Potential Global Distribution of Invasive Alien Species, Anthonomus grandis Boheman, under Current and Future Climate Using Optimal MaxEnt Model
Abstract
1. Introduction
2. Materials and Methods
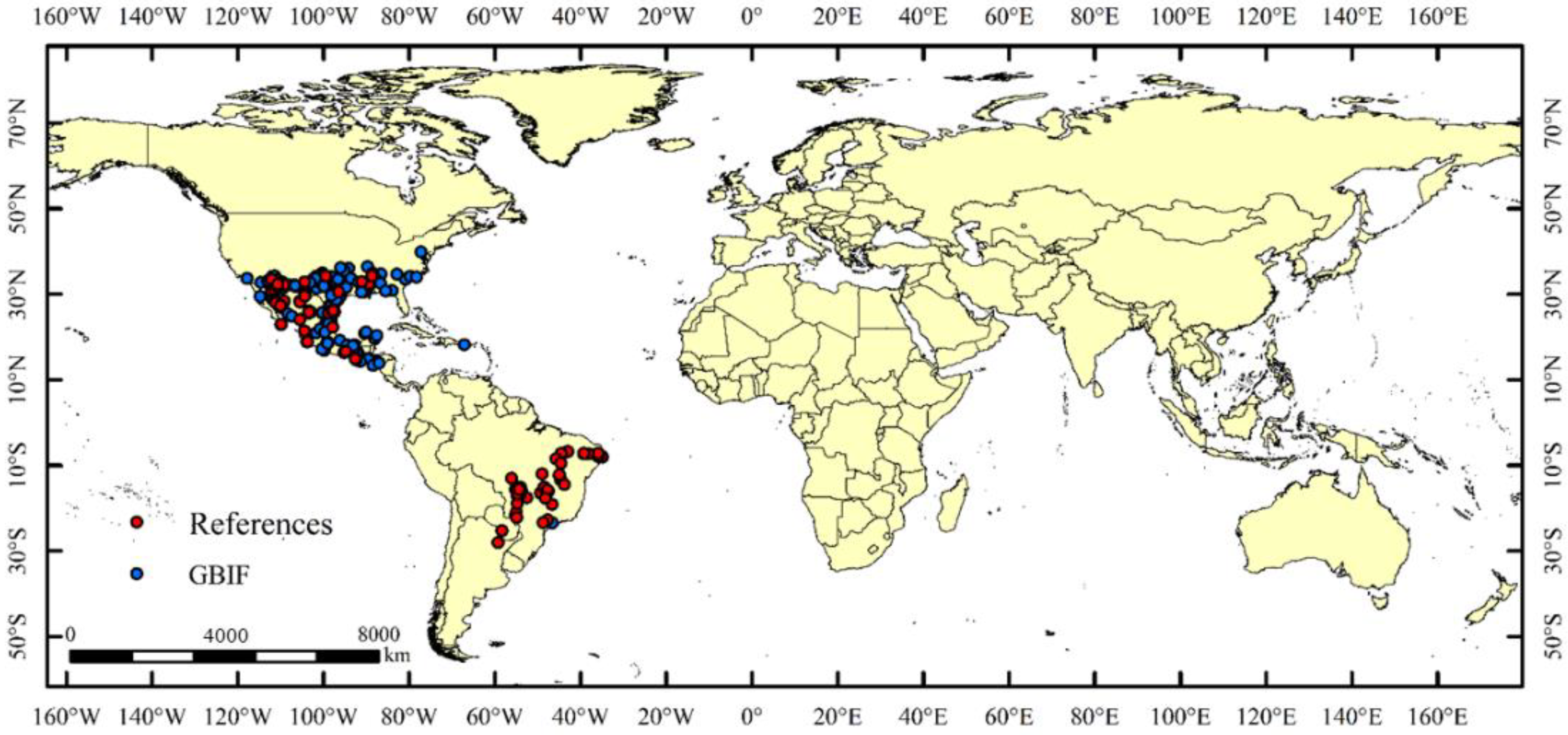
2.1. Global Distribution Records of A. grandis
2.2. Data for Prediction of Environmental Variables
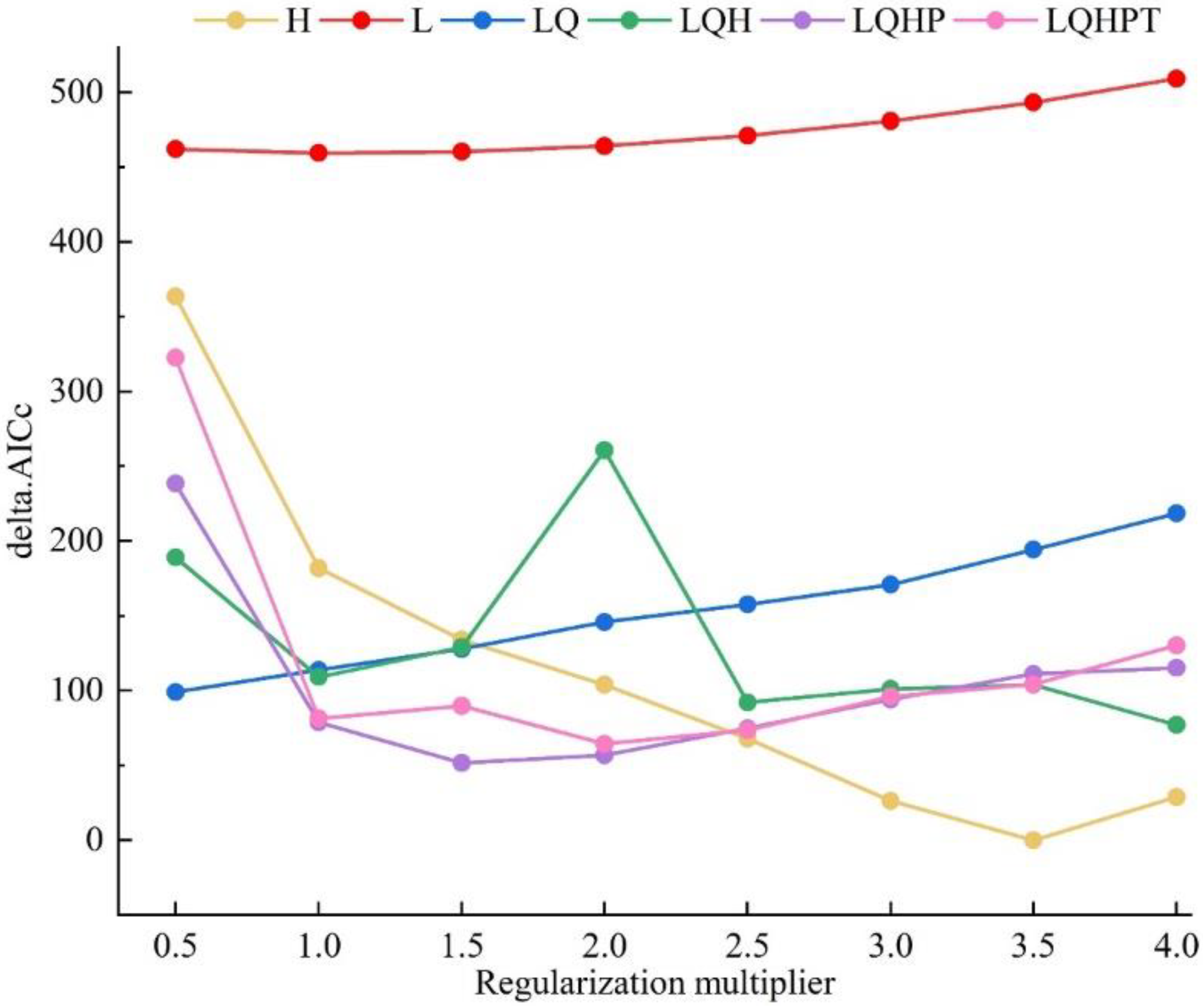
2.3. Model Parameters
2.4. Model Result Evaluation and GIS Analysis
2.5. Centroid Transfer of Suitable Habitat
3. Results
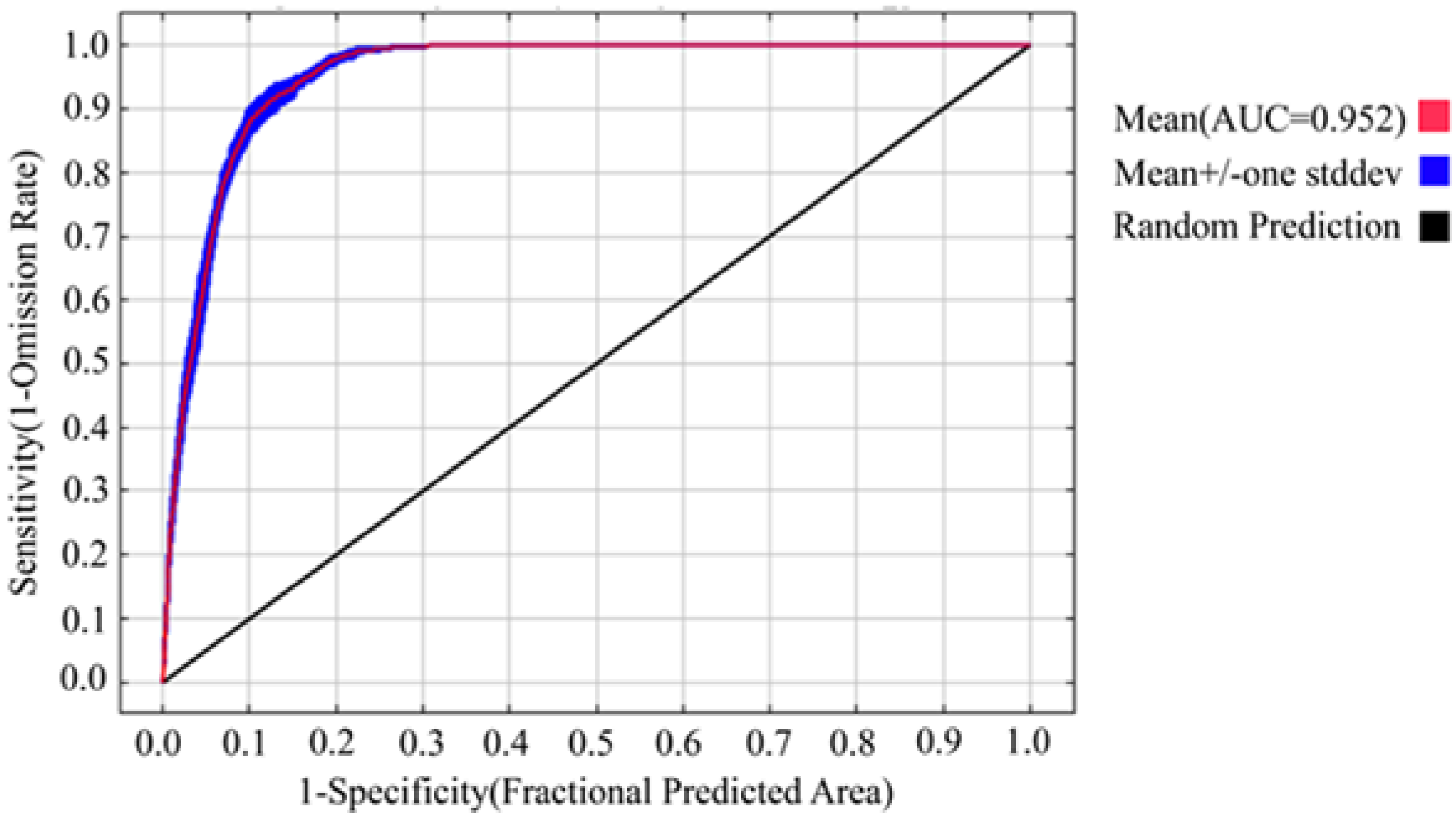
3.1. Model Precision and Importance of Bioclimatic Variable
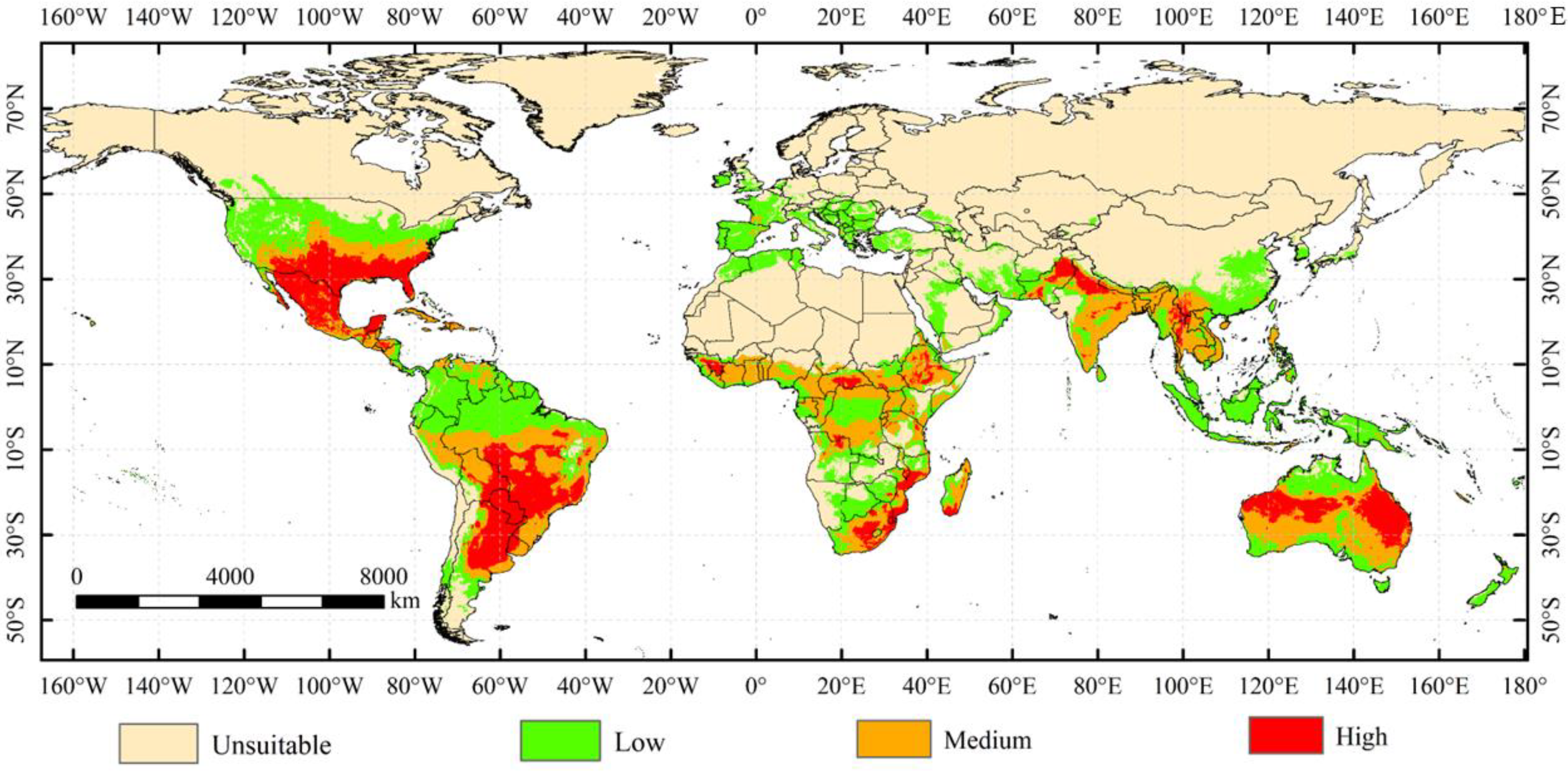
3.2. Potential Distribution of A. grandis under the Current Climate
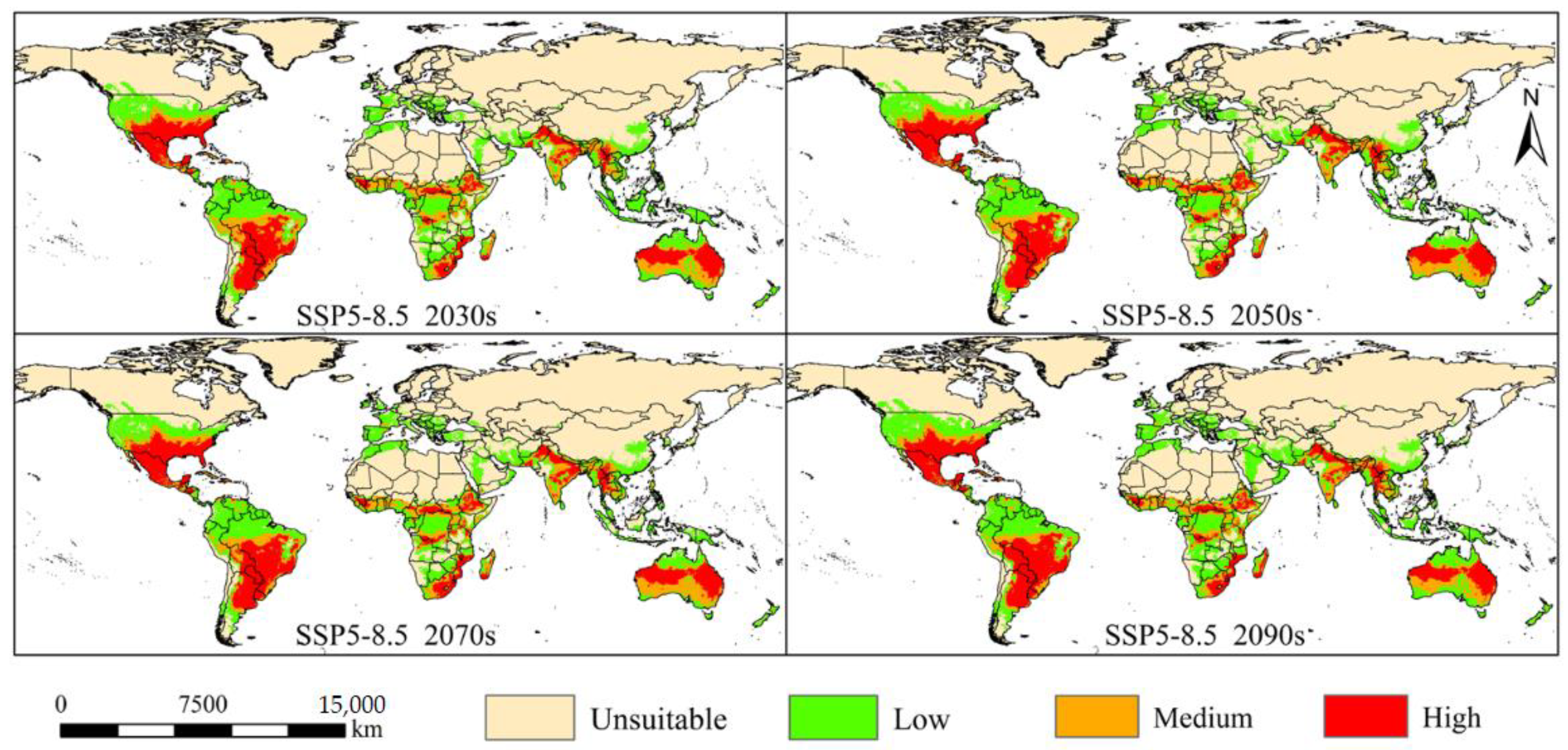
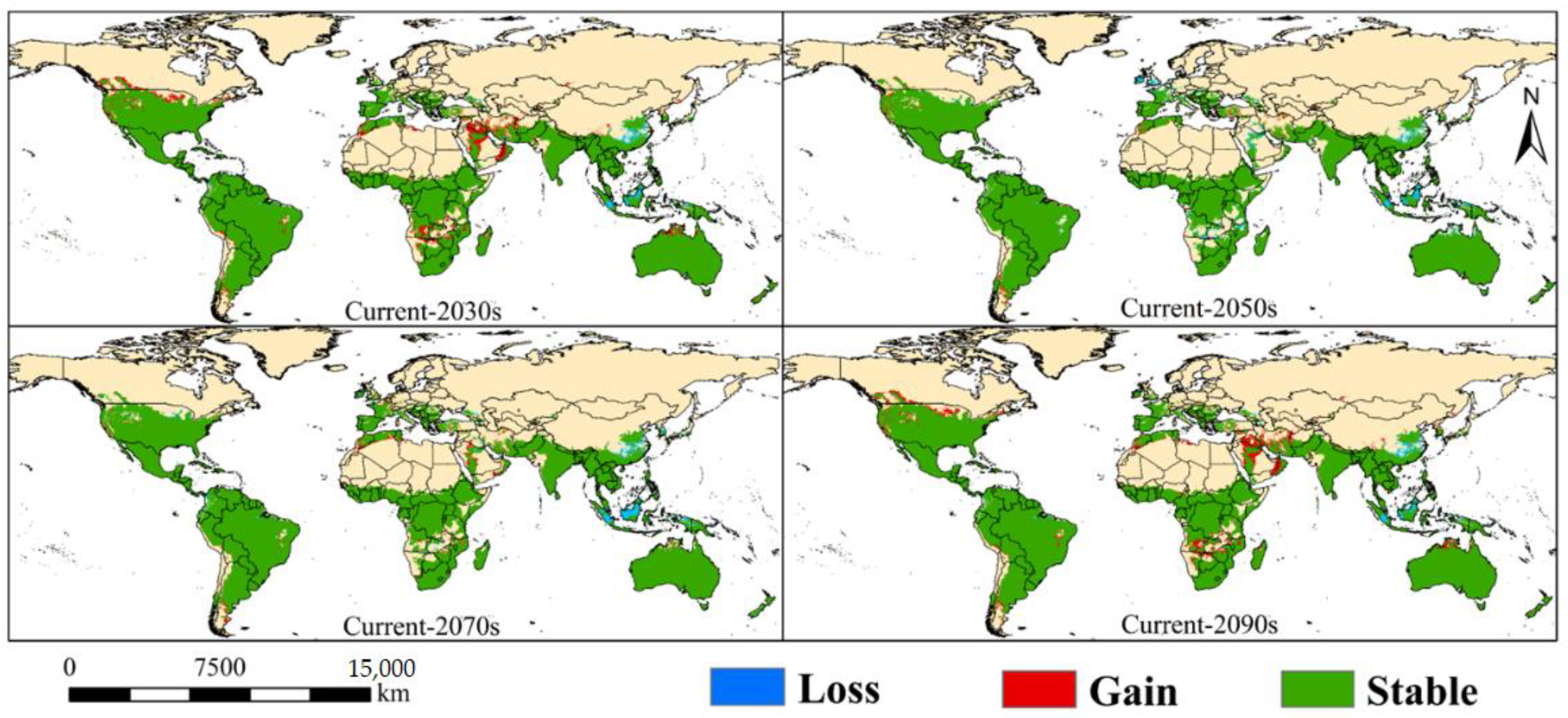
3.3. Changes in Potential Suitable Habitats of A. grandis under the Future Climate
3.3.1. North America
3.3.2. South America
3.3.3. Europe
3.3.4. Africa
3.3.5. Asia
3.3.6. Oceania
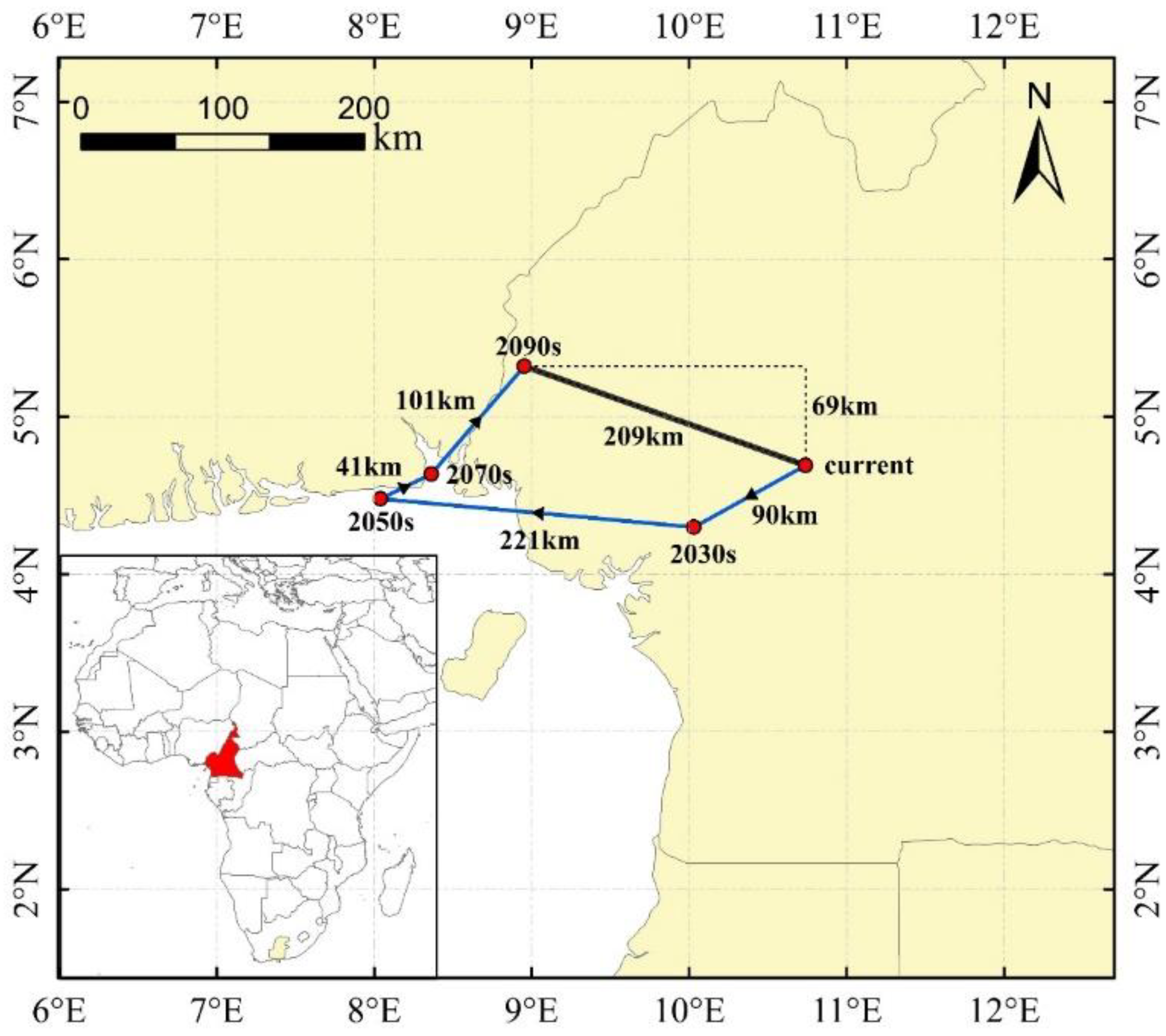
3.4. Centroid Shift of Potential Suitable Global Habitats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Katsanevakis, S.; Coll, M.; Piroddi, C.; Steenbeek, J.; Ben Rais Lasram, F.; Zenetos, A.; Cardoso, A.C. Invading the Mediterranean Sea: Biodiversity patterns shaped by human activities. Front. Mar. Sci. 2014, 1, 32. [Google Scholar] [CrossRef]
- Vergés, A.; Doropoulos, C.; Malcolm, H.A.; Skye, M.; Garcia-Pizá, M.; Marzinelli, E.M.; Campbell, A.H.; Ballesteros, E.; Hoey, A.S.; Vila-Concejo, A.; et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. USA 2016, 113, 13791–13796. