Sorghum–Grass Intercropping Systems under Varying Planting Densities in a Semi-Arid Region: Focusing on Soil Carbon and Grain Yield in the Conservation Systems
Abstract
1. Introduction
2. Materials and Methods
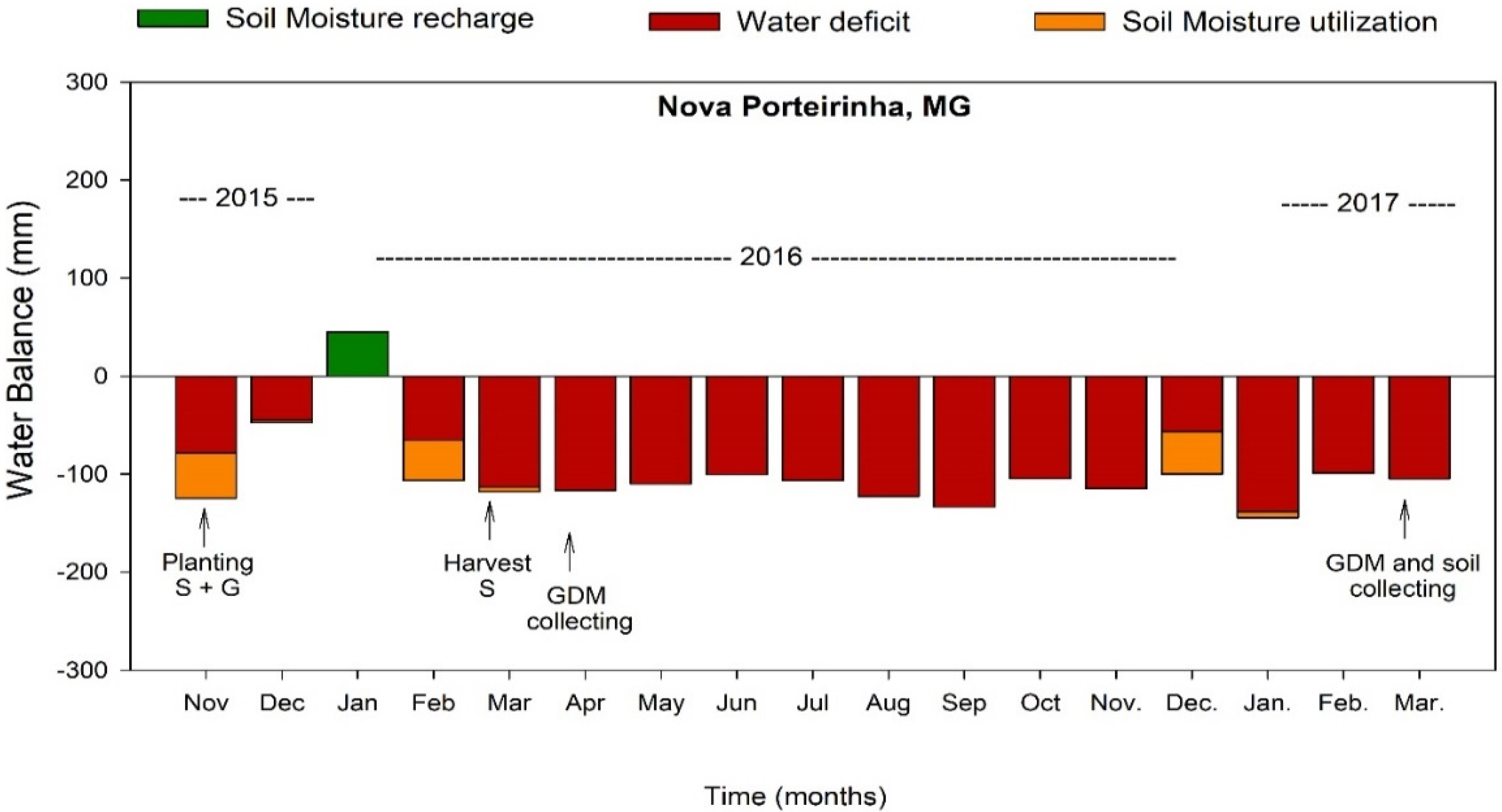
2.1. Site Characterization
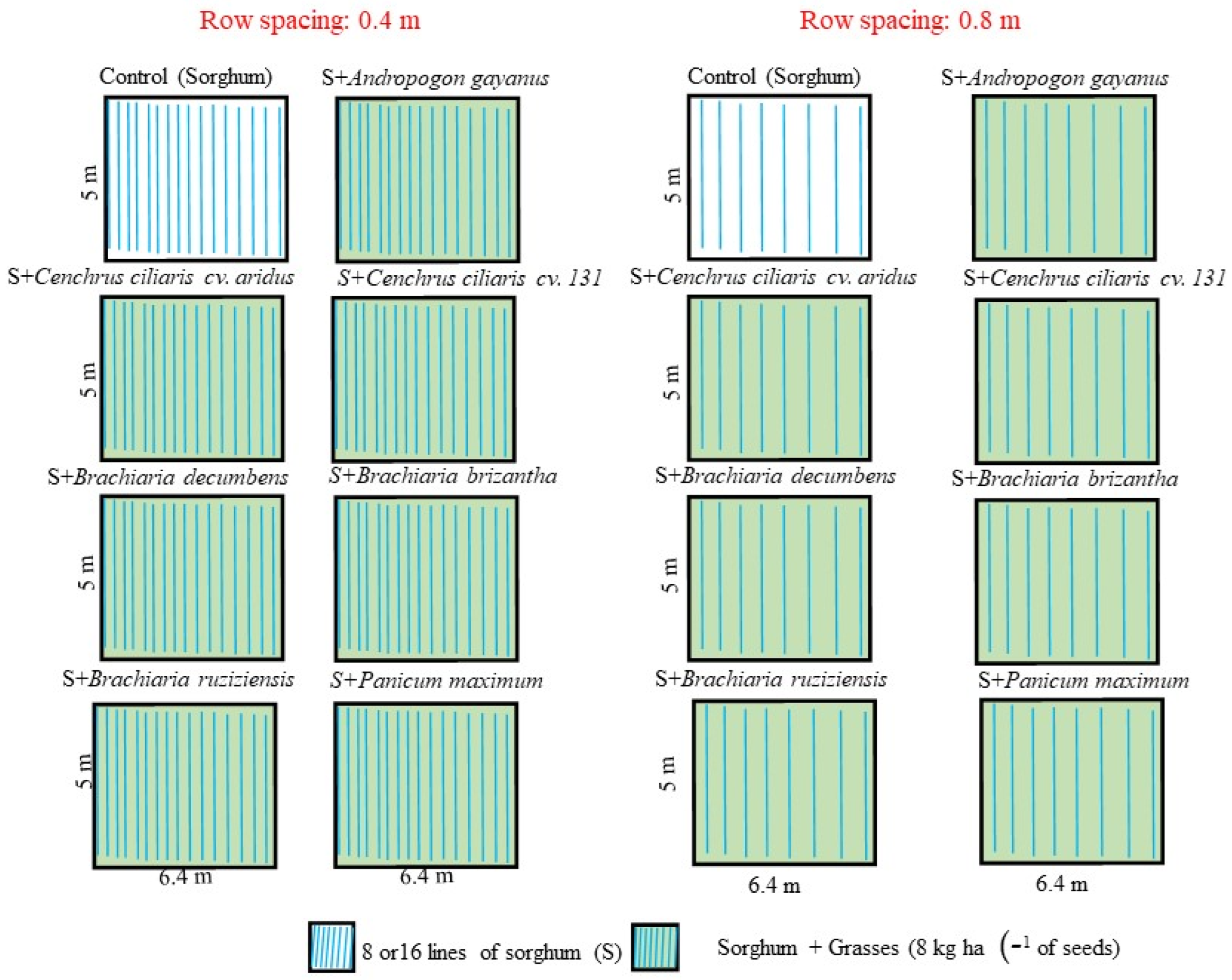
2.2. Experimental Characterization
2.3. Measurements
2.4. Data Analysis
3. Results
3.1. Productivity of Grains and Dry Matter
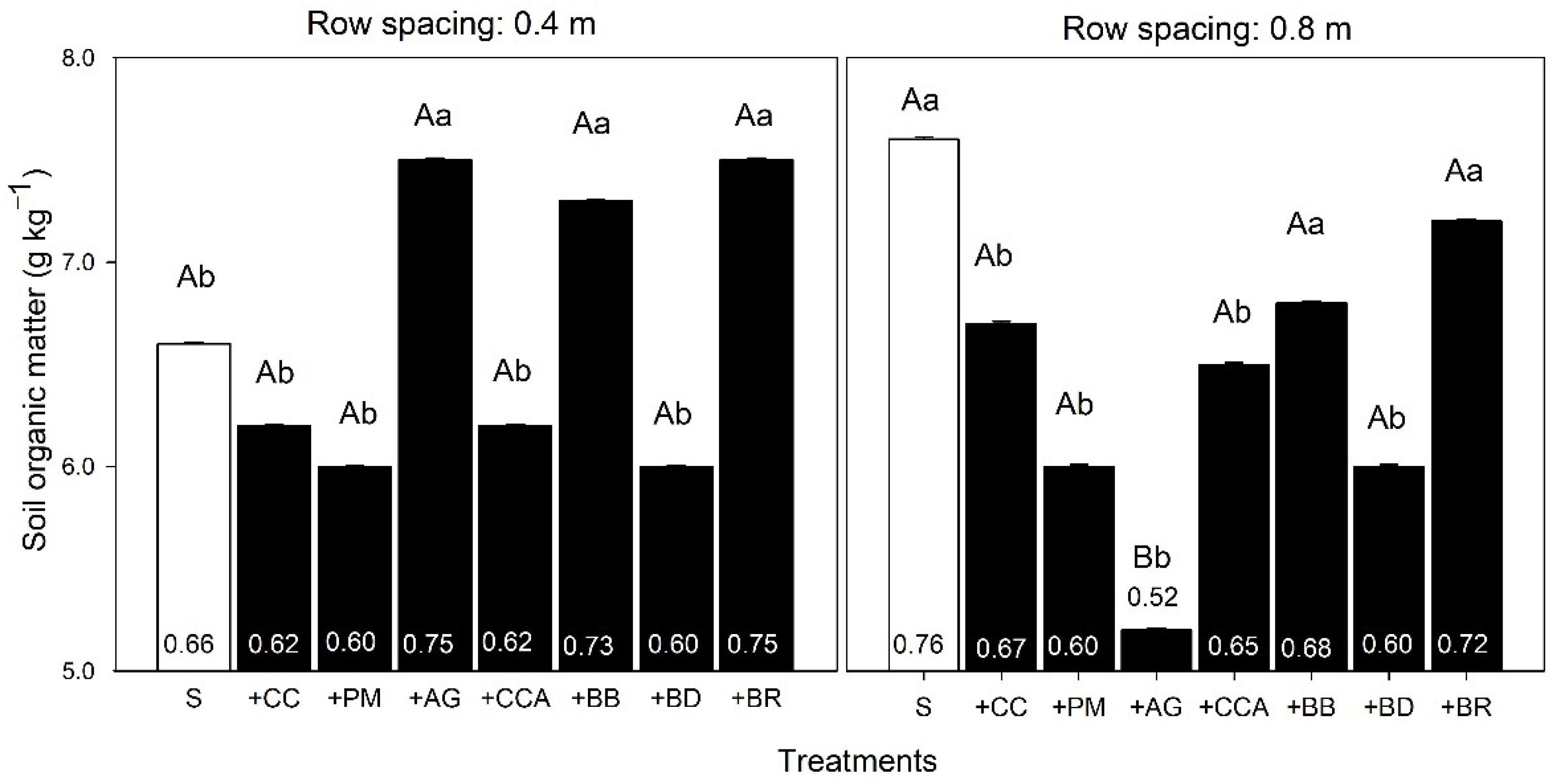
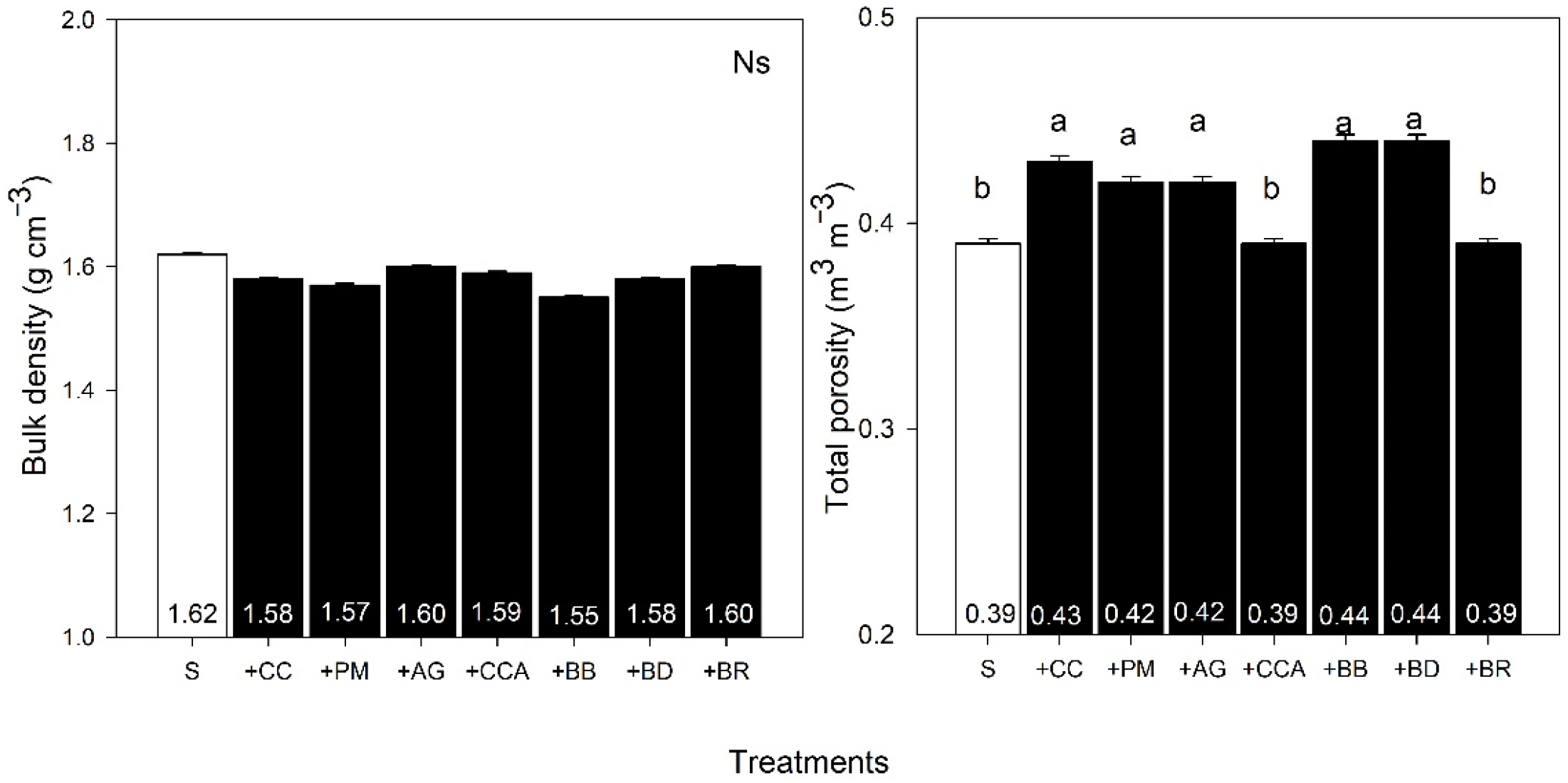
3.2. Soil Carbon and Soil Porosity
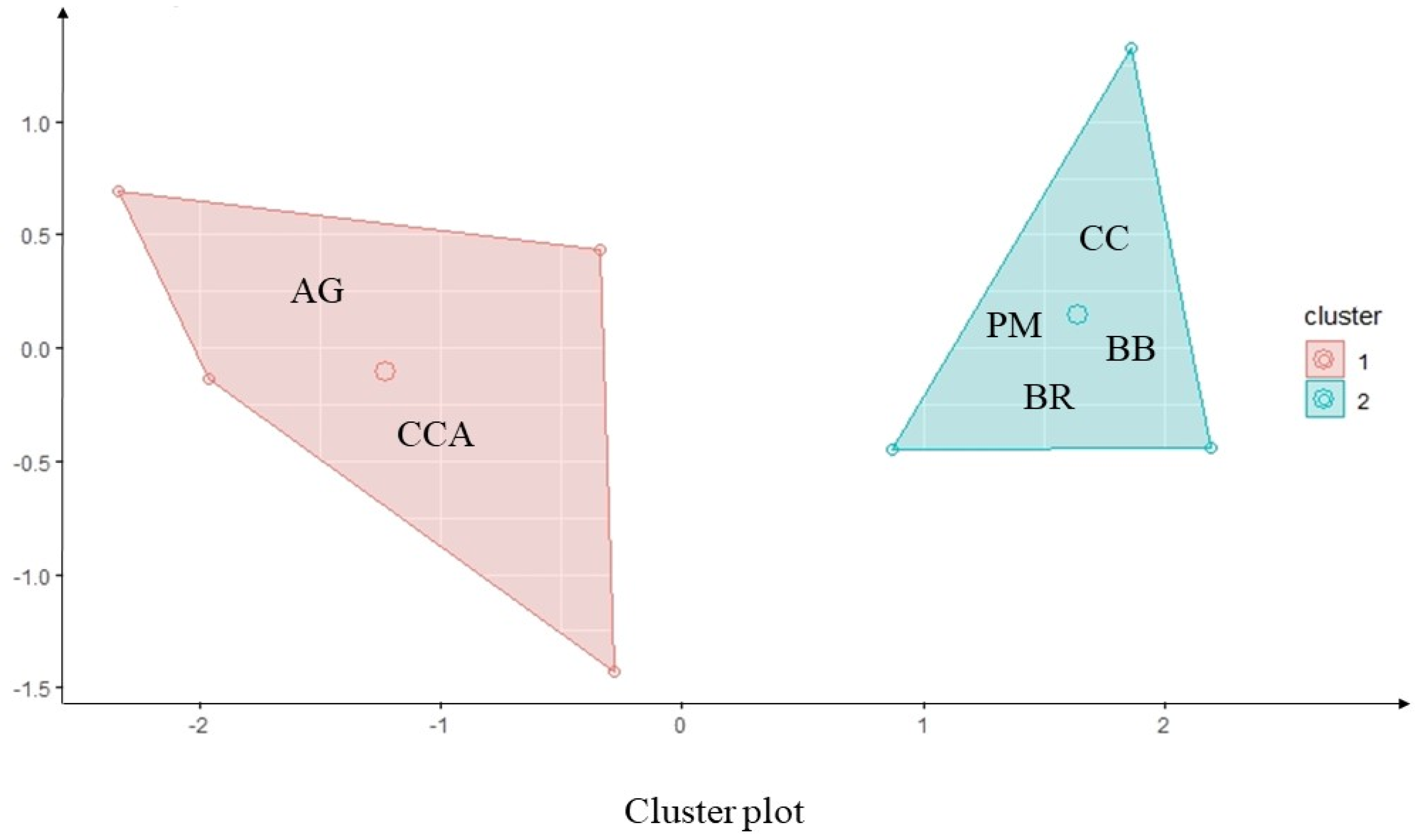
3.3. Cluster Formation
4. Discussion
4.1. Productivity of Grains and Dry Matter
4.2. Carbon and Soil Porosity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brodt, S.; Six, J.; Feenstra, G.; Ingels, C.; Campbell, D. Sustainable agriculture. Nat. Educ. Knowl. 2011, 3, 1. [Google Scholar]
- Wendling, B.; Jucksch, I.; Mendonca, E.S.; Almeida, R.F.; Martins, C.E.; Domingues, L.A.S. Simulation of use and management effects on the soil organic matter pools of the Atlantic Forest biome, Brazil. Biosci. J. 2014, 30, 1278–1290. [Google Scholar]
- FAO—Food and Agriculture Organization of the United Nations. The Economics of Conservation Agriculture. Available online: http://wwwfaoorg/3/Y2781E/y2781e03htm (accessed on 24 January 2020).
