A Field Screening of a Pomegranate (Punica granatum) Ex-Situ Germplasm Collection for Resistance against the False Spider Mite (Tenuipalpus punicae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site, Plant Material and Management
2.2. Field Screening of Mite Infestation on Pomegranate Accessions
2.3. Plant Vegetative Traits
2.4. Phytochemical Traits of the Leaves
2.5. Statistical Analysis
3. Results
3.1. Screening of Pomegranate Accessions
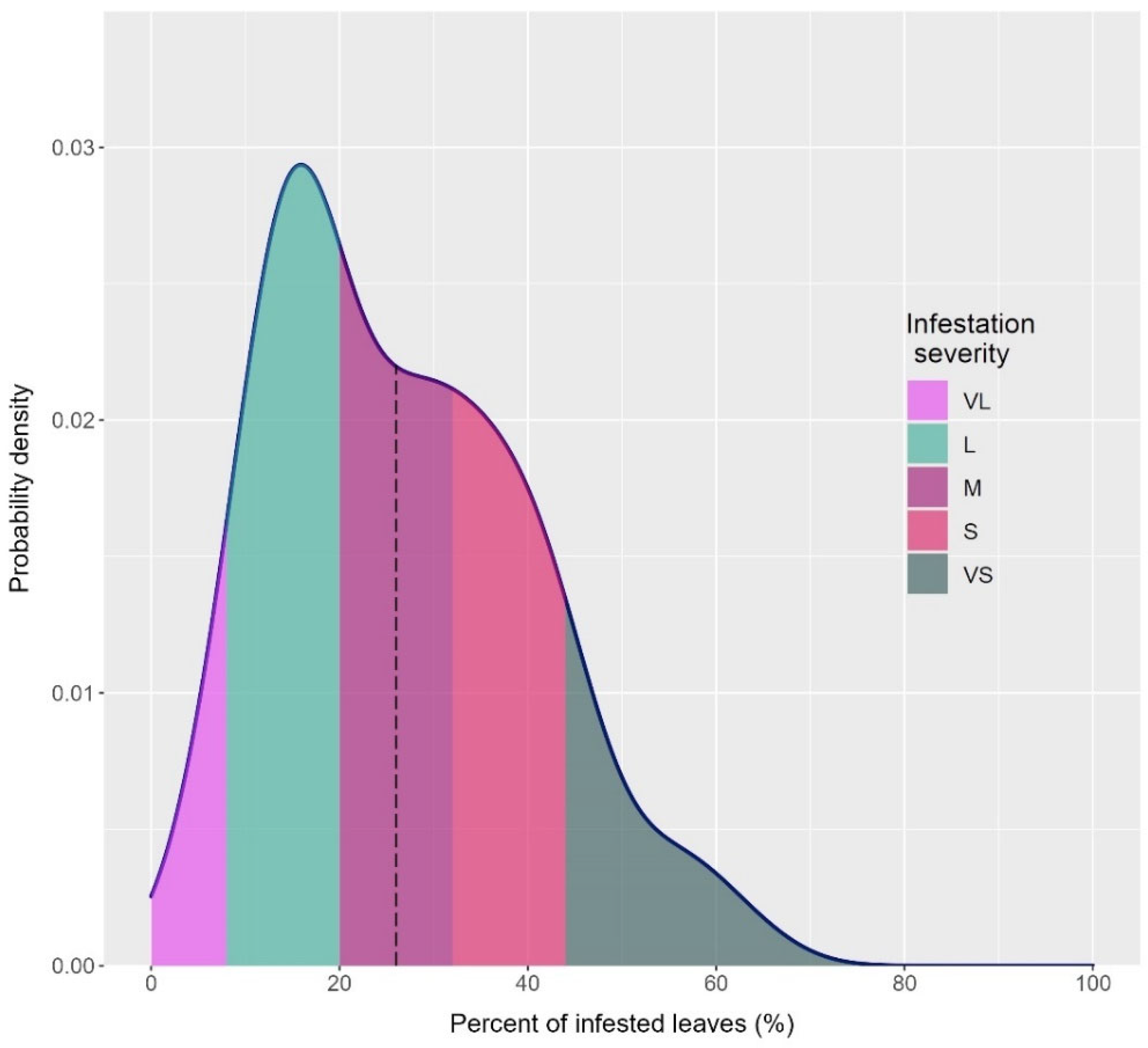
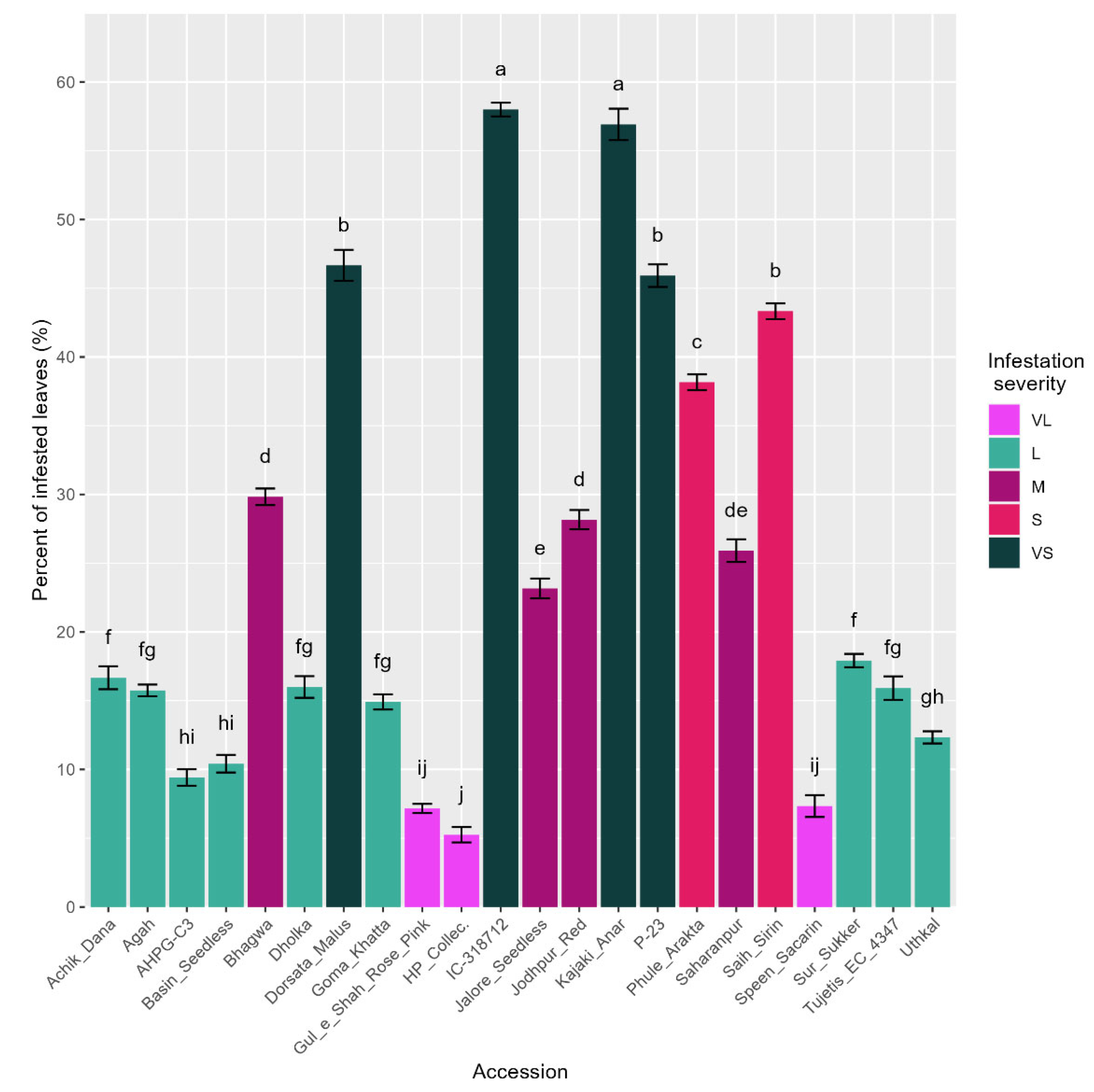
3.2. Mite Infestation Severity in the Selected 22 Accessions
3.3. Vegetative Traits of the 22 Selected Accessions
3.4. Leaf Phytochemical Composition of the 22 Selected Accessions
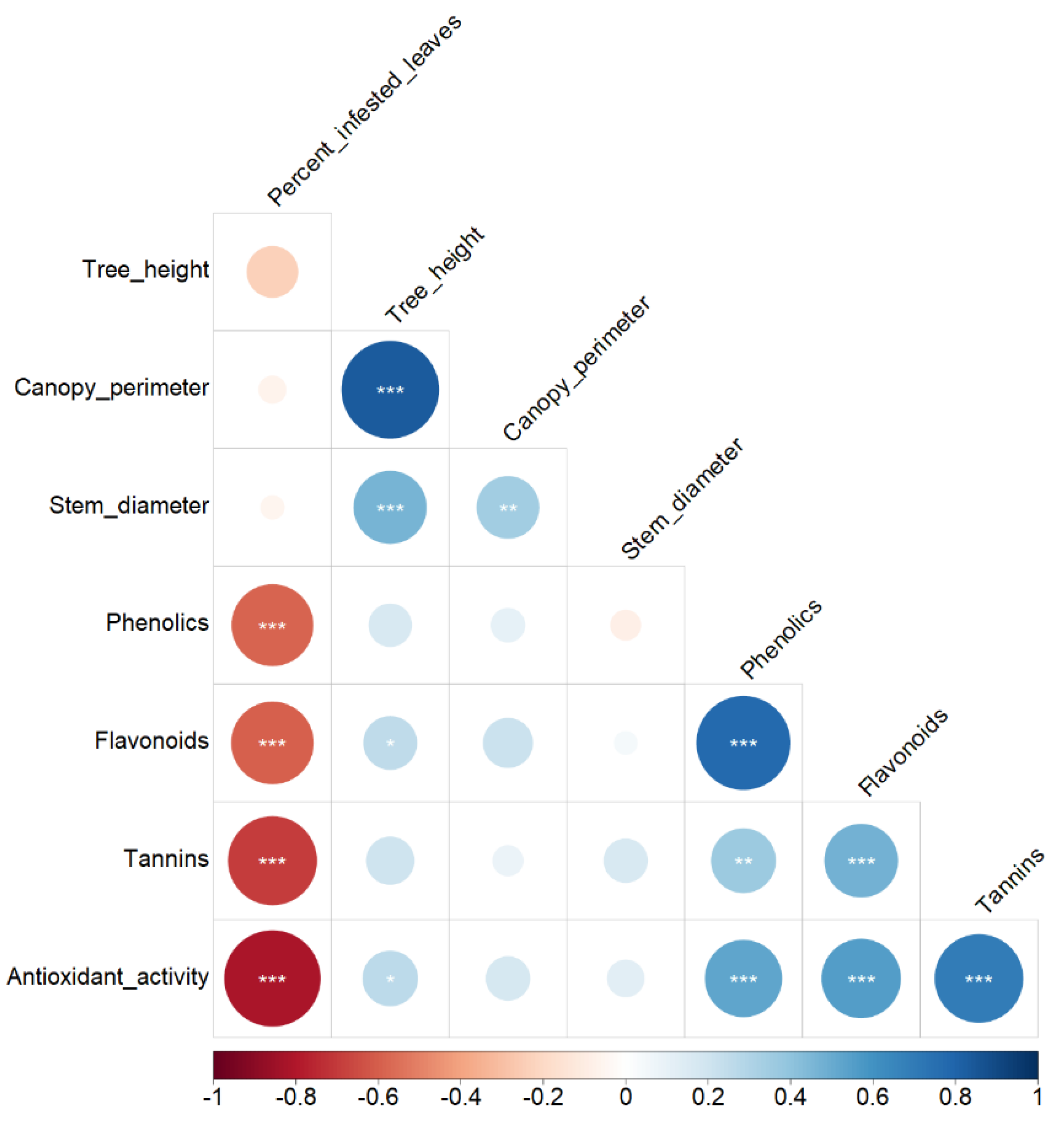
3.5. Correlation Analysis
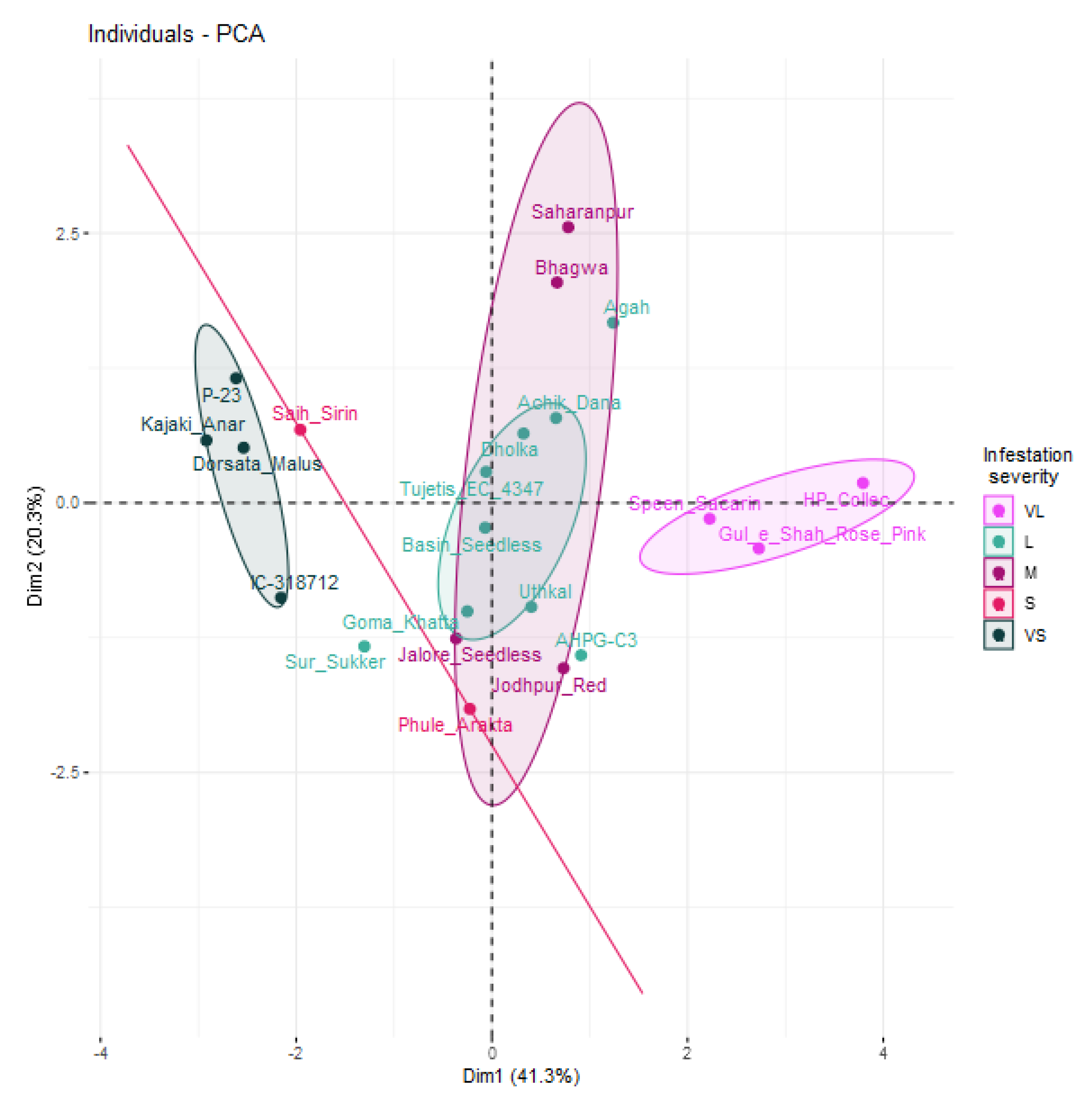
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blumenfeld, A.; Shaya, F.; Hillel, R. Cultivation of pomegranate. Options Méditerranéennes Ser. A 2000, 42, 143–147. [Google Scholar]
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, horticulture, breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar]
- Heber, D.; Schulman, R.N.; Seeram, N.P. Pomegranates: Ancient Roots to Modern Medicine; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Stover, E.; Mercure, E.W. The pomegranate: A new look at the fruit of paradise. HortScience 2007, 42, 1088–1092. [Google Scholar] [CrossRef]
- Rymon, D. Mapping features of the global pomegranate market. Acta Hortic 2011, 890, 599–602. [Google Scholar] [CrossRef]
- Mars, M. Pomegranate plant material: Genetic resources and breeding, a review. Options Mediterraneennes Ser. A 2000, 42, 55–62. [Google Scholar]
- Verma, N.; Mohanty, A.; Lal, A. Pomegranate genetic resources and germplasm conservation: A review. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 120–125. [Google Scholar]
- Zamani, Z.; Sarkhosh, A.; Fatahi, R.; Ebadi, A. Genetic relationships among pomegranate genotypes studied by fruit characteristics and RAPD markers. J. Hortic. Sci. Biotechnol. 2007, 82, 11–18. [Google Scholar] [CrossRef]
- Rana, J.C.; Pradheep, K.; Verma, V. Naturally occurring wild relatives of temperate fruits in Western Himalayan region of India: An analysis. Biodivers. Conserv. 2007, 16, 3963–3991. [Google Scholar] [CrossRef]