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Gozlan, R.E.; Vaissière, A.C.; Assailly, C.; Nuninger, L.; Roiz, D.; Jourdain, F.; Jarić, I.; Courchamp, F. InvaCost, a public database of the economic costs of biological invasions worldwide. Sci. Data. 2020, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Al-Ayedh, H.; Rizwan-ul-Haq, M.; Hussain, A.; Aljabr, A.M. Insecticidal potency of RNAi-based catalase knock down in Rhynchophorus ferrugineus (Oliver) (Coleoptera: Curculionidae). Pest Manag. Sci. 2016, 72, 2118–2127. [Google Scholar] [CrossRef]
- Aghaee, M.A.; Godfrey, L.D. Winter flooding of California rice fields reduces immature populations of Lissorhoptrus oryzophilus (Coleoptera: Curculionidae) in the spring. Pest Manag. Sci. 2017, 73, 1538–1546. [Google Scholar] [CrossRef]
- Lange, F.; Olmstead, A.L.; Rhode, P.W. The impact of the boll weevil, 1892–1932. J. Econ. Hist. 2009, 69, 685–718. [Google Scholar] [CrossRef]
- Scataglini, M.A.; Lanteri, A.A.; Confalonieri, V.A. Diversity of boll weevil populations in South America: A phylogeographic approach. Genetica 2006, 126, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Jeger, M.; Bragard, C.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Gregoire, J.-C.; Jaques Miret, J.A.; Navarro, M.N.; et al. Scientific opinion on the pest categorisation of Anthonomus grandis. EFSA J. 2017, 15, e05074. [Google Scholar] [CrossRef]
- Showler, A.T.; Abrigo, V. Common subtropical and tropical nonpollen food sources of the boll weevil (Coleoptera: Curculionidae). Environ. Entomol. 2007, 36, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Grigolli, J.F.J.; Souza, L.A.; Fernandes, M.G.; Busoli, A.C. Spatial distribution of adult Anthonomus grandis Boheman (Coleoptera: Curculionidae) and damage to cotton flower buds due to feeding and oviposition. Neotrop. Entomol. 2017, 46, 442–451. [Google Scholar] [CrossRef]
- Paim, E.A.; Dias, A.M.; Showler, A.T.; Campos, K.L.; Oliveira, A.A.S.; Grillo, P.P.C.; Bastos, C.S. Cotton row spacing for boll weevil management in low-input production systems. Crop Prot. 2021, 145, 105614. [Google Scholar] [CrossRef]
