 h day/night photoperiod. All the treatments were irrigated with sterile distilled water twice a day. Seeds immersed in distilled water and pots irrigated with distilled water served as control (C).
2.5. Effect of SWE on Rice Seed Germination under Salinity Stress
Rice seeds (seeds/treatment) were primed in respective solutions (10 mL; overnight), dried, and placed in correspondingly labelled sterile petri plates, and incubated (25 ± 2 °C/consecutive 16:8 h LD). The seeds were observed for germination. Parameters associated with germination such as germination percentage (GP, %), average germination time (MGT, hours), germination energy (GE, %), and plantlet vigour index (SVI) as well as seedling growth (radicle–plumule, seedling lengths, cm) were noted [23].
where
- n = number of newly germinated seeds at time T (25 °C)
- T = hours from the beginning of the germination test
- ∑n = final germination.
2.6. Effect of SWE on Rice Salt Alleviation
2.6.1. Physiology
The heights of plant, root, and shoot were measured using a ruler in all treatments, 40 days post-planting of the seedling. At the booting stage, measurement of comparative aqueous content of the shoot (RWC%) along with panicle length (cm) and tiller number (per hill) was performed following the methods described earlier [25]. The ability of SWEs on plants grown under salinity stress in terms of productivity (grain kg/hectare) and yield traits was also estimated by collecting 100 grains from the panicle of each paddy plant per treatment and determining their weight on a standard laboratory weighing scale [26].
2.6.2. Leaf Mortality
The effect of SWE on leaf mortality of rice plants was determined by counting their numbers in each plant in all treatments and the percentage of increase or increase was estimated was compared with plants growing in non-saline conditions [27].
2.6.3. Biochemistry
The effect of CA-LSE on the biochemistry of rice plants grown under salinity stress was studied using standard protocols methods to estimate whole soluble protein (TSP, mg/gFW) and phenolics (TPC, mg/gDW) [28,29]. Photosynthetic characteristics were estimated by measuring the levels of pigments. Chlorophyll (Chl a and b) and carotenoids (Car) and were extracted and estimated (mg/gFW) [30]. Beyer and Fridovich’s method of super-oxide dismutase (SOD) estimation was employed and the level of the antioxidant enzyme was expressed in units (U/mg protein) [31].
Grain quality in terms of biochemistry was determined by estimating the quantities of total soluble sugars (TSS) and starch through the Anthrone method [32]. Grain protein content was also estimated [28]. The ratio of protein to starch contents was also estimated.
2.7. Statistical Analysis
To identify statistically significant variations in means between treatments, ANOVA was utilized. When statistical differences were found, the Tukey–Kramer post-hoc test was used. All experiments employed a maximum of 5 biological replicates. Tests after germination analysis employed only 8 treatments employing the highest concentrations in both salt and SWE (S150 mM and SWE 80%) for comparisons. When p was ≤ 0.05, the differences were considered statistically significant.
3. Results
3.1. Germination Parameters
The germination of rice seeds exposed to salinity stress was severely affected which was evident from their GTC (Figure 2A). Salinity stress deferred the initial germination of rice seeds by 7.73 h compared with control. SWE promoted early germination of rice seeds at 49.54 h compared with control. SWE were able to promote early germination of rice seeds under salinity stress stimulating their germination 36.27 h before that of those grown under salt stress (Figure 3). Consequently, the MGT of rice seeds exposed to salinity stress was also brought down by SWE treatment (Figure 2B). While SFS-treated salinity-exposed rice seeds (SNC) prompted MGT by only 1.89 h compared with control, 50% of S + SWE seeds emerged 23.72 h compared with control (F6,28 = 126.19; p < 0.0001). Seeds treated with 150 mM of salt concentration did not reach 50% of emergence to be carried for MGT calculation.
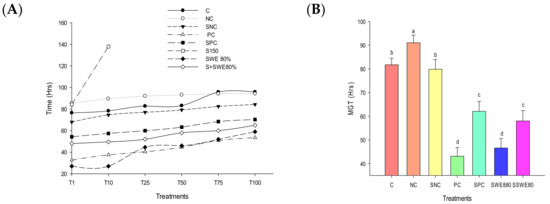
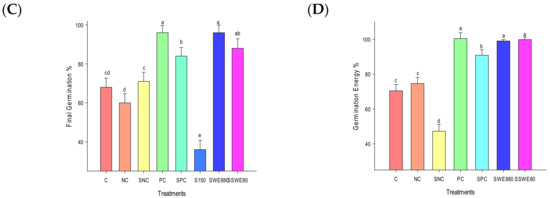
Figure 2.