- Rawat, J.; Tomar, Y.; Rawat, S. Characterization of wild pomegranate (Punica protopunica L.) of Garhwal Himalaya. Progress. Hortic. 2012, 44, 52–54. [Google Scholar]
- Gunnaiah, R.; Jagadeesha, R.C.; Cholin, S.; Prabhuling, G.; Govindaswamy Babu, A.; Fakrudin, B.; Pujer, P.; Murthy, S.B. Genetic diversity assessment and population structure analysis of pomegranate cultivars from different countries and Himalayan wild accessions. J. Hortic. Sci. Biotechnol. 2021, 96, 614–623. [Google Scholar] [CrossRef]
- Cocuzza, G.E.M.; Mazzeo, G.; Russo, A.; Giudice, V.L.; Bella, S. Pomegranate arthropod pests and their management in the Mediterranean area. Phytoparasitica 2016, 44, 393–409. [Google Scholar] [CrossRef]
- Al-Gboory, I.; El-Haidari, H. Studies on some factors affecting the pomegranate false spider mite, Tenuipalpus punicae (Acari: Tenuipalpidae) in Iraq. Prog. Acarol. 1989, 2, 67–72. [Google Scholar]
- Abo-Elmaged, T.M.; Ali, A.-W.M.; El-Wahed, A.; Nazeh, M.; Eraky, S.A.; Abdelgayed, A.S. Population Fluctuations of Mites on Two Pomegranate (Punica granatum) Varieties in Three Suburbs of Assiut Governorate, Egypt. Egypt. Acad. J. Biol. Sci. B. Zool. 2021, 13, 55–63. [Google Scholar] [CrossRef]
- Dhooria, M.S. Fundamentals of Applied Acarology; Springer: London, UK, 2016. [Google Scholar]
- Jeppson, L.R.; Keifer, H.H.; Baker, E.W. Mites Injurious to Economic Plants; University of California Press: Berkeley, CA, USA, 1975. [Google Scholar]
- Dhooria, M.; Sandhu, G. Occurrence of Tenuipalpus punicae Pritchard and Baker (Tenuipalpidae: Acarina) on pomegranate in India. Curr. Sci. 1973, 42, 179–180. [Google Scholar]
- Juan, P.; Martinez, J.; Martinez, J.; Oltra, M.; Ferrandez, M. Current situation of pomegranate growing (Punica granatum L.) in southern Alicante. Chemical control of pests and diseases and financial cost. Options Méditerranéennes. Série A Séminaires Méditerranéens 2000, 42, 157–161. [Google Scholar]
- Wu, S.; Tian, L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef]
- Mincuzzi, A.; Ippolito, A.; Brighenti, V.; Marchetti, L.; Benvenuti, S.; Ligorio, A.; Pellati, F.; Sanzani, S.M. The effect of polyphenols on pomegranate fruit susceptibility to Pilidiella granati provides insights into disease tolerance mechanisms. Molecules 2020, 25, 515. [Google Scholar] [CrossRef]
