Fruit Quality and Contents of Some Bioactive Compounds in Selected Czech Sweet Cherry (Prunus avium L.) Cultivars under Conditions of Central Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytical Methods
- Average fruit mass (g): We measured the average fruit mass (g) on a TP 200 (OHAUS Europe GmbH, Nänikon, Switzerland) analytical balance;
- Average fruit diameter (mm): We measured the diameter of the fruit in two directions with a caliper. Then, we averaged the obtained results from each fruit;
- Average weight of stone (g): We removed the stones from 30 fruits from each repetition, and we then weighed them on a TP 200 (OHAUS Europe GmbH, Nänikon, Switzerland) analytical balance. We averaged the results for each cultivar;
- Stone from the weight of the fruit (%): After determining the weight of the whole fruit and weight of the stone using mathematical calculations, we calculated the percentage of the stone in the fruit for each cultivar;
- Soluble solid content (SSC) (Brix degrees): We refractionally determined the soluble solid content (SCC) (Brix degrees) according to the Polish Standard PN-EN 12143:2000 [33] (developed by the Polish Committee of Standardization) in the juice squeezed out from 30 fruits per replication at a 20 °C temperature. We determined the SSC using an Atago PR-32 digital refractometer (Atago, Tokyo, Japan);
- Fruit firmness (FF): We determined the fruit firmness (FF) as the value of the force needed to deform the fruit by a 3 mm diameter punch probe. We made the determinations using an Instron type 5542 tester (Instron, High Wycombe, UK). We determined the FFs of 20 fruits in three replications. Each fruit was measured twice on each fruit (in the horizontal and vertical planes), with a compression speed of 240 mm−1 during penetration to a 3 mm depth [34]. We express the FF in newtons (N);
- Total (titration) acidity (TA): We determined the total (titration) acidity (TA) according to the Polish Standard PN-EN 12147:2000 [35]. We measured the TA in water extract from an average sample of 30 minced fruits by titrating with 0.1 N sodium hydroxide (NaOH) to the endpoint of a pH of 8.1, using a TitroLine 5000 system (Si Analytics, Mainz, Germany). We express the results as the percentage of anhydrous malic acid;
- Ratio of SSC value to titratable acidity: We based the ratio of the SSC value to the titratable acidity on the SSC and TA values using mathematical calculations. We calculated the SSC/TA ratio for each cultivar;
- External color of fruits: We measured the external color with a Minolta CR-508i colorimeter (Minolta, Osaka, Japan) equipped with a 5 mm measuring head and observer 10° and illuminant D65. We calibrated the meter using the manufacturer’s standard white plate. We quantified the color changes in the L*, a* and b* color spaces. We calculated the hue angle ((h◦ = tan−1 (b*/a*) + 180°) when a* < 0 and b* > 0) and chroma values (C = (a*2 + b*2)1/2) from the a* and b* values. The hue values refer to a color wheel. The red, yellow, green and blue colors were at angles of 0°, 60°, 120° and 240°, respectively. The chroma describes the vividness or dullness of the fruit color, and it is also known as color saturation [36].
- Analysis of total polyphenol content: We conducted the analysis according to the Waterhouse method [37]. We measured the total polyphenol levels using a Marcel s330 PRO spectrophotometer (Marcel S.A., Warsaw, Poland) with Folin–Ciocalteau reagent. We extracted 5 g of material crushed in liquid nitrogen with 50 mL of 100% methanol.We replicated the extraction process twice by pouring the extracts into a 100 mL flask. One by one, we poured 1 mL of extract into a 50 mL flask, and we then added 35 mL of H2O, 2.5 mL of Folin–Ciocalteau reagent and 7.5 mL of 10% NaCO3. We supplemented the above-prepared solution with H2O and incubated it at 25 ± 2 °C for 20 min. We performed the measurements at a 750 nm wavelength. We used gallic acid as a standard at the following concentrations: 0.00, 0.05, 0.15, 0.20, 0.25 and 0.3 g/L. We calculated the polyphenol content using a formula: (105.89 · absorbance2 + 25.318 absorbance)/mass 50. We express the total polyphenol content in milligrams of gallic acid per 100 g−1 FW (fresh weight);
- Analysis of flavonoid content: We performed the analysis using the modified method of Marinova et al. [38]. We crushed 5 g of fruit in liquid nitrogen and used it to determine the flavonoids. We mixed the samples with 25 mL of 80% methanol, and then extracted them for 15 min. We performed the extractions twice. We sequentially added distilled water, 5% NaNO2, 10% AlCl3 and 1M NaOH to the resulting samples at predetermined intervals. We performed the measurements using a Marcel s330 PRO spectrophotometer (Marcel S.A., Warsaw, Poland) at 510 nm. We calculated the flavonoid content using a standard curve (y = 1.86x), performed with quercetin solutions and including the following concentrations: 0.00, 0.20, 0.60, 0.80 and 1.00 g·L. We express the total flavonoid content of the fruit as mg of quercetin equivalents (QE) per 100 g−1 FW (fresh weight);
- Qualitative and quantitative analyses of anthocyanins: We performed the analysis of the separation and contents of the anthocyanins using a Perkin-Elmer 200 series HPLC kit with a Diode Array Detector (DAD), according to modified method of Krupa and Tomala [39]. We performed the separation using a LiChroCART 125-3 (Merck KGaA, Darmstadt, Germany) column with a 1 mL/min flow rate. The column temperature was 25 °C. The mobile phase consisted of (A) water, (B) 20% formic acid and (C) acetonitrile, with variable parameters of the gradients A and C: 0–17.5 min A:B:C = 40:50:10; 17.5–22.5 min A:B:C = 35:50.15; 22.5–32.5 min A:B:C = 45:50:5. We detected the anthocyanins at 520 nm wavelengths by comparing the retention time on the achieved chromatograms with the standard ones. We express the contents of the particular compounds as mg of cyanidin-3-glucoside equivalent in 100 g−1 FW (fresh weight);
- Antioxidant activity: We determined the antioxidant activity according to the method of Saint Criq de Gaulejac et al. [40], which is based on the reduction of free radicals obtained from the DPPH+ (1,1-diphenyl-2-picrylhydrazine, Sigma-Aldrich, Poznan, Poland). We calculated the antioxidant activity based on the absorbance measurements for the proper sample (fruit extract + DPPH+) performed after 20 min at λ = 517 nm in relation to the control sample (H2O + DPPH+). We calculated the flavonoid content using a standard curve (AA = (0.0597·2) − (0.754x) + 1.77), including the following concentrations: 0.01, 0.02, 0.05, 0.1 and 0.2 g·L. We express the results in mg of ascorbic acid equivalent per g of FW (fresh weight).