Effect of SWEs and salt on germination of rice seeds; (A) germination time course; (B) MGT; (C) final GP; (D) GE. Columns denoted by a different letter are significantly different at p ≤ 0.05 in Tukey’s test. Seeds exposed to salt treatment (S150 mM) did not reach 50% of germination.
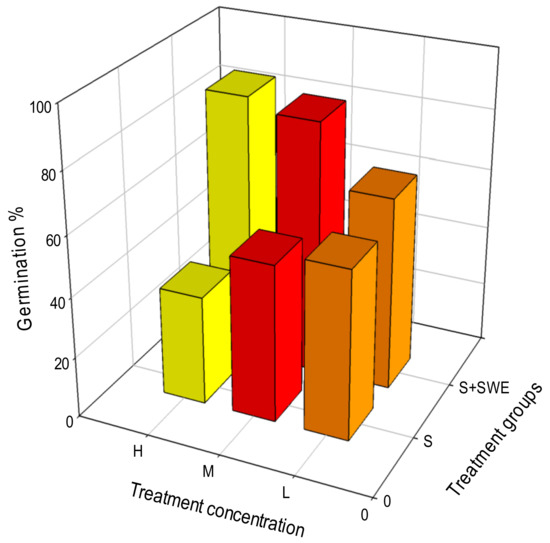
Figure 3.
Efficacy of SWEs on the recovery of GP of rice seeds exposed to salt stress. the X-axis SWE Treatment concentrations (low 40% (brown), Medium 60% (red) and high 80% (yellow)) Y-axis germination%, Z-axis treatment groups.
Salt stress severely affected germination capability of seeds exhibiting 56, 52, and 36% of germination percentage at salt concentrations 80, 100, and 150 mM, respectively. SWE treatments increased GP to 80, 91, and 96% at concentrations, 40, 60, and 80, respectively. SWE were able to significantly increase the GP of seeds under salinity stress to 88 from 36‰ (F6,28 = 80.35; p < 0.0001) (Figure 2C). Germination energy of seeds exposed to salinity stress was severely affected (F6,28 = 213.23; p < 0.0001) with a corresponding impact of salinity strain on the vigour of seedlings was also observed resulting in a very lower vigour index, 133.267. SWEs were able to completely energize the seedlings (99.8%) (Figure 2D, Table 2).

Table 2.
Effect of SWE and salinity stress on SVI of seedlings. Columns denoted by a different letter are significantly different at p ≤ 0.05 in Tukey’s test.
3.2. Growth Parameters
The salt treatments drastically affected the length of radicle and plumule resulting in stunted growths (2.48 and 1.28 cm) which was 48.3 and 44.8% inferior to control (F5,24 = 20.72; p < 0.0001) (Figure 4A). SWE promoted the growths of radicle and plumule in seeds exposed to salinity by increasing their lengths to 6.24 and 9.54 cm from 1.28 (F7,32 = 21.1; p < 0.0001) and 3.72 cm (F7,32 = 83.39; p < 0.0001). A likely reduction in root–shoot lengths of rice plants by salt stress was also noted which was promoted by the influence of SWE (Figure 4B). Salt stress reduced the plant development by negatively influencing the development of roots and shoots decreasing them to 2.82 (F7,32 = 53.78; p < 0.0001) and 3 cm (F7,32 = 58.27; p < 0.0001) in lengths from 4.7 and 9.5 cm. However, the SWE promoted the growths of rhizome and shoots of salt stress-exposed plants to 8.6 and 13.2 cm with a parallel increase in plant height to 22.16 cm from 7.8 cm (F7,32 = 317.75; p < 0.0001). SWE also increased the growth of seedlings exposed to SWE to 9.54 from 3.72 cm (F7,32 = 83.39; p < 0.0001) (Figure 4C).
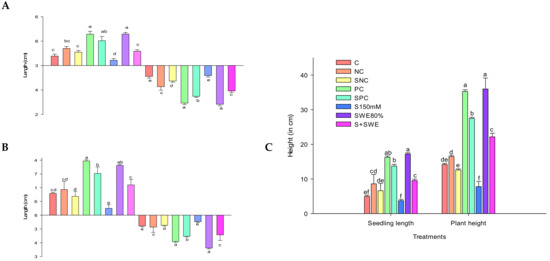
Figure 4.
Effect of SWEs and salt on growth parameters of paddy (cm) (A) radicle–plumule length; (B) Root–shoot height; (C) seedling–plant height. Columns denoted by a different letter are significantly different at p ≤ 0.05 in Tukey’s test.