- Shashi; Garhwal, O.P.; Choudhary, M.R.; Bairwa, L.N.; Kumawat, K.L.; Kumar, P.; Basile, B.; Corrado, G.; Rouphael, Y.; Gora, J.S. Effects of Time of Pruning and Plant Bio-Regulators on the Growth, Yield, Fruit Quality, and Post-Harvest Losses of Ber (Ziziphus mauritiana). Horticulturae 2022, 8, 809. [Google Scholar] [CrossRef]
- Jayalakshmi, K.; Nargund, V.; Raju, J. Screening of pomegranate genotypes for anthracnose disease resistance. J. Mycopathol. Res. 2013, 51, 357–358. [Google Scholar]
- Kumari, N.; Ram, V. Evaluation of pomegranate germplasm for resistance against leaf spot and dry fruit rot (Coniella granati). Int. J. Farm Sci. 2015, 5, 97–104. [Google Scholar]
- Priya, B.T.; Murthy, B.; Gopalakrishnan, C.; Artal, R.B.; Jagannath, S. Identification of new resistant sources for bacterial blight in pomegranate. Eur. J. Plant Pathol. 2016, 146, 609–624. [Google Scholar] [CrossRef]
- Sobhani, M.; Goldansaz, S.H.; Hatami, B.; Hosseini, S.A. A field screening of pomegranate cultivars for resistance to the carob moth, Ectomyelois ceratoniae, and compatibility with its larval parasitoids. Int. J. Pest Manag. 2015, 61, 346–352. [Google Scholar] [CrossRef]
- Berwal, M.; Haldhar, S.; Ram, C.; Saroj, P. Determination of total phenolic & flavonoids and antioxidant activity in Calligonum polygonoides L. from Thar Desert. J. Environ. Biol. 2021, 42, 1347–1354. [Google Scholar]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Rebaya, A.; Belghith, S.I.; Baghdikian, B.; Leddet, V.M.; Mabrouki, F.; Olivier, E.; kalthoum Cherif, J.; Ayadi, M.T. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae). J. Appl. Pharm. Sci. 2015, 5, 052–057. [Google Scholar]
- Maxwell, F.G.; Jennings, P.R. Breeding Plants Resistant to Insects; Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference–performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef]
- Abedi, Z.; Golizadeh, A.; Soufbaf, M.; Hassanpour, M.; Jafari-Nodoushan, A.; Akhavan, H.-R. Relationship between performance of carob moth, Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae) and phytochemical metabolites in various pomegranate cultivars. Front. Physiol. 2019, 10, 1425. [Google Scholar] [CrossRef]
- Carmona, D.; Lajeunesse, M.J.; Johnson, M.T. Plant traits that predict resistance to herbivores. Funct. Ecol. 2011, 25, 358–367. [Google Scholar] [CrossRef]