- Alvalá, R.C.S.; Cunha, A.P.M.A.; Brito, S.S.B.; Seluchi, M.E.; Marengo, J.A.; Moraes, O.L.L.; Carvalho, M.A. Drought monitoring in the Brazilian Semiarid region. An. Acad. Bras. Ciênc. 2017, 91. [Google Scholar] [CrossRef]
- Fortes, C.; Evaristo, A.B.; Barros, A.; Pimentel, L.D. Agronomic performance of biomass sorghum hybrids at edaphoclimatic conditions of Tocantins state. Rev. Energ. Na Agric. 2018, 33, 27–30. [Google Scholar]
- Buso, W.H.D.; Morgado, H.S.; Silva, L.B.; França, A.F.S. Utilização do sorgo forrageiro na alimentação animal. Pubvet 2011, 5, 1145. [Google Scholar] [CrossRef]
- Lima, L.R.; Silva, T.G.F.; Pereira, P.C.; Morais, J.E.F.; Assis, M.C.S. Productive-economic benefit of forage cactus-sorghum intercropping systems irrigated with saline water. Rev. Caatinga 2018, 31, 191–201. [Google Scholar] [CrossRef]
- Silva, J.M.F.; Dutra, A.S.; Câmara, F.T.; Pinto, A.A.; Silva, F.E. Row spacing, plant density, sowing and harvest times for sweet sorghum. Pesqui. Agropecu. Trop. 2017, 47, 408–415. [Google Scholar] [CrossRef][Green Version]
- Fidelis, R.R.; Gonzaga, L.A.M.; Silva, R.R.; Andrade, C.A.O. Desempenho produtivo e nutricional de sorgo forrageiro consorciado com soja em doses de nitrogênio. Comun. Sci. 2016, 7, 204–208. [Google Scholar] [CrossRef]
- Santos, J.M.R.; Silva, M.R.B.; Santos, M.L.S.; Melo, R.F.; Oliveira, A.R. Desenvolvimento e produtividade de sorgo sacarino consorciado com feijão-caupi em barragem subterrânea. In Jornada de Iniciação Científica da Embrapa Semiárido Petrolina Anais Petrolina; Embrapa Semiárido: Petrolina, Brazil, 2016; pp. 173–178. [Google Scholar]