- Loftin, U.C. Living with the boll weevil for fifty years. United States Dep. Agric. Publ. 1946, 3827, 273–291. [Google Scholar]
- Slosser, J.E.; Bordovsky, D.J.; Bevers, S.J. Damage and Costs Associated with Insect Management Options in Irrigated Cotton. J. Econ. Entomol. 1994, 87, 436–445. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Auad, A.M.; Mendes, S.M.; Frizzas, M.R. Economic impact of exotic insect pests in Brazilian agriculture. J. Appl. Entomol. 2013, 137, 1–15. [Google Scholar] [CrossRef]
- Spurgeon, D.W.; Suh, C.P. Morphology, Diet, and Temperature-dependent Host-Free Survival of the Boll Weevil, Anthonomus grandis (Coleoptera: Curculionidae). J. Insect. Sci. 2018, 18, 8. [Google Scholar] [CrossRef]
- Paula, D.P.; Claudino, D.; Timbó, R.V.; Miranda, J.E.; Bemquerer, M.P.; Ribeiro, A.C.; Sujii, E.R.; Fontes, E.M.; Pires, C.S. Reproductive dormancy in boll-weevil from populations of the midwest of Brazil. J. Econ. Entomol. 2013, 106, 86–96. [Google Scholar] [CrossRef]
- Ramasamy, M.; Das, B.; Ramesh, R. Predicting climate change impacts on potential worldwide distribution of fall armyworm based on CMIP6 projections. J. Pest Sci. 2022, 95, 841–854. [Google Scholar] [CrossRef]
- Curtis, A.D.; Joshua, J.T.; Raymond, B.H.; Kimberly, S.S.; Cameron, K.G.; David, C.H.; Paul, R.M. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef]
- Ge, X.Z.; He, S.Y.; Zhu, C.Y.; Wang, T.; Xu, Z.C.; Zong, S.X. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest Manag. Sci. 2018, 75. [Google Scholar] [CrossRef] [PubMed]
- Sutherst, R.W. Pest species distribution modelling: Origins and lessons from history. Biol. Invas. 2014, 16, 239–256. [Google Scholar] [CrossRef]