- Schopf, R. The effect of secondary needle compounds on the development of phytophagous insects. For. Ecol. Manag. 1986, 15, 55–64. [Google Scholar] [CrossRef]
- Duffey, S.S.; Stout, M.J. Antinutritive and toxic components of plant defense against insects. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 1996, 32, 3–37. [Google Scholar] [CrossRef]
- Ferrara, G.; Cavoski, I.; Pacifico, A.; Tedone, L.; Mondelli, D. Morpho-pomological and chemical characterization of pomegranate (Punica granatum L.) genotypes in Apulia region, Southeastern Italy. Sci. Hortic. 2011, 130, 599–606. [Google Scholar] [CrossRef]
- Amri, Z.; Zaouay, F.; Lazreg-Aref, H.; Soltana, H.; Mneri, A.; Mars, M.; Hammami, M. Phytochemical content, fatty acids composition and antioxidant potential of different pomegranate parts: Comparison between edible and non edible varieties grown in Tunisia. Int. J. Biol. Macromol. 2017, 104, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, A.; El Kaibi, M.A.; Abbassi, A.; Horchani, A.; Chekir-Ghedira, L.; Ghedira, K. Phytochemical study and antibacterial and antibiotic modulation activity of Punica granatum (pomegranate) leaves. Scientifica (Cairo) 2020, 8271203. [Google Scholar] [CrossRef]
- Shevale, B.; Kaulgud, S. Population dynamics of pests of pomegranate Punica granatum Linnaeus. In Proceedings of the Advances in IPM for horticultural crops. Proceedings of the First National Symposium on Pest Management in Horticultural Crops: Environmental implications and thrusts, Bangalore, India, 15–17 October 1997; Association for Advancement of Pest Management in Horticultural Ecosystems, Indian Institute of Horticultural Research: Bengaluru, India, 1998; pp. 47–51. [Google Scholar]
- Carroll, D.; Puget, B.; Higbee, B.; Quist, M.; Magallene, O.; Smith, N.; Gjerde, A.; Schneider, K. Pomegranate pest management in the San Joaquin Valley. Assoc. Appl. IPM Ecol. 2006, 1, 21. [Google Scholar]
- Balikai, R.; Kotikal, Y.; Prasanna, P. Status of pomegranate pests and their management strategies in India. Acta Hortic. 2011, 890, 569–583. [Google Scholar] [CrossRef]
- Weston, P.; Johnson, D.A.; Burton, H.; Snyder, J.C. Trichome secretion composition, trichome densities, and spider mite resistance of ten accessions of Lycopersicon hirsutum. J. Am. Soc. Hortic. Sci. 1989, 114, 492–498. [Google Scholar] [CrossRef]