3.3. Physiological Parameters
The detrimental outcome of salt strain-induced upon RWC, panicle length, and tiller numbers of paddy plants were also positively encouraged by the treatments with SWE (Table 3). RWC of paddy leaves were increased from 40.6 to 82.6% (F7,32 = 25.8; p < 0.0001). An increase by SWE in panicle length and tiller numbers from 13.4 cm and 25.2 to 28.39 cm (F7,32 = 25.8; p < 0.0001) and 56.37 (F7,32 = 168.8; p < 0.0001) was also noted (Figure 5). Caryopsis qualities also enhanced by SWEs promoting the grain weight to 3.345 from 1.455 g (F7,32 = 19.43; p < 0.0001). SWEs also promoted yield of paddy plants exposed to salinity stress from 3230.8 to 7743.7 kg/hectare (F7,32 = 10,138.07; p < 0.0001) (Table 3).

Table 3.
Effect of SWE and salinity stress on RWC (%), panicle length (cm), tiller number/hill, 100 grain wt (g), grain yield (kg/hectare) of paddy. Columns denoted by a different letter are significantly different at p ≤ 0.05 in Tukey’s test.

Figure 5.
Effect of SWEs and salt on panicle length (cm).
3.4. Biochemical Parameters
Salt stress resulted in increased rates of leaf mortality (8.4%), which was revoked by SWEs by 85.12% (F7,32 = 30.32; p < 0.0001) (Table 4). Salt stress rigorously derailed the leaf biochemical constituents of paddy plants reducing the levels of TSP, TPC, and SOD from 1.786, 16.16, and 1.76 to 1.21 mg/g FW (F7,32 = 4.34; p < 0.0001), 10.86 mg/g DW (F7,32 = 39.35; p < 0.0001) and 1.3 U/mg protein (F7,32 = 4.19; p < 0.0001), respectively. Consequently, SWEs increased the leaf biochemistry of salt-exposed paddy plants to 1.656 mg/g FW, 25.07 mg/g DW, and 3 U/mg proteins of TSP, TPC, and SOD, respectively (Table 4). Paddy leaf pigments such as chla and b along with carotenoids were also positively influenced by SWE treatments that increased from 1.1704 and 1.165 along with 1.628 mg/g FW to 2.32 (F7,32 = 8.07; p < 0.0001), 1.535 (F7,32 = 2.52; p < 0.0001), and 2.0274 mg/g FW (F7,32 = 4.8; p < 0.0001), respectively (Figure 6).

Table 4.
Effect of SWE and salinity stress on leaf mortality (%), TSP (mg/gFW), TPC (GAE mg/g DW), and SOD (U/mg protein) on paddy leaves. Columns denoted by a different letter are significantly different at p ≤ 0.05 in Tukey’s test.
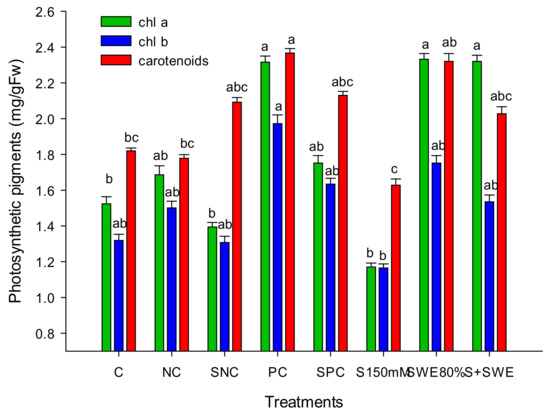
Figure 6.
Effect of SWEs and salt on photosynthetic pigments, chlorophyll (a and b), and carotenoids (mg/g FW) of paddy leaves. Columns denoted by a different letter are significantly different at p ≤ 0.05 in Tukey’s test.
Grain biochemical contents was analysed in terms of carbohydrate content and protein. TSS level was found to be enhanced by salinity stress (17.85%) compared with control (Table 5). Despite the 13.69% decrease in TSS content in SWE-treated rice plants compared with salt-treated plants, TSS content was found to be present 6.14% greater than those exposed to salt stress (F7,32 = 46.5; p < 0.0001). A similar pattern of increase in starch content in grains emerged out of plants exposed to salt stress (18.21%) and those treated with SWE was also observed (0.827%); however, the increase in starch content brought about by SWE application on salt-stressed plants was not statistically significant (p > 0.05). Protein levels in grains of salt-stressed plants was decreased by 7.54%; However, SWE treatments increased grain protein level by 23.12% (F7,32 = 57.28; p < 0.0001). This consequently affected the protein–starch ratio. Salinity stress severely decrease protein–starch ratio by 23.6% and was elevated by SWE treatment to 0.0886 from 49.4 (F7,32 = 53.71; p < 0.0001).

Table 5.
Grain biochemical traits of plants on salinity stress and SWE treatments. Columns denoted by a different letter are significantly different at p ≤ 0.05 in Tukey’s test.