- Raffini, F.; Bertorelle, G.; Biello, R.; D’Urso, G.; Russo, D.; Bosso, L. From nucleotides to satellite imagery: Approaches to identify and manage the invasive pathogen Xylella fastidiosa and its insect vectors in Europe. Sustainability 2020, 12, 4508. [Google Scholar] [CrossRef]
- Carpenter, G.; Gillison, A.N.; Winter, J. Domain: A flexible modeling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Chejara, V.K.; Kriticos, D.J.; Kristiansen, P.; Sindel, B.M.; Whalley, R.D.B.; Nadolny, C. The current and future potential geographical distribution of Hyparrhenia hirta. Weed Res. 2010, 50, 174–184. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.H.; Graham, C.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; J. Hijmans, R.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Elith, J. Species Distribution Modeling with R. Encycl. Biodivers. 2013, 6. [Google Scholar] [CrossRef]
- Guzmán, N.V.; Lanteri, A.A.; Confalonieri, V.A. Colonization ability of two invasive weevils with different reproductive modes. Evol. Ecol. 2012, 26, 1371–1390. [Google Scholar] [CrossRef]
- Ramos, R.S.; Kumar, L.; Shabani, F.; Picanço, M.C. Mapping global risk levels of Bemisia tabaci in areas of suitability for open field tomato cultivation under current and future climates. PLoS ONE 2018, 13, e0198925. [Google Scholar] [CrossRef] [PubMed]
- Santana Jr, P.A.; Kumar, L.; Da Silva, R.S.; Pereira, J.L.; Picanco, M.C. Assessing the impact of climate change on the worldwide distribution of Dalbulus maidis (DeLong) using MaxEnt. Pest Manag. Sci. 2019, 75, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; McPherson, J. An R package for conducting spatially independent evaluations and estimating optimal model complexity for MaxEnt ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Riahi, K.; van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of MaxEnt. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Warren, D.L.; Wright, A.N.; Seifert, S.N.; Shaffer, H.B. Incorporating model complexity and spatial sampling bias into ecological niche models of climate change risks faced by 90 California vertebrate species of concern. Divers. Distrib. 2014, 20, 334–343. [Google Scholar] [CrossRef]
- Altamiranda-Saavedra, M.; Amat, E.; Gómez-P, L.M. Influence of montane altitudinal ranges on species distribution models; evidence in Andean blow flies. PeerJ 2020, 8, e10370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Watson, G.W.; Zhang, R. The potential distribution of an invasive mealybug Phenacoccus Solenopsis and its threat to cotton in Asia. Agric. For. Entomol. 2010, 12, 403–416. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lin, C.; Wang, Y.; Zhang, Y.; Ji, L. Ecological niche complexity of invasive and native cryptic species of the Bemisia tabaci species complex in China. J. Pest Sci. 2022, 95, 1245–1259. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking receiver operating characteristic analysis application in ecological niche modeling. Ecol. Modell. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. An evaluation of alternative algorithms for fitting species distribution models using logistic regression. Ecol. Modell. 2000, 128, 127–147. [Google Scholar] [CrossRef]
- Bean, W.T.; Stafford, R.; Brashares, J.S. The effects of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography 2012, 35, 250–258. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in MaxEnt: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Liu, Y.L.; Qin, H.; Meng, Q.X. Prediction on spatial migration of suitable distribution of Elaeagnus mollis under climate change conditions in Shanxi Province, China. Ying Yong