2.2. Statistical Analysis
3. Results
3.1. Fruit Quality
3.1.1. Fruit Size (Weight and Diameter)
3.1.2. Stone Weight
3.1.3. Proportion of Seed in Fruit
3.1.4. Firmness
3.1.5. Soluble Solid Content (SSC)
3.1.6. Titration Acidity (TA)
3.1.7. SSC/TA Ratio
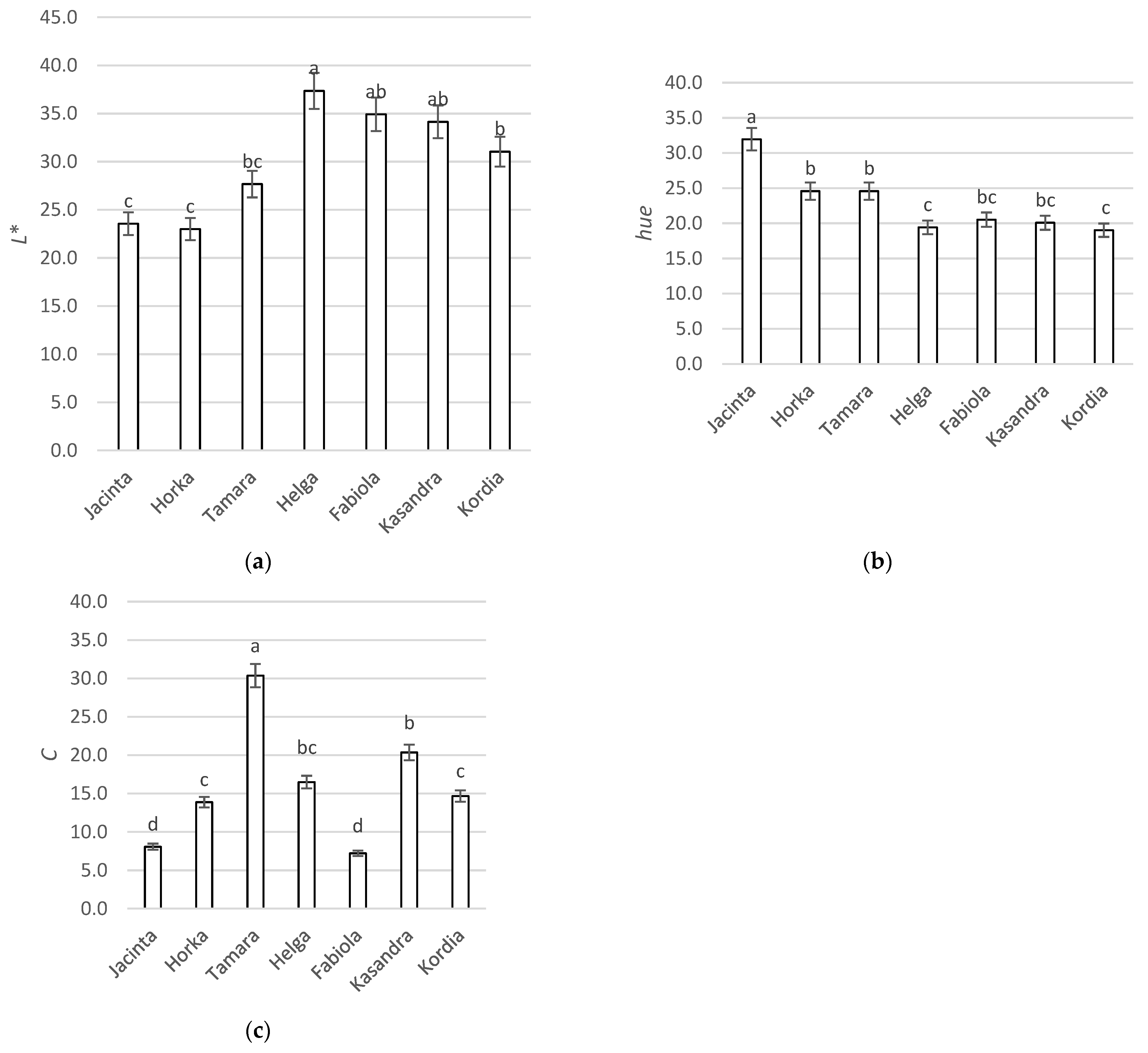
3.2. External Color
3.2.1. Value of L*
3.2.2. Value of Hue
3.2.3. Value of Chroma
3.3. Contents of Bioactive Compounds in Fruits
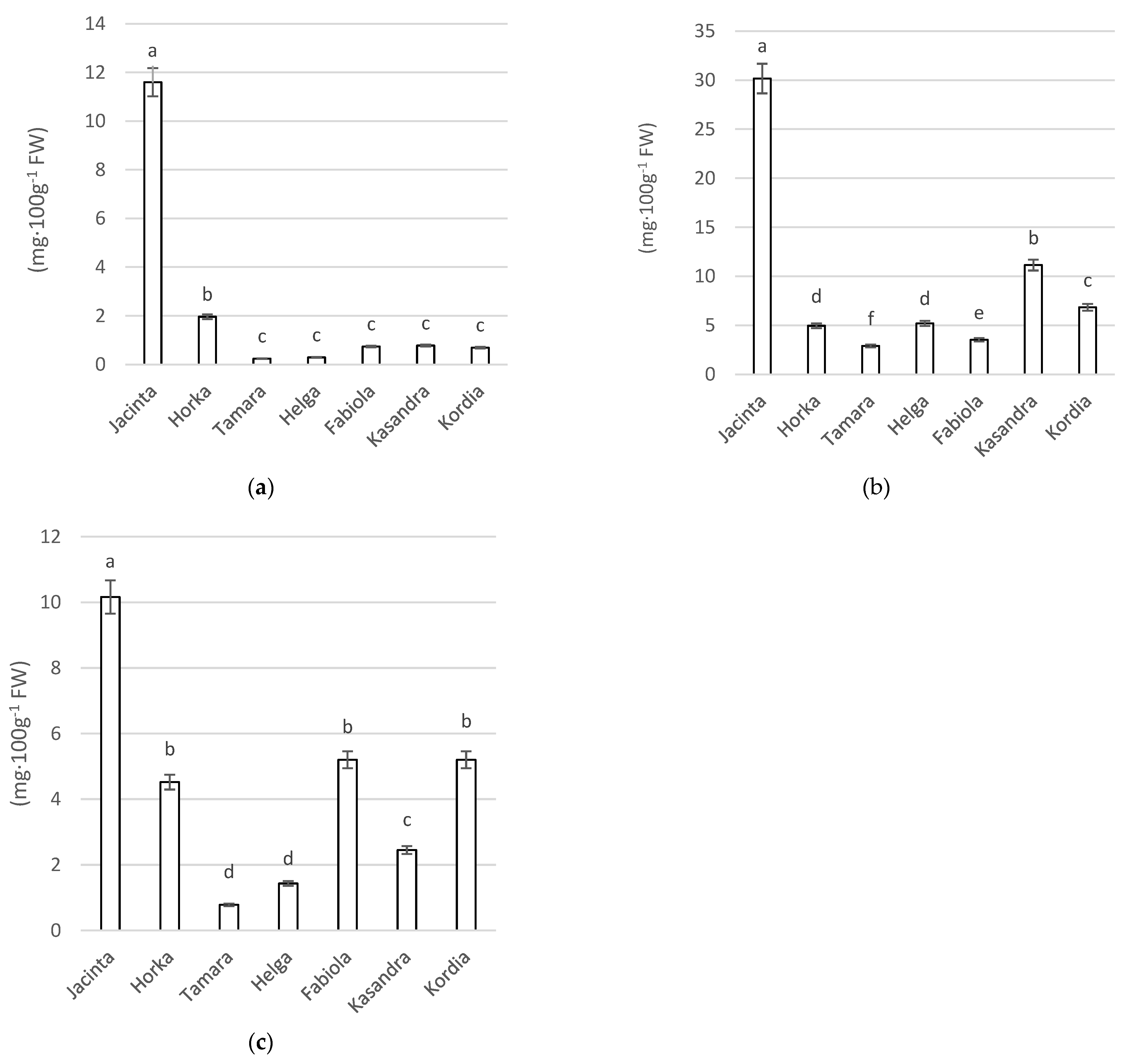
3.3.1. Analysis of Total Polyphenol Content
3.3.2. Analysis of Flavonoid Content
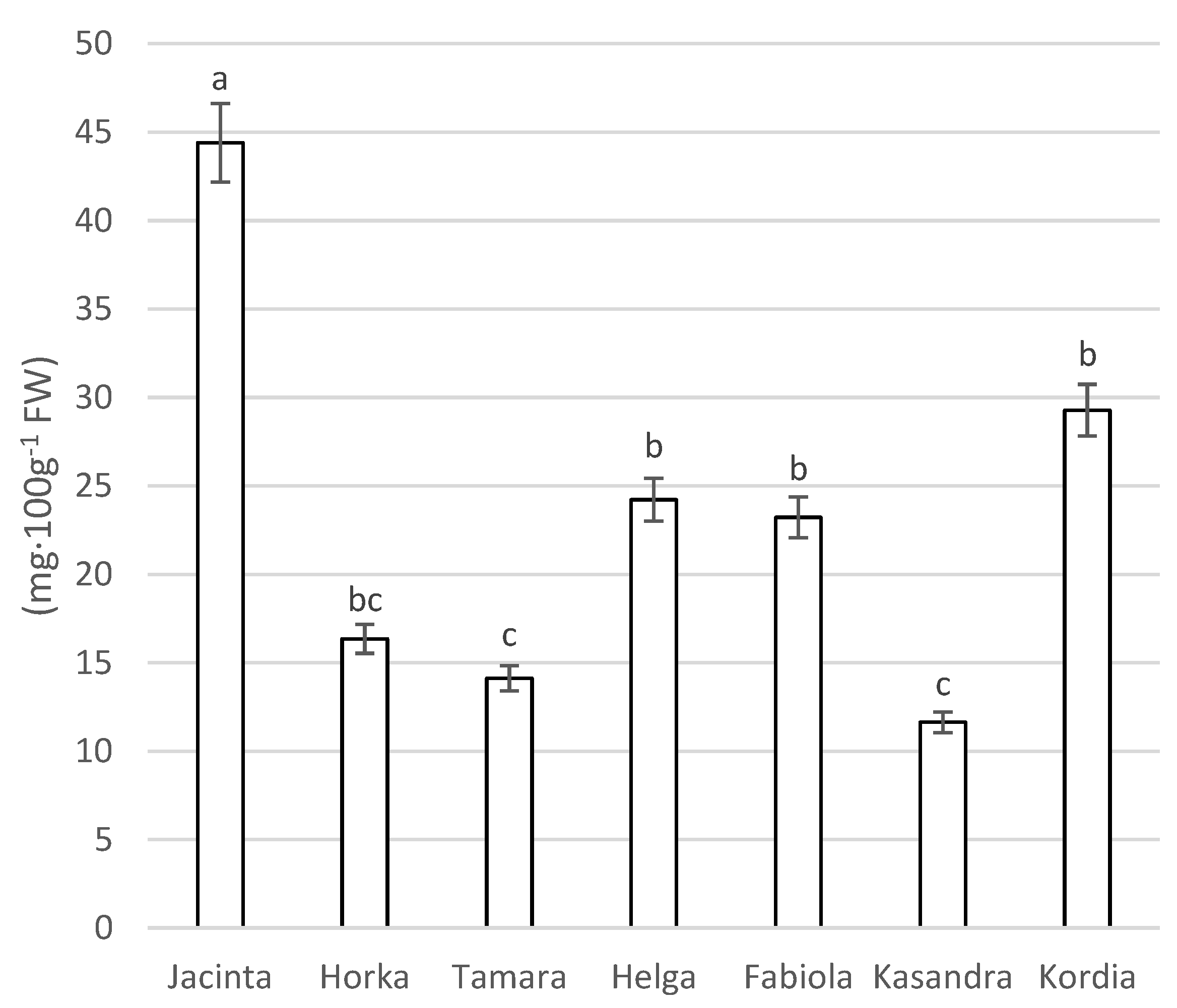
3.3.3. Analysis of Anthocyanin Content
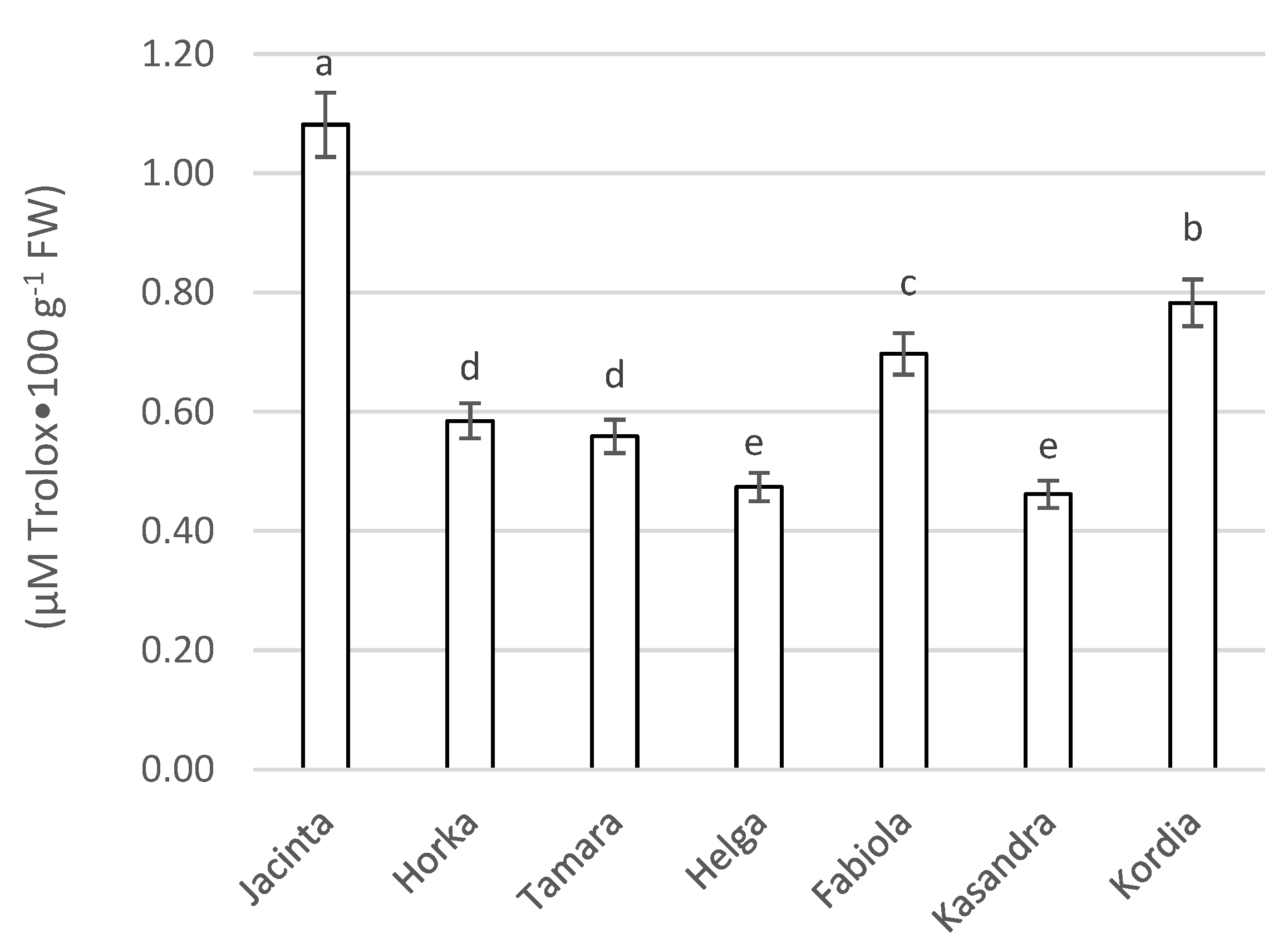
3.3.4. Antioxidant Capacity
3.4. Correlation Coefficient Values between Selected Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karagiannis, E.; Sarrou, E.; Michailidis, M.; Tanou, G.; Ganopoulos, I.; Bazakos, C.; Kazantzis, K.; Martens, S.; Xanthopoulou, A.; Molassiotis, A. Fruit quality trait discovery and metabolic profiling in sweet cherry genebank collection in Greece. Food Chem. 2021, 342, 128315. [Google Scholar] [CrossRef] [PubMed]
- Ates, U.; Ozturk, B. Fruit Quality Characteristics of Different Sweet Cherry (Prunus avium L.) Cultivars Grown in Ordu Province of Turkey. Karadeniz Fen Bilim. Derg. 2022, 12, 168–177. [Google Scholar] [CrossRef]
- Esti, M.; Cinquante, L.; Sinesio, F.; Moneta, E.; Matteo, M. Physicochemical and sensory fruit characteristics of two sweet cherry cultivars after cool storage. Food Chem. 2002, 76, 399–405. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Kolniak-Ostek, J.; Oziembłowski, M.; Ticha, A.; Hyšpler, R.; Zadak, Z.; Židová, P.; Paprstein, F. Comparison of old cherry cultivars grown in Czech Republic by chemical composition and bioactive compounds. Food Chem. 2017, 228, 136–142. [Google Scholar] [CrossRef]