4. Discussion
Salty conditions are known to limit plant development; saline soils and saline irrigation pose major issues for vegetable crop productivity [33]. With increasing salt, salt stress can delay and limit plant development differentiation, as well as lower the fresh weight of leaf, stem, and root tissue [34]. Poor germination of O. sativa control seeds is uncommon for a well-domesticated crop, since most crop species demonstrate quick germination and can reach 100% germination under ideal conditions [35]. A sustainable eco-friendly technique of using seaweed extracts was devised to minimize both the agricultural constraint and to increase rice output. SWE had an influence on the sprouting and development of rice.
Seed emergence is hampered by the equipoise, which is bordered by the embryo’s development abilities as well as the endosperm’s mechanical resistance, which needs to be weakened for germination [36]. The endosperm cap is weakened by a succession of enzymes and phytohormones, which accelerate cell development in the embryo and alter the radicle’s emergence. As a result, seed germination augmentation practices such as seed instructing are increasingly being used to boost crop growth and production.
Until recently, seaweeds were being investigated as possible crop development besides produce enhancers, with the goal of replacing chemical fertilizers due to their superior efficiencies, larger action range, eco-friendliness, and cost effectiveness. Seaweeds have been observed to have remarkable plant growth encouraging potentials, including enhanced plant height, root, and shoot lengths, and are therefore classified as plant growth biostimulants, according to Craigie et al. [37]. The radicle and plumule are crucial in determining the underpinning of a plant in the field since they are the major developmental plant growth phase. Seeds with a prominent radicle and plumule germinate quickly and have increased competence [19]. Plant establishment efficiency is also specified by lengthier radicles. Seeds with a shorter radicle–plumule may have problems transporting nutrients to the embryo [38].
Seed priming effects of SWEs were instituted to ensure a favourable impact on early emergence and plant growth. SWEs not only spurred early seed emergence but also raised seed GP, whereas increasing salt treatments delayed seed emergence and lowered GP. The toxic effects of Na+ and Cl in the germination process might be to blame for the decrease in germination [39]. Salt stress appears to influence seed germination by limiting seed water absorption, causing protein synthesis abnormalities, and causing excessive nutrient pool usage [40]. Regardless of the circumstance, greater salt concentrations inhibited more than 80% of seed germination, and SWEs increased the GP by 59.09%. SWEs are known to have beneficial impacts on the germination of a variety of crops [41].
The proportion of seeds that germinate quickly is expressed as germination energy. While salt stress depleted the germination energy completely, SWEs enhanced the GE to its maximal degree (80–90%). The relative increase in seed emergence is linked to the seed eminence, which appears to enhance by SWE treatment. Furthermore, Amabika and Sujatha [42] demonstrated that priming red gram seeds with extract of the seaweed, Sargassum myriocystum increased the seedling vigour index of emerged seedlings. This thereby proves the fact that higher SVI is a good indicator of healthy seedlings.
Sangare et al. [43] indicated that the true performance ability of a seed can be elucidated by comparing its SVI with that of a control. Higher SVI of SWE-treated seeds denote an increase in seed quality. Other characteristics of SWE-treated seeds showed greater seedling-plant height, radicle-root, plumule-shoot lengths, and dry-wet weight compared with control. Enhanced seed germination and growth rates of brinjal and tomato, as well as chilli, were connected with increased SVI after seed priming with SWE of Ulva lactuca, Padina pavonic, and S. johnstonii [44]. This beneficial impact might be accredited to various growth-regulating chemicals found in SWEs, such as kinetin, gibberellic acid, and ethylene, which have been linked to the reversal of seed dormancy [45]. The growth hormones found in SWE may serve a crucial part in initiating the creation of hydrolytic enzymes from scratch. These phytohormones may cause abscisic acid inhibitors to seep from the seeds, improving germination rates [46].
Yang and Guo [47] found that the principal response to salinity stress is a suppression of shoot and root vegetative development, which was seen in the current experiment. The same discoveries were established by Khosravinejad et al. [48], who observed a substantial reduction in shoot lengthening in barley when NaCl treatment was increased. Compared with plants in a saline state, liquid extract delivers the highest outcomes in terms of plant development under salt stress conditions by a considerable increase. Seaweed elements such as macro- and microelement nutrients, vitamins, amino acids, auxins, and cytokinins, which impact cellular metabolism in plants and lead to increased growth, can be connected to improved wheat vegetative development [49]. Researchers from all around the world have confirmed that seaweeds boost plant development [50,51,52].