Sheng Tai Xue Bao 2019, 30, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Reyes, U.J.; Jones, R.W.; Raszick, T.J.; Ruiz-Arce, R.; Sword, G.A. Potential distribution of wild Host Plants of the Boll Weevil (Anthonomus grandis) in the United States and Mexico. Insects 2022, 13, 337. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Sétamou, M.; Sappington, T.W.; Liu, T.-X.; Coleman, R.J.; Armstrong, J.S. Temperature-dependent development and reproduction of the boll weevil (Coleoptera: Curculionidae). Insect Sci. 2005, 12, 449–459. [Google Scholar] [CrossRef]
- De Lima, I.S.; Degrande, P.E.; Miranda, J.E.; dos Santos, W.J. Evaluation of the boll weevil Anthonomus grandis Boheman (Coleoptera: Curculionidae) suppression program in the State of Goiás, Brazil. Neotrop. Entomol. 2013, 42, 82–88. [Google Scholar] [CrossRef]
- Ben-David, A. About the relationship between ROC curves and Cohen’s kappa. Eng. Appl. Artif. Intell. 2008, 21, 874–882. [Google Scholar] [CrossRef]
- Spurgeon, D.W.; Suh, C.P.-C. Temperature influences on diapause induction and survival in the boll weevil (Coleoptera: Curculionidae). J. Insect Sci. 2017, 17, 124. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Sparks, A.N., Jr.; Norman, J.W., Jr.; Coleman, R.; Bradford, J.M.; Yang, C.; Sappington, T.W.; Showler, A. Chemical cotton stalk destruction for maintenance of host-free periods for the control of overwintering boll weevil in tropical and subtropical climates. Pest Manag. Sci. 2007, 63, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, B.; Neumann, P.; Schweiger, O. Global warming promotes biological invasion of a honey bee pest. Glob. Chang. Biol. 2019, 25, 3571–3994. [Google Scholar] [CrossRef]