- Antognoni, F.; Potente, G.; Mandrioli, R.; Angeloni, C.; Freschi, M.; Malaguti, M.; Hrelia, S.; Lugli, S.; Gennari, F.; Muzzi, E.; et al. Fruit Quality Characterization of New Sweet Cherry Cultivars as a Good Source of Bioactive Phenolic Compounds with Antioxidant and Neuroprotective Potential. Antioxidants 2020, 9, 677. [Google Scholar] [CrossRef]
- Di Matteo, A.; Russo, R.; Graziani, G.; Ritieni, A.; Di Vaio, C. Characterization of autochthonous sweet cherry cultivars (Prunus avium L.) of southern Italy for fruit quality, bioactive compounds and antioxidant activity. J. Sci. Food Agric. 2017, 97, 2782–2794. [Google Scholar] [CrossRef]
- Romano, G.S.; Cittadini, E.D.; Pugh, B.; Schouten, R. Sweet cherry quality in the horticultural production chain. Stewart Postharvest Rev. 2006, 6, 2. Available online: https://inta.gob.ar/sites/default/files/script-tmp-inta_4_sweet_cherry_quality_in_the_horticultural_prod.pdf (accessed on 1 July 2022). [CrossRef]
- Wani, A.A.; Singh, P.; Gul, K.; Wani, M.H.; Langowski, H.C. Sweet cherry (Prunus avium): Critical factors affecting the composition and shelf life. Food Packag. Shelflife 2014, I, 86–99. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Usenik, V.; Fabcic, J.; Stampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Maro, A.D.; Petriccione, M.; Galasso, S.; Piccolella, S.; Giuseppe, A.; Scortichini, M.; Monaco, P. Chemical composition, nutritional value and antioxidant properties of autochthonous Prunus avium cultivars from Campania Region. Food Res. Int. 2014, 64, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jiang, Q.; Lin, J.; Li, X.; Sun, C.; Chen, K. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Dhandevi, P.E.M.; Jeewon, R. Fruit and vegetable intake: Benefits and progress of nutrition education interventions-narrative review article. Iran. J. Public Health 2015, 44, 1309–1321. [Google Scholar]