- Almeida, R.F.; Mikhael, J.E.R.; Franco, F.O.; Santana, L.M.F.; Wendling, B. Measuring the labile and recalcitrant pools of carbon and nitrogen in forested and agricultural soils: A study under tropical conditions. Forests 2019, 10, 544. [Google Scholar] [CrossRef]
- Mattos, J.L.S.; Gomide, J.A.; Martinez, C.A. Effect of water deficit and flooding on the growth of Brachiaria species in the field. Rev. Bras. Zootec. 2005, 34, 755–764. [Google Scholar] [CrossRef]
- Oliveira, A.R.F.; Araujo, L.C.; Ludkiewicz, M.G.Z.; Zagato, L.Q.S.D.; Galindo, F.S.; Maruno, T.C. Productivity, composition morphological and chemical the grass marandu intercropped with forage sorghum for renewal of degraded pastures in closed. Rev. Cult. Agron. 2017, 26, 69–81. [Google Scholar]
- Albuquerque, C.J.B.; Oliveira, R.M.; Silva, K.M.J.; Alves, D.D.; Alvarenga, R.C.; Borges, G.L.F.N. Intercropping of tropical forage crops with grain sorghum in two localities of Minas Gerais state. Rev. Bras. Milho Sorgo 2013, 12, 1–9. [Google Scholar] [CrossRef]
- Ashoori, N.; Abdi, M.; Golzardi, F.; Ajalli, F.; Ilkaee, M.N. Forage potential of sorghum-clover intercropping systems in semi-arid conditions. Bragantia 2021, 80, e1421. [Google Scholar] [CrossRef]
- Müller, M.M.L.; Guimarães, M.F.; Desjardins, T.; Mitja, D. The relationship between pasture degradation and soil properties in the Brazilian Amazon: A case study. Agric. Ecosyst. Environ. 2004, 103, 279–288. [Google Scholar] [CrossRef]