- Daniel, G.-T.; Alex, C.-A.; Wesley, D.; Andrés, L.-N.; Sánchez-Guillén Rosa, A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. Camb. Philos. Soc. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Gao, X.Y.; Zhao, Q.; Wei, J.F.; Zhang, H.F. Study on the Potential Distribution of Leptinotarsa decemlineata and Its Natural Enemy Picromerus bidens Under Climate Change. Front. Ecol. Evol. 2022, 9, 786436. [Google Scholar] [CrossRef]
- Turbelin, A.J.; Diagne, C.; Hudgins, E.J.; Moodley, D.; Kourantidou, M.; Novoa, A.; Haubrock, P.J.; Bernery, C.; Gozlan, R.E.; Francis, R.A.; et al. Introduction pathways of economically costly invasive alien species. Biol. Invasions. 2022, 24, 2061–2079. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.; Richardson, D.M. A proposed unifed framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef]
- Lehan, N.E.; Murphy, J.R.; Thorburn, L.P.; Bradley, B.A. Accidental introductions are an important source of invasive plants in the continental United States. Am. J. Bot. 2013, 100, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Essl, F.; Bacher, S.; Blackburn, T.M.; Booy, O.; Brundu, G.; Brunel, S.; Cardoso, A.C.; Eschen, R.; Gallardo, B.; Galil, B.; et al. Crossing frontiers in tackling pathways of biological invasions. Bioscience 2015, 65, 769–782. [Google Scholar] [CrossRef]
- Raszick, T.J. Boll Weevil Eradicationin: A Success Story of Science in the Service of Policy and Industry. Ann. Entomol. Soc. Am. 2021, 114, 702–708. [Google Scholar] [CrossRef]
- McCullough, D.G.; Work, T.T.; Cavey, J.F.; Liebhold, A.M.; Marshall, D. Interceptions of nonindigenous plant pests at US ports of entry and border crossings over a 17-year period. Biol. Invasions. 2006, 8, 611–630. [Google Scholar] [CrossRef]
- Caton, B.P.; Dobbs, T.T.; Brodel, C.F. Arrivals of hitchhiking insect pests on international cargo aircraft at Miami International Airport. Biol. Invasions. 2006, 8, 765–785. [Google Scholar] [CrossRef]