- Wang, S.Y. Effect of pre-harvest conditions on antioxidant capacity in fruits. Acta Hortic. 2006, 712, 299–306. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, H.J. The roles of polyphenols in cancer chemoprevention. Biofactors 2006, 26, 105–121. [Google Scholar] [CrossRef]
- Kampa, M.; Nifli, A.P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol. 2007, 159, 79–113. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Tabaszewska, M. Potential of sweet cherry (Prunus avium L.) by-products: Bioactive compounds and antioxidant activity of leaves and petioles. Eur. Food Res. Technol. 2019, 245, 763–772. [Google Scholar] [CrossRef]
- Mozetič, B.; Trebše, P.; Simčič, M.; Hribar, J. Changes of anthocyanins and hydroxycinnamic acids affecting the skin colour during maturation of sweet cherries (Prunus avium L.). Swiss Soc. Food Sci. Technol. 2004, 37, 123–128. [Google Scholar] [CrossRef]
- Mozetič, B.; Simčič, M.; Trebše, P. Anthocyanins and hydroxycinnamic acids of Lambert Compact cherries (Prunus avium L.) after cold storage and 1-methylcyclopropene treatment. Food Chem. 2006, 97, 302–309. [Google Scholar] [CrossRef]
- Gao, L.; Mazza, G. Characterization, quantitation, and distribution of anthocyanins and colorless phenolics in sweet cherries. J. Agric. Food Chem. 1995, 43, 343–346. [Google Scholar] [CrossRef]
- Usenik, V.; Fajt, N.; Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. Sweet cherry pomological and biochemical characteristics influenced by rootstock. J. Agric. Food Chem. 2010, 58, 4928–4933. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhong, F.; Tian, R.; Zhang, K.; Zhang, X.; Li, T. Comparative study of phenolic compounds and antioxidant activity in different species of cherries. J. Food Sci. 2011, 76, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, N.; Wang, Y.; Jiang, D.; Feng, X. Characterization of Phenolic Compounds from Early and Late Ripening Sweet Cherries and Their Antioxidant and Antifungal Activities. J. Agric. Food Chem. 2017, 65, 5413–5420. [Google Scholar] [CrossRef] [PubMed]
- Simeone, A.; Nota, P.; Ceccarelli, D.; Del Toro, A.; Piazza, G.; De Salvador, F.R.; Caboni, E.; Krupa, T. Anthocyanins in blueberry cultivars: Effect of the growing area. Acta Hortic. 2012, 926, 713–716. [Google Scholar] [CrossRef]
- Whiting, M.D.; Lang, G.; Ophardt, D. Rootstock and training system affect sweet cherry growth, yield and fruit quality. HortScience 2005, 40, 582–586. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and health: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- PN-EN 12143; Soki Owocowe i Warzywne—Oznaczanie Zawartosci Substancji Rozpuszczalnych Metoda Refraktometryczna. Polish Committee of Standardization: Warsaw, Poland, 2000. (In Polish)
- Szpadzik, E.; Krupa, T.; Niemiec, W.; Jadczuk-Tobjasz, E. Yielding and fruit quality of selected sweet cherry (Prunus avium) cultivars in the conditions of central Poland. Acta Sci. Pol. Hortorum Cultus 2019, 18, 117–126. [Google Scholar] [CrossRef]
- PN-EN 12147; Soki Owocowe i warzywne—Oznaczanie kwasowosci miareczkowej. Polish Committee of Standardization: Warsaw, Poland, 2000. (In Polish)
- Stefaniak, J.; Sawicka, M.; Krupa, T.; Latocha, P.; Łata, B. Effect of kiwiberry pre-storage treatments on the fruit quality during cold storage. Zemdirb.-Agric. 2017, 104, 235–242. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons: New York, NY, USA, 2001; pp. I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. Available online: https://dl.uctm.edu/journal/node/j2005-3/Marinova.pdf (accessed on 1 July 2022).