- Arruda, E.M.; De-Almeida, R.F.; Domingues, L.A.S.; Silva Junior, A.C.; Moraes, E.R.; Barro, L.R.; Sousa, J.L.O.; Lana, R.M.Q. Soil porosity and density in sugarcane cultivation under different tillage systems. Afr. J. Agric. Res. 2016, 11, 2689–2696. [Google Scholar]
- Martins, F.P.; Almeida, R.F.; Mikhael, J.E.R.; Queiroz, I.D.S.; Teixeira, W.G.; Borges, E.N. Correlação do COT e porosidade em Latossolo com diferentes usos e manejos na região de Uberaba, MG. Rev. Agrogeoambient. 2014, 7, 81–90. [Google Scholar] [CrossRef][Green Version]
- Almeida, R.F.; Nave, E.R.; Mota, R.P. Soil quality: Enzymatic activity of soil β-glucosidase. Glob. Sci. Res. J. 2015, 3, 146–150. [Google Scholar]
- Mikhael, J.E.R.; Almeida, R.F.; Franco, F.O.; Camargo, R.O.; Wendling, B. Recalcitrant carbon and nitrogen in agriculture soils with residue accumulation and fertilization under tropical conditions. Biosci. J. 2019, 35, 732–740. [Google Scholar] [CrossRef]
- Almeida, R.F.; Sanches, B.C. Availability of soil organic carbon in the Brazilian Cerrado. Sci. Agrar. Parana. 2014, 13, 259–264. [Google Scholar]
- Ferraz-Almeida, R.; da Silva, N.L.; Wendling, B. How does N mineral fertilizer influence the crop residue N credit? Nitrogen 2020, 1, 99–110. [Google Scholar] [CrossRef]