| Accession | Infestation (%) | ISC | Accession | Infestation (%) | ISC |
|---|---|---|---|---|---|
| A K Anar | 22.5 ± 1.4 opqrstuv | M | Jalore Red | 32.5 ± 1.4 hijklm | S |
| Achik Dana | 17.5 ± 1.4 tuvwxyz | L | Jalore Seedless | 23.3 ± 0.8 nopqrstu | M |
| Agah | 16.7 ± 0.8 uvwxyzA | L | Jodhpur coll. | 37.5 ± 1.4 efghi | S |
| AH-PG-H3 | 32.5 ± 1.4 wxyzABCD | L | Jodhpur Red | 27.5 ± 1.4 klmnopqr | M |
| AHPG-C1 | 14.2 ± 0.8 BCDE | L | Jyoti | 25.8 ± 0.8 lmnopqrs | M |
| AHPG-C3 | 8.3 ± 0.8 wxyzABCD | L | Kabul | 42.5 ± 1.4 def | S |
| AHPG-C4 | 14.2 ± 0.8 mnopqrst | M | Kabul Kohinoor | 14.2 ± 0.8 wxyzABCD | L |
| AHPG-H1 | 25.0 ± 1.4 ghijkl | S | Kajaki Anar | 55.0 ± 1.4 bc | VS |
| AHPG-H2 | 33.3 ± 0.8 hijklm | S | Kalisirin | 17.5 ± 1.4 tuvwxyz | L |
| AHPG-H4 | 14.2 ± 0.8 wxyzABCD | L | Kandhari | 15.8 ± 1.7 uvwxyzAB | L |
| Alah | 30.0 ± 2.9 ijklmno | M | Khog | 37.5 ± 1.4 efghi | S |
| Banaras collect. | 37.5 ± 1.4 efghi | S | Kurvi | 38.3 ± 1.7 efgh | S |
| Basin Seedling | 57.5 ± 1.4 ab | VS | Malta | 16.7 ± 0.8 uvwxyzA | L |
| Basin Seedless | 9.2 ± 0.8 ABCDE | L | MR 599 | 15.8 ± 0.8 uvwxyzAB | L |
| Bedana Sedana | 21.7 ± 0.8 pqrstuvw | M | Mridula | 37.5 ± 1.4 efghi | S |
| Bedana Suri | 63.3 ± 2.2 a | VS | Muskat | 40.8 ± 0.8 defg | S |
| Bedana Thin Skin | 29.2 ± 0.8 jklmnop | M | P-13 | 29.2 ± 0.8 jklmnop | M |
| Bhagwa | 29.2 ± 0.8 jklmnop | M | P-21 | 14.2 ± 0.8 wxyzABCD | L |
| Boseka Link | 15.8 ± 0.8 uvwxyzAB | L | P-23 | 44.2 ± 0.8 de | VS |
| Coimb. White | 43.3 ± 1.7 def | S | P-26 | 25.8 ± 0.8 lmnopqrs | M |
| Crenedo de Elecho | 12.5 ± 1.4 xyzABCDE | L | Patna-5 | 37.5 ± 1.4 efghi | S |
| Dholka | 15.0 ± 1.4 vwxyzABC | L | Phule Arakta | 37.5 ± 1.4 efghi | S |
| Dorsata Malus | 47.5 ± 1.4 cd | VS | Ruby | 17.5 ± 1.4 tuvwxyz | L |
| EC-12613 | 20.8 ± 0.8 qrstuvw | M | Saharanpur | 25.0 ± 1.4 mnopqrst | M |
| EC-62812 | 30.8 ± 0.8 hijklmn | M | Saih Sirin | 42.5 ± 1.4 def | S |
| G-137 | 30.8 ± 0.8 hijklmn | M | Sirin | 11.7 ± 0.8 yzABCDE | L |
| Ganesh | 28.3 ± 2.2 jklmnopq | M | Speen Danedar | 29.2 ± 0.8 jklmnop | M |
| GKVK-1 | 11.7 ± 0.8 yzABCDE | L | Speen Sacarin | 7.5 ± 1.4 CDE | VL |
| Goma Khatta | 14.2 ± 0.8 wxyzABCD | L | Sur Sukker | 20.0 ± 1.4 rstuvwx | L |
| Gul-e-Shah | 14.2 ± 0.8 wxyzABCD | L | Surat Anar | 15.8 ± 2.2 uvwxyzAB | L |