Although salt stress reduced rice plant protein and phenolic contents, SWE enhanced both biochemical properties by 22 and 56.8%, respectively. Wheat plants treated with Fucus spiralis LSEs showed a similar rise in TSP and TPC [45]. All kinds of stressors cause an increase in the synthesis of hazardous oxygen derivatives. Plants have effective mechanisms for scavenging active oxygen species, which protect them from oxidative processes that are harmful to them [53]. Antioxidant enzymes show a vital part in the defensive mechanisms of the plant system. The administration of SWE enhanced the synthesis of the SOD enzyme that was previously reduced by salt stress in the current investigation. The application of extracts of F. spiralis to wheat plants resulted in a comparable rise in SOD activity [45]. Salinity levels have a big impact on relative water content [54]. Plants exposed to salt at higher levels had much decreased water content in their leaves. Plants under salinity stress had their RWC increased by SWEs. Bulgari et al. [55] stated that similar applications of ascorbic acid and abscisic acid, as well as seaweed extracts, have been demonstrated to protect plants against water loss and retain water. Plant growth, tiller number, length of panicle, seed weight, and caryopsis traits all decrease with increasing saline levels, according to Abdullah et al. [56], which correlates with our findings. The treatment with SWEs, which cumulatively improved panicle length and augmented the number of tillers/hills, improved all the above characteristics.
In our research, SWEs had a comparable favourable effect on grain weight, yield, and photosynthetic pigments. Reduced chlorophyll concentration, fragmentation of chloroplast membranes, besides disruption of biochemical activities, are all examples of abiotic stressors that have a noteworthy influence on plant photosynthesis. NaCl stress dramatically reduced chlorophyll content in paddy, according to the findings of this study. The seaweed extract was shown to increase plant chlorophyll content by promoting its production. Under NaCl stress, the polysaccharides of the algae Lessonia nigrescens (LNP) greatly boosted chlorophyll levels in plants. LNP treatment decreased lipid peroxidation and alleviated the salt-induced loss of chlorophyll content [57]. The high content of macro- and micronutrients, growth stimulators, bioactive ingredients, and unique plant growth promoting products in the alga can be accredited to the helpful outcome of algal amendment besides also their stress-dismissing influence on rice yield [58].
The varied effects of the treatments changed the biochemistry and grain weight. Despite the fact that TSS and starch levels increased, rice plants treated with SWE had higher protein contents. The total protein–starch ratio of grains treated with SWE, which previously reduced due to salt stress, rose as a result. Thitisaksakul et al. previously established that salt stress increases the starch content of rice grains [59]. However, it was suggested that genotypes affected how much biochemical material accumulated in rice grains in response to salt stress [60]. Proteins are thought to be the factors that determine the sensory quality of rice [61]. According to reports, rice grain proteins can change depending on the salinity. It is considered that the more protein a grain contains, the less likely it is to break during the milling process [62]. In light of nutrition, storage, and marketability, increased rice grain protein content encouraged by SWE treatment under saline stress is a desirable rice quality. Soil salinity has been reported to enact a negative impact on the plants in all stages of development, affecting the plant growth, development, functionality, and eventually the crop’s quality and yield. Application of SWE was hence tested for its salt alleviation capacity right from germination and throughout the growth of rice plant. It was evident that the SE promoted seed germination that was severely constrained by salinity stress and eventually producing plants of greater height and vigour. This can be correlated to the enhanced biochemical attributes that contributed to increase in the synthesis of vital plant biochemical such as protein, phenols, antioxidant enzyme, and chlorophyll pigments. An increase in water retention in leaves treated with SWE exposed to salt stress and lower leaf mortality rates dues to SWE treatments clearly indicates that SWE was successful in conferring protection to rice plants against salt-induced toxicities. An increase in grain yield and weight along with grain biochemical attributes such as protein and protein:starch also indicates that SWE had exerted a profound effect in promoting the productivity of plant as a result of enhanced plant functionalities. Hence the current study proposes that SWE promotes effective use of plant resources, increases plant development, and increases tolerance to unfavourable environmental circumstances.
5. Conclusions
These discoveries propose that seaweed extracts can help rice plants cope with salt stress by boosting rice seed germination and plant development. The extracts significantly enhanced panicle length and tiller numbers, both of which were badly harmed by salt stress. SWE also improved the content of photosynthetic pigments. The treatment of SWE had a good effect on the caryopsis characteristics and yield. SWE significantly reduced leaf death rates while also boosting protein, phenolic content, and antioxidant enzyme levels. Extracts from the halotolerant macroalgae C. antennina can help plants deal with salt stress, according to our findings.