- Ferraz-Almeida, R.; Silveira, C.H.; Mota, R.P.; Moitinho, M.; Arruda, E.M.; Mendonça, E.S.; La Scala, N.; Wendling, B. For how long does the quality and quantity of residues in the soil affect the carbon compartments and CO2-C emissions? J. Soils Sediments 2016, 16, 2354–2364. [Google Scholar] [CrossRef]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. Manual de Métodos de Análise de Solo, 2nd ed.; Embrapa—CNPS: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis Part 3 Chemical Methods Soil Science of America and American Society of Agronomy; Black, C.A., Ed.; Wiley: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- CONAB—Companhia Nacional de Abastecimento. Sorgo. Available online: https://wwwconabgovbr/info-agro/analises-do-mercado-agropecuario-e-extrativista/analises-do-mercado/historico-mensal-de-sorgo/item/download/24901_d14bbb75a7ea3485fc1180409183ac2b (accessed on 10 January 2019).
- Albuquerque, C.J.B.; Von Pinho, R.G.; Rodrigues, J.A.S.; Brant, R.S.; Mendes, M.C. Row spacing and sowing density for grain sorghum in semi-arid. Bragantia 2011, 70, 278–285. [Google Scholar] [CrossRef][Green Version]
- Von Pinho, R.G.V.; Vasconcelos, R.C. Sorghum Planting Lavras; UFLA: Lavras, Brazil, 2002; p. 76. [Google Scholar]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. Sorgo granífero Embrapa—Milho e Sorgo. Sete Lagoas, Brazil, 2008. Available online: https://www.embrapa.br/en/busca-de-solucoes-tecnologicas/-/produto-servico/3547/sorgo-granifero-brs-373 (accessed on 15 October 2022).