| Gul-e-Shah Red | 14.2 ± 0.8 DE | VL | Surkh Anar | 35.8 ± 0.8 fghij | S |
| Gul-e-Shah Rose Pink | 6.7 ± 0.8 wxyzABCD | L | Tebest | 19.2 ± 2.2 stuvwxy | L |
| Gulsa Red | 21.7 ± 0.8 pqrstuvw | M | Tujetis EC 4347 | 15.0 ± 1.4 vwxyzABC | L |
| HP Collec. | 5.8 ± 0.8 E | VL | Uthkal | 10.8 ± 0.8 zABCDE | L |
| IC-318712 | 55.8 ± 0.8 ab | VS | Yercaud | 47.5 ± 1.4 cd | VS |
| IIHR 12/1 | 34.2 ± 0.8 ghijk | S | Yercaud Local | 44.2 ± 0.8 de | VS |
| IIHR 19/10 | 42.5 ± 1.4 def | S |
| Source of Variation | Plant Height (m) | Canopy Perimeter (m) | Stem Diameter (cm) |
|---|---|---|---|
| Growing season (GS) | |||
| 2019 | 1.30 ± 0.03 b | 3.50 ± 0.09 b | 1.48 ± 0.04 b |
| 2020 | 1.59 ± 0.03 a | 4.74 ± 0.11 a | 1.78 ± 0.05 a |
| Significance | *** | *** | *** |
| Accession (A) | |||
| Achik Dana | 1.12 ± 0.04 j | 3.28 ± 0.13 kl | 1.43 ± 0.09 defgh |
| Agah | 1.48 ± 0.02 defgh | 3.26 ± 0.12 kl | 1.36 ± 0.10 fgh |
| AHPG-C3 | 1.59 ± 0.14 bcd | 4.63 ± 0.54 d | 1.57 ± 0.09 defg |
| Basin Seedless | 1.33 ± 0.02 i | 4.07 ± 0.19 fg | 1.81 ± 0.09 bcd |
| Bhagwa | 1.90 ± 0.03 a | 5.75 ± 0.18 a | 1.98 ± 0.10 abc |
| Dholka | 1.52 ± 0.07 cdef | 4.49 ± 0.25 de | 1.25 ± 0.07 gh |
| Dorsata Malus | 1.40 ± 0.05 fghi | 3.90 ± 0.46 ghi | 1.68 ± 0.10 cdef |
| Goma Khatta | 1.54 ± 0.03 cde | 4.70 ± 0.23 cd | 1.67 ± 0.10 cdef |
| Gul-e-Shah Rose Pink | 1.47 ± 0.11 defgh | 3.77 ± 0.16 ghij | 1.39 ± 0.09 efgh |
| HP Collec. | 1.49 ± 0.05 defg | 4.27 ± 0.27 ef | 1.81 ± 0.12 bcd |
| IC-318712 | 0.99 ± 0.02 k | 3.16 ± 0.07 l | 1.57 ± 0.12 defg |
| Jalore Seedless | 1.37 ± 0.02 ghi | 3.65 ± 0.26 ij | 2.28 ± 0.09 a |
| Jodhpur Red | 1.61 ± 0.04 bc | 4.68 ± 0.21 d | 1.51 ± 0.10 defg |
| Kajaki Anar | 1.12 ± 0.03 j | 3.19 ± 0.09 l | 1.46 ± 0.07 defgh |
| P-23 | 1.32 ± 0.09 i | 4.26 ± 0.51 ef | 1.44 ± 0.08 defgh |
| Phule Arakta | 1.70 ± 0.03 b | 5.36 ± 0.11 b | 1.83 ± 0.11 bcd |
| Saharanpur | 1.40 ± 0.06 fghi | 4.02 ± 0.40 fgh | 2.01 ± 0.10 abc |
| Saih Sirin | 1.37 ± 0.11 ghi | 3.70 ± 0.31 hij | 1.46 ± 0.10 defgh |
| Speen Sacarin | 1.51 ± 0.07 cdef | 4.07 ± 0.14 fg | 1.78 ± 0.09 bcde |
| Sur Sukker | 1.42 ± 0.16 efghi | 3.83 ± 0.45 ghij | 1.27 ± 0.08 gh |
| Tujetis EC 4347 | 1.36 ± 0.17 hi | 3.55 ± 0.51 jk | 1.08 ± 0.08 h |
| Uthkal | 1.83 ± 0.21 a | 5.04 ± 0.74 bc | 2.11 ± 0.14 ab |
| Significance | *** | *** | *** |
| GS × A | *** | *** | n.s. |