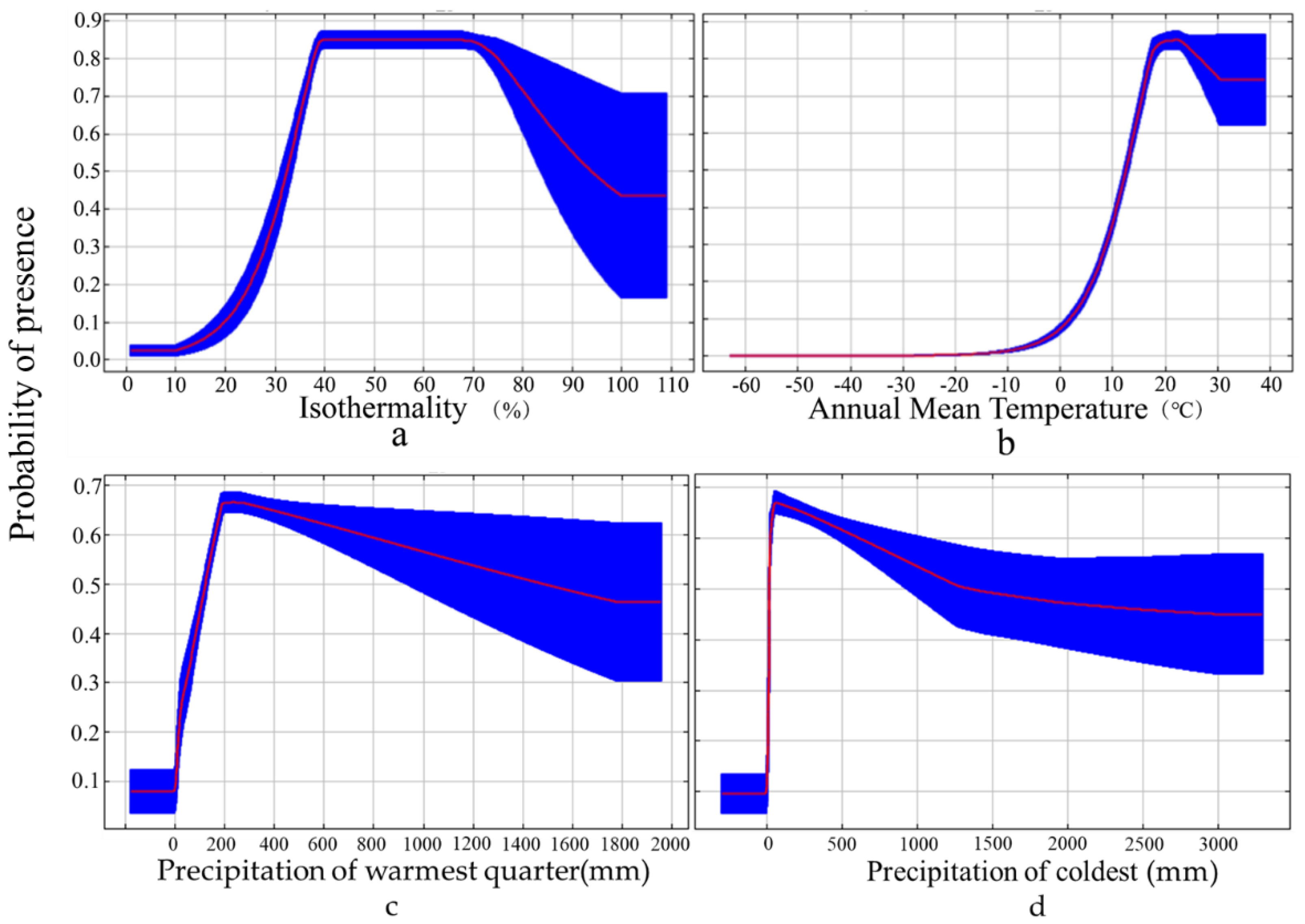

| Variable | Description | Contribution (%) |
|---|---|---|
| Bio1 | Annual Mean Temperature (°C) | 7.2 |
| Bio2 | Mean Diurnal Range (°C) | 10.5 |
| Bio3 | Isothermality (%) | 51.4 |
| Bio7 | Temperature Annual Range (°C) | 1.1 |
| Bio15 | Precipitation Seasonality (mm) | 0.2 |
| Bio17 | Precipitation of Driest Quarter (mm) | 1.1 |
| Bio18 | Precipitation of Warmest Quarter (mm) | 17.5 |
| Bio19 | Precipitation of Coldest Quarter (mm) | 11.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Yu, W.; Zhao, H.; Xian, X.; Jing, K.; Yang, N.; Lu, X.; Liu, W. Potential Global Distribution of Invasive Alien Species, Anthonomus grandis Boheman, under Current and Future Climate Using Optimal MaxEnt Model. Agriculture 2022, 12, 1759. https://doi.org/10.3390/agriculture12111759
Jin Z, Yu W, Zhao H, Xian X, Jing K, Yang N, Lu X, Liu W. Potential Global Distribution of Invasive Alien Species, Anthonomus grandis Boheman, under Current and Future Climate Using Optimal MaxEnt Model. Agriculture. 2022; 12(11):1759. https://doi.org/10.3390/agriculture12111759
Chicago/Turabian StyleJin, Zhenan, Wentao Yu, Haoxiang Zhao, Xiaoqing Xian, Kaiting Jing, Nianwan Yang, Xinmin Lu, and Wanxue Liu. 2022. "Potential Global Distribution of Invasive Alien Species, Anthonomus grandis Boheman, under Current and Future Climate Using Optimal MaxEnt Model" Agriculture 12, no. 11: 1759. https://doi.org/10.3390/agriculture12111759
APA StyleJin, Z., Yu, W., Zhao, H., Xian, X., Jing, K., Yang, N., Lu, X., & Liu, W. (2022). Potential Global Distribution of Invasive Alien Species, Anthonomus grandis Boheman, under Current and Future Climate Using Optimal MaxEnt Model. Agriculture, 12(11), 1759. https://doi.org/10.3390/agriculture12111759