- Srinivasarao, C.; Deshpande, A.N.; Venkateswarlu, B.; Lal, R.; Singh, A.K.; Kundu, S.; Vittal, K.P.R.; Mishra, P.K.; Prasad, J.V.N.S.; Mandal, U.K.; et al. Grain yield and carbon sequestration potential of post monsoon sorghum cultivation in Vertisols in the semi-arid tropics of central India. Geoderma 2012, 175–176, 90–97. [Google Scholar] [CrossRef]
- Júnior, I.D.O.A.; Santos, S.R.D.; Kondo, M.K.; Oliveira, P.M.D.; Júnior, V.R.R. Response of forage sorghum to water availability in a typic quartzipsamment. Rev. Caatinga 2019, 32, 1015–1026. [Google Scholar] [CrossRef]
- De Araujo, L.C.; Santos, P.M.; Mendonca, F.C.; Mourão, G. Establishment of Brachiaria brizantha cv. Marandu, under levels of soil water availability in stages of growth of the plants. Rev. Bras. Zootec. 2011, 40, 1405–1411. [Google Scholar] [CrossRef]
- Santos, P.M.; da Cruz, P.G.; de Araujo, L.C.; Pezzopane, J.R.M.; do Valle, C.B.; Pezzopane, C.G. Response mechanisms of Brachiaria brizantha cultivars to water deficit stress. Rev. Bras. Zootec. 2013, 42, 767–773. [Google Scholar] [CrossRef]
- Philp, J.N.M.; Vance, W.; Bell, R.W.; Chhay, T.; Boyd, D.; Phimphachanhvongsod, V.; Denton, M.D. Forage options to sustainably intensify smallholder farming systems on tropical sandy soils: A review. Agron. Sustain. Dev. 2019, 39, 30. [Google Scholar] [CrossRef]
- Baumhardt, R.L.; Howell, T.A. Seeding Practices, Cultivar Maturity, and Irrigation Effects on Simulated Grain Sorghum Yield. Agron. J. 2006, 98, 462–470. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Tripathi, A.K.; Bandyopadhyay, K.K.; Manna, M.C. Assessment of nutrient competition and nutrient requirement in soybean/sorghum intercropping system. Eur. J. Agron. 2009, 1, 43–50. [Google Scholar] [CrossRef]
- Peerzada, A.M.; Ali, H.H.; Chauhan, B.S. Weed management in sorghum [Sorghum bicolor (L.) Moench] using crop competition: A review. Crop Prot. 2017, 95, 74–80. [Google Scholar] [CrossRef]
- Gazola, R.N.; de Melo, L.M.M.; Dinalli, R.P.; Teixeira Filho, M.C.M.; Garcia, C.M.P. Sowing depths of brachiaria in intercropping with corn in no tillage planting. Energ. Na Agric. 2013, 33, 157–166. [Google Scholar] [CrossRef]
- Ramos, S.J.; Faquin, V.; Rodrigues, C.R.; Silva, C.A.; Boldrin, P.F. Biomass production and phosphorus use of forage grasses fertilized with two phosphorus sources. Rev. Bras. Ciênc. Solo 2009, 33, 335–343. [Google Scholar] [CrossRef]
- Zavaschi, E.; de Abreu Faria, L.; Ferraz-Almeida, R.; do Nascimento, C.A.C.; Pavinato, P.S.; Otto, R.; Vitti, A.C.; Vitti, G.C. Dynamic of P flux in tropical acid soils fertilized with humic acid–complexed phosphate. J. Soil Sci. Plant Nutr. 2020, 1, 1937–1948. [Google Scholar] [CrossRef]
- Almeida, R.F.; Queiroz, I.D.S.; Mikhael, J.E.R.; Oliveira, R.C.; Borges, E.N. Enriched animal manure as a source of phosphorus in sustainable agriculture. Int. J. Recycl. Org. Waste Agric. 2019, 8, 203–210. [Google Scholar] [CrossRef]
- Giongo, V.; Salviano, A.M.; Dos Santos, B.R.C.; Leal, E.F. Phosphorus fertilization and growth of buffel grass (Cenchrus ciliares L.) cultivars. Rev. Bras. Eng. Agríc. Ambient. 2015, 19, 34–38. [Google Scholar] [CrossRef][Green Version]
- Porto, E.M.V.; Manoel, T.V.C.; David Alves, D.; Lima, M.V.G.; Ferreira da Silva, M. Structural components of variety of grass buffel submitted to nitrogen. Agropecu. Cient. Semiárido 2014, 10, 90. [Google Scholar]
- Ferrazza, R.A.; Lopes, M.A.; Albuquerque, C.J.B. Bioeconomic evaluation of a sorghum consortium with different forage species for an integrated crop-livestock system in nova porteirinha, MG. Forragicultura Pastagens 2016, 73, 94–102. [Google Scholar]
- Amado, T.J.C.; Bayer, C.; Conceição, P.C.; Spagnollo, E.; Campos, B.H.C.; Veiga, M. Potential of carbon accumulation in no-till soils with intensive use and cover crops in Southern Brazil. J. Environ. Qual. 2006, 35, 1599–1607. [Google Scholar] [CrossRef]