| Accession | Total Phenolics (mg GAE/g DW) | Flavonoids (mg catechin equi./g DW) | Tannins (mg catechin equi./g DW) | Antioxidant Activity (mg AAE/g DW) |
|---|---|---|---|---|
| Achik Dana | 46.31 ± 0.38 abc | 3.04 ± 0.04 defg | 8.02 ± 0.05 c | 460.6 ± 3.2 d |
| Agah | 47.37 ± 0.47 ab | 3.28 ± 0.04 cde | 6.76 ± 0.04 fg | 445.3 ± 1.7 de |
| AHPG-C3 | 49.44 ± 0.42 a | 3.66 ± 0.05 bc | 3.45 ± 0.02 n | 497.1 ± 2.2 bc |
| Basin Seedless | 36.18 ± 0.50 g | 2.47 ± 0.07 i | 8.62 ± 0.03 b | 509.6 ± 2.5 bc |
| Bhagwa | 43.74 ± 0.34 bcd | 3.35 ± 0.08 cd | 5.67 ± 0.04 i | 392.3 ± 3.0 ghi |
| Dholka | 45.50 ± 0.32 abcd | 3.19 ± 0.03 def | 5.89 ± 0.03 i | 410.2 ± 4.2 fgh |
| Dorsata Malus | 36.27 ± 0.63 g | 2.66 ± 0.08 ghi | 4.19 ± 0.04 l | 309.3 ± 2.7 m |
| Goma Khatta | 38.50 ± 0.43 efg | 2.84 ± 0.06 efghi | 6.56 ± 0.03 gh | 458.0 ± 2.7 d |
| Gul-e-Shah Rose Pink | 48.94 ± 0.41 a | 3.72 ± 0.05 bc | 8.11 ± 0.04 c | 571.5 ± 3.2 a |
| HP Collec. | 49.00 ± 0.68 a | 4.48 ± 0.05 a | 9.87 ± 0.04 a | 522.1 ± 3.0 b |
| IC-318712 | 43.33 ± 1.93 bcd | 2.87 ± 0.03 efghi | 3.86 ± 0.02 m | 289.7 ± 2.7 m |
| Jalore Seedless | 42.68 ± 0.39 bcde | 2.83 ± 0.02 fghi | 7.25 ± 0.03 de | 387.1 ± 3.5 hij |
| Jodhpur Red | 45.73 ± 0.47 abcd | 3.03 ± 0.04 defg | 7.39 ± 0.07 d | 426.2 ± 2.7 ef |
| Kajaki Anar | 34.10 ± 0.58 g | 2.51 ± 0.02 hi | 4.16 ± 0.04 lm | 337.8 ± 1.9 l |
| P-23 | 37.92 ± 0.41 fg | 2.61 ± 0.02 ghi | 4.78 ± 0.03 k | 298.1 ± 1.5 m |
| Phule Arakta | 42.34 ± 0.24 cdef | 3.27 ± 0.10 cdef | 5.08 ± 0.03 jk | 365.6 ± 3.9 jk |
| Saharanpur | 42.33 ± 0.54 cdef | 3.15 ± 0.12 def | 7.00 ± 0.04 ef | 487.8 ± 2.4 c |
| Saih Sirin | 38.59 ± 0.52 efg | 2.70 ± 0.05 ghi | 4.80 ± 0.04 k | 350.0 ± 3.3 kl |
| Speen Sacarin | 45.63 ± 0.45 abcd | 4.05 ± 0.05 ab | 8.89 ± 0.04 b | 460.5 ± 9.2 d |
| Sur Sukker | 41.36 ± 0.53 def | 2.93 ± 0.03 defgh | 5.32 ± 0.05 j | 372.3 ± 2.8 ijk |
| Tujetis EC 4347 | 44.25 ± 0.34 bcd | 3.35 ± 0.05 cd | 6.99 ± 0.04 ef | 368.5 ± 2.6 ijk |
| Uthkal | 44.80 ± 0.47 abcd | 3.27 ± 0.06 cdef | 6.40 ± 0.03 h | 415.3 ± 4.2 fg |
| Significance | *** | *** | *** | *** |
| Principal Component | Eigenvalue | Variance Explained (%) | Correlation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant Height | Canopy Perimeter | Stem Diameter | Total Phenolics | Flavonoids | Tannins | Antioxidant Activity | |||