- Krupa, T.; Tomala, K. Antioxidant capacity, anthocyanin content profile in ‘Bluecrop’ blueberry fruit. Veg. Crops Res. Bull. 2007, 66, 129–141. [Google Scholar] [CrossRef]
- Saint-Cricq De Gaulejac, N.; Provost, C.; Vivas, N. Comparative study of polyphenol scavenging activities assessed by different methods. J. Agric. Food Chem. 1999, 47, 425–431. [Google Scholar] [CrossRef]
- Grandi, M.; Lugli, S.; Correale, R. Fruit quality changes in postponed picking of new cherry cultivars. Acta Hortic. 2017, 1161, 599–602. [Google Scholar] [CrossRef]
- Correia, S.; Aires, A.; Queirós, F.; Carvalho, R.; Schouten, R.; Silva, A.P.; Gonçalves, B. Climate conditions and spray treatments induce shifts in health promoting compounds in cherry (Prunus avium L.) fruits. Sci. Hortic. 2020, 263, 109147. [Google Scholar] [CrossRef]
- Zhang, C.; Whiting, M. Improving ‘Bing’ sweet cherry fruit quality with plant growth regulators. Sci. Hortic. 2011, 127, 341–346. [Google Scholar] [CrossRef]
- Predieri, S.; Dris, R.; Rapparini, F. Influence of growing conditions on yield and quality of cherry: II. Fruit quality. Food Agric. Environ. 2004, 2, 307–309. [Google Scholar]
- Whiting, M.D.; Ophardt, D.; McFerson, J.R. Chemical blossom thinners vary in their effect on sweet cherry fruit set, yield, fruit quality, and crop value. Horttechnology 2006, 16, 66–70. [Google Scholar] [CrossRef]
- Kappel, F.; Fischer-Fleming, B.; Hogue, E. Fruit characteristics and sensory attributes of an ideal sweet cherry. HortScience 1996, 31, 443–446. [Google Scholar] [CrossRef]
- Wermund, U.; Holland, A.; Reardon, S. Cracking susceptibility of sweet cherries in the United Kingdom in relation to calcium application and covering systems. Acta Hortic. (ISHS) 2005, 667, 475–482. [Google Scholar] [CrossRef]
- Bandi, A.; Thiesz, R.; Ferencz, L.; Bandi, M.J. Some physical and biochemical compositions of the sweet cherry (Prunus avium L.) fruit. Acta Univ. Sapientiae Agric. Environ. 2010, 2, 5–16. Available online: http://www.acta.sapientia.ro/acta-agrenv/C2/agrenv2-1.pdf (accessed on 1 July 2022).
- Vavra, R. Sweet cherry fruit characteristic in covered orchards. Acta Hortic. Et Regiotect. 2020, 23, 5–7. [Google Scholar] [CrossRef]
- Imrak, B.; Kuden, A.; Sarıer, A. Researches on 0900 Ziraat cherry cultivar prevent from fruit cracking. Int. J. Agric. Nat. Sci. 2018, 1, 142–145. Available online: https://www.ijans.org/index.php/ijans/article/view/435 (accessed on 1 July 2022).
- Crisosto, C.H.; Garner, D.; Doyle, J.; Day, K.R. Relationship between fruit respiration, bruising susceptibility, and temperature in sweet cherries. HortScience 1993, 28, 132–135. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Johnson, S.; DeJong, T. Orchard factors affecting postharvest stone fruit quality. HortScience 1997, 32, 820–823. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Buchanan, D.A.; Fellman, J.K. Volatile compounds emitted by sweet cherries (Prunus avium cv. Bing) during fruit development and ripening. J. Agric. Food Chem. 1992, 40, 471–474. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Sturm, K.; Fajt, N. Rootstocks affect leaf mineral composition and fruit quality of ‘Lapins’ sweet cherry. Acta Hortic. 2005, 667, 247–252. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Petkovsek, M.M.; Kastelec, D. The effect of fruit size and fruit colour on chemical composition in ‘Kordia’ sweet cherry (Prunus avium L.). J. Food Compos. Anal. 2015, 38, 121–130. [Google Scholar] [CrossRef]
- Saracoglu, O.; Ozturk, B.; Yildiz, K.; Kucuker, E. Pre-harvest methyl jasmonate treatments delayed ripening and improved quality of sweet cherry fruits. Sci. Hortic. 2017, 226, 19–23. [Google Scholar] [CrossRef]
- Aglar, E.; Ozturk, B.; Guler, S.K.; Karakaya, O.; Uzun, S.; Saracoglu, O. Effect of modified atmosphere packaging and ‘Parka’ treatments on fruit quality characteristics of sweet cherry fruits (Prunus avium L. ‘0900 Ziraat’) during cold storage and shelf life. Sci. Hortic. 2017, 222, 162–168. [Google Scholar] [CrossRef]
- Aglar, E.; Saracoglu, O.; Karakaya, O.; Ozturk, B.; Gun, S. The relationship between fruit color and fruit quality of sweet cherry (Prunus avium L. cv. ‘0900 Ziraat’). Turk. J. Food Agric. Sci. 2019, 1, 1–5. Available online: https://dergipark.org.tr/en/download/article-file/824387 (accessed on 1 July 2022).