- Almeida, R.A.; Haddad Silveira, C.; Mikhael, J.E.R.; Oliveira Franco, F.; Teixeira Ribeiro, B.S.; Mendonça, E.; Wendling, B. CO2 emissions from soil incubated with sugarcane straw and nitrogen fertilizer. Afr. J. Biotechnol. 2014, 13, 3376–3384. [Google Scholar]
- Maia, P.R.; Fernandes, A.R.; Melo, V.S.; Santos, E.R.; da Silva, G.B. Nutrient recycling of sorghum straw and soil biological attributes in Eastern Amazon. Ciênc. Agrotecnol. 2012, 36, 518–525. [Google Scholar] [CrossRef][Green Version]
- Morais, Y.C.B.; Araújo Mdo, S.B.; Moura, M.S.B.; Galvíncio, J.D.; Miranda, R.Q. Analysis of carbon sequestration in Caatinga areas of Pernambucano Semiarid. Rev. Bras. Meteorol. 2017, 32, 585–599. [Google Scholar] [CrossRef]
- Amézquita, M.C.; Ibrahim, M.; Llanderal, T.; Buurman, P.; Amézquita, E. Carbon Sequestration in Pastures, Silvo-Pastoral Systems and Forests in Four Regions of the Latin American Tropics. J. Sustain. For. 2004, 21, 31–49. [Google Scholar] [CrossRef]
- Lal, R.; Kimble, J.M. Conservation tillage for carbon sequestration. Nutr. Cycl. Agroecosyst. 1997, 49, 243–253. [Google Scholar] [CrossRef]
- Joris, H.A.W.; Vitti, A.C.; Ferraz-Almeida, R.; Otto, R.; Cantarella, H. Long-term N fertilization reduces uptake of N from fertilizer and increases the uptake of N from soil. Sci. Rep. 2020, 10, 18834. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.J.; Assefa, Y.; Haag, L.A.; Thompson, C.R.; Stone, L.R. Long-Term tillage on yield and water use of grain sorghum and winter wheat. Agron. J. 2018, 110, 269–280. [Google Scholar] [CrossRef]
- Govindasamy, P.; Mowrer, J.; Rajan, N.; Provin, T.; Hons, F.; Bagavathiannan, M. Influence of long-term (36 years) tillage practices on soil physical properties in a grain sorghum experiment in Southeast Texas. Arch. Agron. Soil Sci. 2020, 1, 11. [Google Scholar] [CrossRef]
| Soil Layers | |||
|---|---|---|---|
| Soil Chemical | 0.0–0.1 m | 0.1–0.2 m | 0.2–0.3 m |
| pH (H2O) | 6.5 ± 0.04 | 6.7 ± 0.04 | 6.7 ± 0.04 |
| Organic matter (g kg−1) | 6.0 ± 1.0 | 4.0 ± 0.67 | 4.0 ± 0.33 |
| P (mg dm−3) | 72.7 ± 6.99 | 57.4 ± 5.52 | 40.0 ± 3.85 |
| K (mg dm−3) | 143 ± 0.83 | 145.0 ± 0.84 | 148 ± 0.85 |
| Ca (cmolc dm−3) | 1.9 ± 0.06 | 1.7 ± 0.05 | 1.6 ± 0.05 |
| Mg (cmolc dm−3) | 0.8 ± 0.00 | 0.8 ± 0.00 | 0.8 ± 0.00 |
| Al (cmolc dm−3) | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
| B (mg dm−3) | 0.2 ± 0.00 | 0.2 ± 0.00 | 0.2 ± 0.00 |
| Zn (mg dm−3) | 7.9 ± 0.95 | 5.0 ± 0.60 | 4.0 ± 0.48 |
| Fe (mg dm−3) | 55.6 ± 1.11 | 61.5 ± 1.22 | 62.0 ± 1.23 |
| Mn (mg dm−3) | 24.0 ± 1.29 | 18.8 ± 1.01 | 18.0 ± 0.96 |
| Cu (mg dm−3) | 1.3 ± 0.06 | 1.0 ± 0.04 | 1.1 ± 0.05 |
| Soil physical | |||
| Total porosity (m3 m−3) | 0.49 ± 0.01 | 0.43 ± 0.01 | 0.44 ± 0.01 |
| Macroporosity (m3 m−3) | 0.23 ± 0.00 | 0.22 ± 0.00 | 0.24 ± 0.00 |
| Microporosity (m3 m−3) | 0.25 ± 0.01 | 0.21 ± 0.01 | 0.19 ± 0.01 |
| Bulk density (kg m−3) | 1.40 ± 0.01 | 1.40 ± 0.01 | 1.45 ± 0.01 |
| Systems of Production | Difference of Grain Yield (Mg ha−1) | |
|---|---|---|
| 0.4 m | 0.8 m | |
| Sorghum + AG | −1.76 (−57%) | +0.86 (+39%) |
| Sorghum + CCA | +0.23 (+7%) | −0.54 (−24%) |
| Sorghum + CC | −0.95 (−31%) | −0.58 (−26%) |
| Sorghum + BD | −1.94 (−63%) | −0.23 (−10%) |
| Sorghum + BB | −0.93 (−30%) | −0.22 (−10%) |
| Sorghum + BR | −0.89 (−29%) | +0.86 (+39%) |
| Sorghum + PM | −1.77 (−57%) | −1.85 (−84%) |
| Systems of Production | Grain Yield (Mg ha−1) | |||
| 0.4 m | 0.8 m | |||
| Crop-year 1 | ||||
| Sorghum | 3.07 ± 0.12 aA | 2.19 ± 0.10 bB | ||
| Sorghum + AG | 1.31 ± 0.05 cB | 3.05 ± 0.14 aA | ||
| Sorghum + CCA | 3.30 ± 0.13 aA | 1.65 ± 0.08 dB | ||
| Sorghum + CC | 2.12 ± 0.09 bA | 1.61 ± 0.07 dB | ||
| Sorghum + BD | 1.13 ± 0.05 dB | 1.96 ± 0.09 cA | ||
| Sorghum + BB | 2.14 ± 0.09 bA | 1.97 ± 0.09 cB | ||
| Sorghum + BR | 2.18 ± 0.09 bB | 3.05 ± 0.14 aA | ||
| Sorghum + PM | 1.30 ± 0.05 cA | 0.34 ± 0.02 eB | ||
| Average | 2.06 ± 0.08 * | 1.97 ± 0.09 * | ||
| Grass Dry Matter, DM (Mg ha−1) | ||||