Author Contributions
Conceptualization, K.M.-P.C. and S.S.-N.; Methodology, K.M.-P.C. and G.-S.P.; Validation, K.M.-P.C., A.G. and S.S.-N.; Investigation, G.-S.P., P.M. (Pauldurai Malarvizhi), A.G. and P.M. (Ponnusamy Murugan); Formal analysis, A.D.-A., P.M. (Pauldurai Malarvizhi), M.J., H.S. and R.R.; Data curation, K.M.-P.C., S.S.-N., A.G. and V.S.-R.; writing—original draft preparation, K.M.-P.C.; writing—review and editing, A.A.-M., P.K. and S.S.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Department of Science and Technology (DST-FIST), India under FIST program (SR/FIST/LS-1/2019/522). This research work was partially supported by Chiang Mai University, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S.; et al. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef]
- Kalaivani, K.; Kalaiselvi, M.M.; Senthil-Nathan, S. Effect of Methyl Salicylate (MeSA) induced changes in rice plant (Oryza sativa) that affect growth and development of the rice leaffolder, Cnaphalocrocis medinalis. Physiol. Mol. Plant Pathol. 2018, 101, 116–126. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Yasuor, H.; Yermiyahu, U.; Ben-Gal, A. Consequences of irrigation and fertigation of vegetable crops with variable quality water: Israel as a case study. Agric. Water Manag. 2020, 242, 106362. [Google Scholar] [CrossRef]
- Choudhary, O.P.; Grattan, S.R.; Minhas, P.S. Sustainable crop production using saline and sodic irrigation waters. In Alternative Farming Systems, Biotechnology, Drought Stress and Ecological Fertilisation; Springer: Berlin/Heidelberg, Germany, 2011; pp. 293–318. [Google Scholar]
- Singh, A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Bosch, A.; Rigol-Sanchez, J.P.; Vallejos, A.; Andreu, J.M.; Ceron, J.C.; Molina-Sanchez, L.; Sola, F. Impacts of agricultural irrigation on groundwater salinity. Environ. Earth Sci. 2018, 77, 197. [Google Scholar] [CrossRef]
- FAO. Management of Salt Affected Soils: ‘Soil Management’ under ‘FAO SOILS PORTAL’. In Food and Agriculture Organization’ of the ‘United Nations’. Rome. 2020. Available online: http://www.fao.org/soils-portal/soil-management/management-of-some-problemsoils/salt-affected-soils/more-information-on-salt-affected-soils/en/ (accessed on 15 November 2022).
- Hossain, M.A.; Araki, H.; Takahashi, T. Poor grain filling induced by waterlogging is similar to that in abnormal early ripening in wheat in Western Japan. Field Crops Res. 2011, 123, 100–108. [Google Scholar] [CrossRef]
- Luo, X.; Dai, Y.; Zheng, C.; Yang, Y.; Chen, W.; Wang, Q.; Chandrasekaran, U.; Du, J.; Liu, W.; Shu, K. The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol. 2021, 229, 950–962. [Google Scholar] [CrossRef]
- Kalaivani, K.; Maruthi-Kalaiselvi, M.; Senthil-Nathan, S. Seed treatment and foliar application of methyl salicylate (MeSA) as a defense mechanism in rice plants against the pathogenic bacterium, Xanthomonas oryzae pv. oryzae. Pest. Biochem. Physiol. 2021, 171, 104718. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and biochemical responses of Glycine max (L.) Merr. to the use of seaweed extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef]
- Stirk, W.A.; Rengasamy, K.R.; Kulkarni, M.G.; van Staden, J. Plant biostimulants from seaweed: An Overview. Chem. Biol. Plant Biostimulants 2020, 2, 31–55. [Google Scholar]
- Bohra, A.; Jha, U.C.; Jha, R.; Naik, S.J.; Maurya, A.K.; Patil, P.G. Genomic interventions for biofortification of food crops. In Quality Breeding in Field Crops; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–21. [Google Scholar]
- Nakashima, K.; Yanagihara, S.; Muranaka, S.; Oya, T. Development of Sustainable Technologies to Increase Agricultural Productivity and Improve Food Security in Africa. Jpn. Agric. Res. Q. 2022, 56, 7–18. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Abiotic stress effects on performance of horticultural crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef]
- Reddy, A.M.; Francies, R.M.; Rasool, S.N.; Reddy, V.R. Breeding for tolerance stress triggered by salinity in rice. Int. J. Appl. Biol. Pharm. Technol. 2014, 5, 167–176. [Google Scholar]
- Rasel, H.M.; Hasan, M.R.; Ahmed, B.; Miah, M.S. Investigation of soil and water salinity, its effect on crop production and adaptation strategy. Int. J. Water Res. Environ. Eng. 2013, 5, 475–481. [Google Scholar]