- Brown, S.K.; Bourne, M.C. Assessment of components of fruit firmness in selected sweet cherry genotypes. HortScience 1988, 23, 902–904. [Google Scholar] [CrossRef]
- Guyer, D.E.; Sinha, N.K.; Tung-Sung, C.; Cash, J.N. Physicochemical and sensory characteristics of selected Michigan sweet cherry (Prunus avium L.) cultivars. J. Food Qual. 1993, 16, 355–370. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Ritenour, M.A. Testing the reliability of skin color as an indicator of quality for early season “Brooks” (Prunus avium L.) cherry. Postharvest Biol. Technol. 2002, 24, 147–154. [Google Scholar] [CrossRef]
- Poll, L.; Petersen, M.B. Influence on harvest year and harvest time on soluble solids, titrateable acid, anthocyanin content and aroma components in sour cherry (Prunus cerasus L. cv. “Stevnsbær”). Eur. Food Res. Technol. 2003, 216, 212–216. [Google Scholar] [CrossRef]
- Palou, L.; Crisosto, C.H.; Garner, D.; Basinal, L.M. Effect of continuous exposure to exogenous ethylene during cold storage on postharvest decay development and quality attributes of stone fruits and table grapes. Postharvest Biol. Technol. 2003, 27, 243–254. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Tudela, J.A.; Luchsinger, L.; Artés-Hdez, F.; Artés, F. ‘Ambrunés’ sweet cheery quality factors change during ripening. Acta Hortic. 2005, 667, 529–534. [Google Scholar] [CrossRef]
- Serrano, M.; Díaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillen, F.; Martínez-Romero, D.; Valverde, J.M.; Valero, D. Maturity stage at harvest determines the fruit quality and antioxidant potential after storage of sweet cherry cultivars. J. Agric. Food Chem. 2009, 57, 3240–3246. [Google Scholar] [CrossRef] [PubMed]
- González-Gómez, D.; Lozano, M.; Fernández-León, M.F.; Bernalte, M.J.; Ayuso, M.C.; Rodríguez, A.B. Sweet cherry phytochemicals: Identification and characterization by HPLC-DAD/ESI-MS in six sweet-cherry cultivars grown in Valle del Jerte (Spain). J. Food Compos. Anal. 2010, 23, 533–539. [Google Scholar] [CrossRef]
- Serra, A.T.; Duarte, R.O.; Bronze, M.R.; Duarte, C.M.M. Identification of bioactive response in traditional cherries from Portugal. Food Chem. 2011, 125, 318–325. [Google Scholar] [CrossRef]
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical Constituents and Antioxidant Activity of Sweet Cherry at Different Ripening Stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Zapata, P.J.; Guillén, F.; Serrano, M. Sensory, nutritive and functional properties of sweet cherry as affected by cultivar and ripening stage. Food Sci. Technol. Int. 2009, 15, 535–543. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Hernández, A.; López-Corrales, M.; Córdoba, M.D.G. Physicochemical and sensorial characterisation of four sweet cherry cultivars grown in Jerte Valley (Spain). Food Chem. 2012, 133, 1551–1559. [Google Scholar] [CrossRef]
- Gonçalves, B.; Landbo, A.K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar] [CrossRef]
- Schmitz-Eiberger, M.A.; Blanke, M.M. Bioactive components in forced sweet cherry fruit (Prunus avium L.), antioxidative capacity and allergenic potential as dependent on cultivation under cover. Lebensm.-Wiss. Und-Technol. 2012, 46, 388–392. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Anthocyanin and phenolic composition of fresh and processed cherries. J. Food Sci. 2004, 69, 173–183. [Google Scholar] [CrossRef]
- Faniadis, D.; Drogoudi, P.D.; Vasilakakis, M. Effects of cultivar, orchard elevation, and storage on fruit quality characters of sweet cherry (Prunus avium L.). Sci. Hortic. 2010, 125, 301–304. [Google Scholar] [CrossRef]
- Knapová, P.; Bílková, A. Antioxidation Characteristics of sweet cherry varieties bred in Holovousy. VPO 2022, 28, 10–18. Available online: https://vpovsuo.cz/wp-content/uploads/2022/05/Knapova_VPO_28_1_22.pdf (accessed on 1 July 2022).