| Crop-Year 1 | Crop-Year 2 | |||
| 0.4 m | 0.8 m | 0.4 m | 0.8 m | |
| Sorghum | - | - | - | - |
| Sorghum + AG | 2.00 ± 0.13 cA | 1.34 ± 0.09 cB | 0.40 ± 0.03 cA | 0.27 ± 0.02 cA |
| Sorghum + CCA | 0.80 ± 0.05 dA | 0.53 ± 0.04 dB | 0.16 ± 0.01 dA | 0.17 ± 0.01 cA |
| Sorghum + CC | 5.26 ± 0.35 aA | 3.51 ± 0.23 aB | 0.23 ± 0.02 cA | 0.15 ± 0.01 cA |
| Sorghum + BD | 1.14 ± 0.08 dA | 0.76 ± 0.05 dA | 0.76 ± 0.05 bA | 0.50 ± 0.03 bB |
| Sorghum + BB | 3.77 ± 0.25 bA | 2.51 ± 0.17 bB | 1.05 ± 0.07 aA | 0.70 ± 0.04 aB |
| Sorghum + BR | 3.98 ± 0.26 aA | 2.65 ± 0.18 bB | 0.80 ± 0.05 bA | 0.53 ± 0.03 bB |
| Sorghum + PM | 4.69 ± 0.31 aA | 3.12 ± 0.21 aB | 0.94 ± 0.06 aA | 0.63 ± 0.04 aB |
| Average | 3.09 ± 0.20 * | 2.06 ± 0.14 * | 0.62 ± 0.04 * | 0.42 ± 0.03 * |
| ANOVA | ||||
| Grain yield | DM (year 1) | DM (year 2) | ||
| psystem | ≤0.01 | ≤0.01 | ≤0.01 | |
| prow spacing | ≤0.01 | ≤0.01 | ≤0.01 | |
| pinteraction | ≤0.01 | ≤0.01 | ≤0.01 | |
| Systems of Production | 0.4 m | 0.8 m |
|---|---|---|
| Macroporosity (m3 m−3) | ||
| Sorghum | 0.11 ± 0.002 bA | 0.11 ± 0.002 bA |
| Sorghum + AG | 0.12 ± 0.003 bA | 0.08 ± 0.002 bB |
| Sorghum + CCA | 0.11 ± 0.002 bA | 0.12 ± 0.003 aA |
| Sorghum + BB | 0.11 ± 0.002 bA | 0.14 ± 0.003 aB |
| Sorghum + CC | 0.12 ± 0.003 bA | 0.14 ± 0.003 aA |
| Sorghum + BD | 0.16 ± 0.004 aA | 0.13 ± 0.003 aA |
| Sorghum + BR | 0.11 ± 0.002 bA | 0.11 ± 0.002 bA |
| Sorghum + PM | 0.16 ± 0.004 aA | 0.11 ± 0.002 bB |
| Microporosity (m3 m−3) | ||
| Sorghum | 0.26 ± 0.004 bA | 0.29 ± 0.003 aA |
| Sorghum + AG | 0.30 ± 0.004 bB | 0.34 ± 0.004 aA |
| Sorghum + CCA | 0.30 ± 0.004 bA | 0.25 ± 0.003 aA |
| Sorghum + BB | 0.36 ± 0.005 aA | 0.28 ± 0.003 aB |
| Sorghum + CC | 0.31 ± 0.005 bA | 0.29 ± 0.003 aA |
| Sorghum + BD | 0.27 ± 0.004 bA | 0.31 ± 0.003 aA |
| Sorghum + BR | 0.27 ± 0.004 bA | 0.29 ± 0.003 aA |
| Sorghum + PM | 0.26 ± 0.004 bB | 0.31 ± 0.003 aA |
| ANOVA | ||
| Macroporosity | Microporosity | |
| psystem | ≤0.01 | ≤0.01 |
| prow spacing | ≤0.01 | ≤0.01 |
| pinteraction | ≤0.01 | ≤0.01 |
| Variables | Average | |
|---|---|---|
| Cluster 1 | Cluster 2 | |
| Sorghum yield (Mg ha−1) | 2.33 ± 0.21 A | 1.84 ± 0.75 B |
| Dry matter (Mg ha−1) | 1.42 ± 0.83 B | 4.32 ± 0.37 A |
| Bulk density (g cm−3) | 1.60 ± 0.01 A | 1.58 ± 0.02 A |
| Soil porosity (m3 m−3) | 0.42 ± 0.02 A | 0.42 ± 0.02 A |
| Cluster information | ||
| Sum of squares | 1.16 | 2.11 |
| Number of members | 3 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz-Almeida, R.; Albuquerque, C.J.B.; Camargo, R.; Lemes, E.M.; Soares de Faria, R.; Quintão Lana, R.M. Sorghum–Grass Intercropping Systems under Varying Planting Densities in a Semi-Arid Region: Focusing on Soil Carbon and Grain Yield in the Conservation Systems. Agriculture 2022, 12, 1762. https://doi.org/10.3390/agriculture12111762
Ferraz-Almeida R, Albuquerque CJB, Camargo R, Lemes EM, Soares de Faria R, Quintão Lana RM. Sorghum–Grass Intercropping Systems under Varying Planting Densities in a Semi-Arid Region: Focusing on Soil Carbon and Grain Yield in the Conservation Systems. Agriculture. 2022; 12(11):1762. https://doi.org/10.3390/agriculture12111762
Chicago/Turabian StyleFerraz-Almeida, Risely, Carlos Juliano Brant Albuquerque, Reginaldo Camargo, Ernane Miranda Lemes, Renato Soares de Faria, and Regina Maria Quintão Lana. 2022. "Sorghum–Grass Intercropping Systems under Varying Planting Densities in a Semi-Arid Region: Focusing on Soil Carbon and Grain Yield in the Conservation Systems" Agriculture 12, no. 11: 1762. https://doi.org/10.3390/agriculture12111762
APA StyleFerraz-Almeida, R., Albuquerque, C. J. B., Camargo, R., Lemes, E. M., Soares de Faria, R., & Quintão Lana, R. M. (2022). Sorghum–Grass Intercropping Systems under Varying Planting Densities in a Semi-Arid Region: Focusing on Soil Carbon and Grain Yield in the Conservation Systems. Agriculture, 12(11), 1762. https://doi.org/10.3390/agriculture12111762