| Dim. 1 | 2.89 | 41.3 | 0.416 NS | 0.146 NS | −0.043 NS | 0.805 *** | 0.862 *** | 0.759 *** | 0.854 *** |
| Dim. 2 | 1.42 | 20.3 | −0.001 NS | 0.851 *** | 0.825 *** | −0.114 NS | −0.042 NS | 0.066 NS | −0.012 NS |
| Dim. 3 | 1.00 | 14.3 | 0.636 ** | 0.289 NS | −0.388 NS | −0.438 * | −0.312 NS | 0.201 NS | 0.171 NS |
| Dim. 4 | 0.82 | 11.8 | 0.637 ** | −0.124 NS | 0.200 NS | 0.205 NS | 0.205 NS | −0.446 * | −0.283 NS |
| Dim. 5 | 0.43 | 6.2 | 0.123 NS | −0.375 NS | 0.330 NS | −0.175 NS | −0.067 NS | 0.356 NS | −0.063 NS |
| Dim. 6 | 0.27 | 3.8 | 0.017 NS | −0.116 NS | 0.135 NS | 0.029 NS | −0.206 NS | −0.209 NS | 0.385 NS |
| Dim. 7 | 0.16 | 2.3 | 0.032 NS | 0.027 NS | −0.013 NS | 0.269 NS | −0.262 NS | 0.097 NS | −0.097 NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haldhar, S.M.; Kumar, R.; Corrado, G.; Berwal, M.K.; Gora, J.S.; Thaochan, N.; Samadia, D.K.; Hussain, T.; Rouphael, Y.; Kumar, P.; et al. A Field Screening of a Pomegranate (Punica granatum) Ex-Situ Germplasm Collection for Resistance against the False Spider Mite (Tenuipalpus punicae). Agriculture 2022, 12, 1686. https://doi.org/10.3390/agriculture12101686
Haldhar SM, Kumar R, Corrado G, Berwal MK, Gora JS, Thaochan N, Samadia DK, Hussain T, Rouphael Y, Kumar P, et al. A Field Screening of a Pomegranate (Punica granatum) Ex-Situ Germplasm Collection for Resistance against the False Spider Mite (Tenuipalpus punicae). Agriculture. 2022; 12(10):1686. https://doi.org/10.3390/agriculture12101686
Chicago/Turabian StyleHaldhar, Sharavan Manbhar, Ramesh Kumar, Giandomenico Corrado, Mukesh Kumar Berwal, Jagan Singh Gora, Narit Thaochan, Dilip Kumar Samadia, Tajamul Hussain, Youssef Rouphael, Pradeep Kumar, and et al. 2022. "A Field Screening of a Pomegranate (Punica granatum) Ex-Situ Germplasm Collection for Resistance against the False Spider Mite (Tenuipalpus punicae)" Agriculture 12, no. 10: 1686. https://doi.org/10.3390/agriculture12101686
APA StyleHaldhar, S. M., Kumar, R., Corrado, G., Berwal, M. K., Gora, J. S., Thaochan, N., Samadia, D. K., Hussain, T., Rouphael, Y., Kumar, P., & Basile, B. (2022). A Field Screening of a Pomegranate (Punica granatum) Ex-Situ Germplasm Collection for Resistance against the False Spider Mite (Tenuipalpus punicae). Agriculture, 12(10), 1686. https://doi.org/10.3390/agriculture12101686