- Pessarakli, M.; Szabolcs, I. Soil salinity and sodicity as particular plant/crop stress factors. In Handbook of Plant and Crop Stress, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 3–21. [Google Scholar]
- Shi, W.; Yin, X.; Struik, P.C.; Xie, F.; Schmidt, R.C.; Jagadish, K.S. Grain yield and quality responses of tropical hybrid rice to high night-time temperature. Field Crops Res. 2016, 190, 18–25. [Google Scholar] [CrossRef]
- Liu, H.; Hussain, S.; Zheng, M.; Sun, L.; Fahad, S.; Huang, J.; Cui, K.; Nie, L. Progress and constraints of dry direct-seeded rice in China. J. Food Agric. Environ. 2014, 12, 465–472. [Google Scholar]
- Stanley-Raja, V.; Senthil-Nathan, S.; Chanthini, K.M.P.; Sivanesh, H.; Ramasubramanian, R.; Karthi, S.; Shyam-Sundar, N.; Vasantha-Srinivasan, P.; Kalaivani, K. Biological activity of chitosan inducing resistance efficiency of rice (Oryza sativa L.) after treatment with fungal based chitosan. Sci. Rep. 2021, 11, 20488. [Google Scholar] [CrossRef]
- Chanthini, K.M.; Stanley-Raja, V.; Thanigaivel, A.; Karthi, S.; Palanikani, R.; ShyamSundar, N.; Sivanesh, H.; Soranam, R.; Senthil-Nathan, S. Sustainable agronomic strategies for enhancing the yield and nutritional quality of wild tomato, Solanum Lycopersicum (l) var Cerasiforme Mill. Agronomy 2019, 9, 311. [Google Scholar] [CrossRef]
- Jisha, K.C.; Puthur, J.T. Seed priming with BABA (β-amino butyric acid): A cost-effective method of abiotic stress tolerance in Vigna radiata (L.) Wilczek. Protoplasma 2016, 253, 277–289. [Google Scholar] [CrossRef]
- Radanielson, A.M.; Angeles, O.; Li, T.; Ismail, A.M.; Gaydon, D.S. Describing the physiological responses of different rice genotypes to salt stress using sigmoid and piecewise linear functions. Field Crops Res. 2018, 220, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Fujita, M.; Islam, M.N.; Ahamed, K.U.; Nahar, K. Performance of four irrigated rice varieties under different levels of salinity stress. Int. J. Integr. Biol. 2019, 6, 85–90. [Google Scholar]
- Thitisaksakul, M.; Tananuwong, K.; Shoemaker, C.F.; Chun, A.; Tanadul, O.U.; Labavitch, J.M.; Beckles, D.M. Effects of timing and severity of salinity stress on rice (Oryza sativa L.) yield, grain composition, and starch functionality. J. Agric. Food Chem. 2015, 63, 2296–2304. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford assay for determining protein concentration. Cold Spring Harb. Protoc. 2020, 4, 102269. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.S.; Miller, E.E.; Pratt, D.E. Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Manaf, H.H. Beneficial effects of exogenous selenium, glycine betaine and seaweed extract on salt stressed cowpea plant. Ann. Agric. Sci. 2016, 61, 41–48. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequence of minor changes in conditions. Ann. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Maness, N. Extraction and analysis of soluble carbohydrates. In Plant Stress Tolerance; Humana Press: Totowa, NJ, USA, 2010. [Google Scholar]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Bonomelli, C.; Celis, V.; Lombardi, G.; Mártiz, J. Salt stress effects on avocado (Persea americana Mill.) plants with and without seaweed extract (Ascophyllum nodosum) application. Agronomy 2018, 8, 64. [Google Scholar] [CrossRef]
- Kalaivani, K.; Kalaiselvi, M.M.; Senthil-Nathan, S. Effect of methyl salicylate (MeSA), an elicitor on growth, physiology and pathology of resistant and susceptible rice varieties. Sci. Rep. 2016, 6, 34498. [Google Scholar] [CrossRef]
- Seethalakshmi, S.; Umarani, R. Biochemical changes during imbibition stages of seed priming in tomato. Int. J. Chem. Stud. 2018, 6, 454–456. [Google Scholar]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Chanthini, K.M.P.; Senthil-Nathan, S.; Stanley-Raja, V.; Thanigaivel, A.; Karthi, S.; Sivanesh, H.; Sundar, N.S.; Palanikani, R.; Soranam, R. Chaetomorpha antennina (Bory) Kützing derived seaweed liquid fertilizers as prospective bio-stimulant for Lycopersicon esculentum (Mill). Biocatal. Agric. Biotechnol. 2019, 20, 101190. [Google Scholar] [CrossRef]