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruit and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]

| Month | Sum of Rainfall [mm·m−2] | Average Temp. [°C] |
|---|---|---|
| March | 18.3 | 4.0 |
| April | 55.3 | 6.5 |
| May | 62.3 | 12.4 |
| June | 69.2 | 19.7 |
| July | 118.8 | 21.7 |
| August | 140.1 | 17.2 |
| Cultivar | Fruit Weight [g] | Fruit Diameter [mm] | Stone Weight [g] | Proportion of Seed in the Fruit [%] | Firmness [N] | SSC [°Bx] | TA [% of Malic Acid] | SSC/TA Ratio |
|---|---|---|---|---|---|---|---|---|
| Jacinta Horka Tamara Helga Fabiola KasandraKordia | 12.21 ± 0.3 A,* 11.90 ± 0.06 A 12.58 ± 0.5 A 7.82 ± 0.3 C 12.58 ± 0.6 A 9.65 ± 0.2 B 9.60 ± 0.3 B | 30.60 ± 0.1 A 30.64 ± 0.3 A 30.80 ± 0.3 A 26.20 ± 0.2 C 30.53 ± 0.6 A 28.60 ± 0.4 B 28.43 ± 0.3 B | 0.55 ± 0.03 A 0.46 ± 0.01 B,C 0.52 ± 0.02 A,B 0.43 ± 0.02 C 0.51 ± 0.01 A,B 0.49 ± 0.02 B 0.43 ± 0.03 C | 4.52 ± 0.2 B 3.86 ± 0.1 C 4.10 ± 0.2 B,C 5.50 ± 0.1 A 4.02 ± 0.2 C 5.08 ± 0.2 A 4.50 ± 0.2 B | 4.26 ± 0.2 E 6.95 ± 0.3 B,C 7.51 ± 0.3 B 6.43 ± 0.4 C 8.52 ± 0.4 A 5.59 ± 0.3 D 8.29 ± 0.07 A | 16.30 ± 0.4 A 15.78 ± 0.4 A 13.30 ± 0.2 C 13.50 ± 0.2 C 14.33 ± 0.7 B 13.93 ± 0.3 B,C 13.53 ±0.2 C | 0.75 ± 0.02 A 0.77 ± 0.01 A 0.52 ± 0.04 C 0.57 ± 0.01 B,C 0.51 ± 0.08 C 0.54 ± 0.01 C 0.65 ± 0.02 B | 21.74 ± 1.0 B 20.63 ± 0.6 B 25.50 ± 2.1 A,B 23.78 ± 0.4 A,B 28.75 ± 2.4 A 25.70 ± 0.9 A,B 20.85 ± 0.9 B |
| Flavonoids | DPPH | Peonidin-3-rutinoside | Cyanidin-3-rutinoside | Cyanidin-3-galactoside | Color hue | Color C | Color L* | Diameter | Weight | Firmness | SSC | Titration Acidity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenols | 0.799 * | 0.800 * | 0.609 * | 0.555 * | 0.730 * | 0.335 | −0.480 * | −0.361 | 0.008 | −0.002 | −0.141 | 0.364 | 0.560 * |
| Flavonoids | - | 0.871 * | 0.774 * | 0.731 * | 0.810 * | 0.463 * | −0.598 * | −0.234 | 0.048 | 0.091 | −0.380 | 0.456 * | 0.479 * |
| DPPH | - | - | 0.847 * | 0.767 * | 0.925 * | 0.611 * | −0.563 * | −0.492 * | 0.410 | 0.417 | −0.296 | 0.586 * | 0.533 * |
| Peonidin-3-rutinoside | - | - | - | 0.953 * | 0.851 * | 0.777 * | −0.464 * | −0.564 * | 0.349 | 0.339 | −0.721 * | 0.757 * | 0.609 * |
| Cyanidin-3-rutinoside | - | - | - | - | 0.788 * | 0.673 * | −0.397 | −0.416 | 0.178 | 0.148 | −0.813 * | 0.637 * | 0.511 * |
| Cyanidin-3-galactoside | - | - | - | - | - | 0.591 * | −0.745 * | −0.482 * | 0.392 | 0.371 | −0.377 | 0.752 * | 0.637 * |
| Color hue | - | - | - | - | - | - | −0.179 | −0.720 * | 0.510 * | 0.496 * | −0.531 * | 0.749 * | 0.455 * |
| Color C | - | - | - | - | - | - | - | 0.001 | −0.048 | −0.083 | 0.109 | −0.609 * | −0.372 |
| Color L* | - | - | - | - | - | - | - | - | −0.650 * | −0.579 * | 0.274 | −0.669 * | −0.677 * |
| Diameter | - | - | - | - | - | - | - | - | - | 0.974 * | 0.042 | 0.462 * | 0.283 |
| Weight | - | - | - | - | - | - | - | - | - | - | 0.078 | 0.430 | 0.188 |
| Firmness | - | - | - | - | - | - | - | - | - | - | - | −0.501 * | −0.388 |
| SSC | - | - | - | - | - | - | - | - | - | - | - | - | 0.674 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpadzik, E.; Krupa, T.; Molska-Kawulok, K.; Przybyłko, S. Fruit Quality and Contents of Some Bioactive Compounds in Selected Czech Sweet Cherry (Prunus avium L.) Cultivars under Conditions of Central Poland. Agriculture 2022, 12, 1859. https://doi.org/10.3390/agriculture12111859
Szpadzik E, Krupa T, Molska-Kawulok K, Przybyłko S. Fruit Quality and Contents of Some Bioactive Compounds in Selected Czech Sweet Cherry (Prunus avium L.) Cultivars under Conditions of Central Poland. Agriculture. 2022; 12(11):1859. https://doi.org/10.3390/agriculture12111859
Chicago/Turabian StyleSzpadzik, Ewa, Tomasz Krupa, Karolina Molska-Kawulok, and Sebastian Przybyłko. 2022. "Fruit Quality and Contents of Some Bioactive Compounds in Selected Czech Sweet Cherry (Prunus avium L.) Cultivars under Conditions of Central Poland" Agriculture 12, no. 11: 1859. https://doi.org/10.3390/agriculture12111859
APA StyleSzpadzik, E., Krupa, T., Molska-Kawulok, K., & Przybyłko, S. (2022). Fruit Quality and Contents of Some Bioactive Compounds in Selected Czech Sweet Cherry (Prunus avium L.) Cultivars under Conditions of Central Poland. Agriculture, 12(11), 1859. https://doi.org/10.3390/agriculture12111859