- Zhumabekova, Z.; Xu, X.; Wang, Y.; Song, C.; Kurmangozhinov, A.; Sarsekova, D. Effects of Sodium Chloride and Sodium Sulfate on Haloxylon ammodendron Seed Germination. Sustainability 2020, 12, 4927. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Begum, M.; Bordoloi, B.C.; Singha, D.D.; Ojha, N.J. Role of seaweed extract on growth, yield and quality of some agricultural crops: A review. Agric. Rev. 2018, 39, 321–326. [Google Scholar] [CrossRef]
- Amabika, S.; Sujatha, K. Effect of priming with seaweed extracts on germination and vigour under different water holding capacities. Seaweed Res. Utiln. 2015, 37, 37–44. [Google Scholar]
- Sangare, S.K.; Compaore, E.; Buerkert, A.; Vanclooster, M.; Sedogo, M.P.; Bielders, C.L. Field-scale analysis of water and nutrient use efficiency for vegetable production in a West African urban agricultural system. Nutr. Cycl. Agroecosyst. 2012, 92, 207–224. [Google Scholar] [CrossRef]
- Patel, R.V.; Pa, K.Y. Effect of hydropriming and biopriming. Res. J. Agric. For. 2017, 5, 1–14. [Google Scholar]
- Latique, S.; Mohamed Aymen, E.; Halima, C.; Chérif, H.; Mimoun, E.K. Alleviation of salt stress in durum wheat (Triticum durum L.) seedlings through the application of liquid seaweed extracts of Fucus spiralis. Commun. Soil Sci. Plant Anal. 2017, 48, 2582–2593. [Google Scholar] [CrossRef]
- du Jardin, P.; Xu, L.; Geelen, D. Agricultural Functions and Action Mechanisms of Plant Biostimulants (PBs) an Introduction. Chem. Biol. Plant Biostimulants 2020, 1–30. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Khosravinejad, F.; Heydari, R.; Farboodnia, T. Effect of salinity on organic solutes contents in barley. Pak. J. Biol. Sci. 2019, 12, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo-Herrera, D.A.; Hernández-Carmona, G.; Muñoz-Ochoa, M.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E. Monthly variation in the chemical composition and biological activity of Sargassum horridum. Bot. Mar. 2018, 61, 91–102. [Google Scholar] [CrossRef]
- Arioli, T.; Mattner, S.W.; Winberg, P.C. Applications of seaweed extracts in Australian agriculture: Past, present and future. J. Appl. Phycol. 2015, 27, 2007–2015. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Oude Essink, G.H.; Van Baaren, E.S.; De Louw, P.G. Effects of climate change on coastal groundwater systems: A modeling study in the Netherlands. Water Resour. Res. 2010, 46, 1–16. [Google Scholar] [CrossRef]
- Shahi, C.; Bargali, K.; Bargali, S.S. Assessment of salt stress tolerance in three varieties of rice (Oryza sativa L.). Progress. Agric. 2015, 6, 50–56. [Google Scholar]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Abdullah, Z.K.; Khan, M.A.; Flowers, T.J. Causes of sterility in seed set of rice under salinity stress. J. Agron. Crop Sci. 2001, 187, 25–32. [Google Scholar] [CrossRef]
- Zou, P.; Lu, X.; Zhao, H.; Yuan, Y.; Meng, L.; Zhang, C.; Li, Y. Polysaccharides derived from the brown algae Lessonia nigrescens enhance salt stress tolerance to wheat seedlings by enhancing the antioxidant system and modulating intracellular ion concentration. Front. Plant Sci. 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Ward, F.; Deyab, M.; El-katony, T. Biochemical Composition and Bioactivity of Dictyota from Egypt; LAP LAMBERT Academic Publishing: Sunnyvale, CA, USA, 2017. [Google Scholar]
- Irakoze, W.; Prodjinoto, H.; Nijimbere, S.; Rufyikiri, G.; Lutts, S. NaCl and Na2SO4 salinities have different impact on photosynthesis and yield-related parameters in rice (Oryza sativa L.). Agronomy 2020, 10, 864. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, A.; Safdar, L.B.; Zafar, M.M.; Rui, Y.; Shakeel, A.; Shaukat, A.; Ashraf, M.; Gong, W.; Yuan, Y. Salt stress induces physiochemical alterations in rice grain composition and quality. J. Food Sci. 2020, 85, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Calingacion, M.; Laborte, A.; Nelson, A.; Resurreccion, A.; Concepcion, J.C.; Daygon, V.D.; Mumm, R.; Reinke, R.; Dipti, S.; Bassinello, P.Z.; et al. Diversity of global rice markets and the science required for consumer-targeted rice breeding. PLoS ONE 2014, 9, e85106. [Google Scholar] [CrossRef]
- Lee, I.S.; Lee, J.O.; Ge, L. Comparison of terrestrial laser scanner with digital aerial photogrammetry for extracting ridges in the rice paddies. Surv. Rev. 2009, 41, 253–267. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).