Comparison Study on Wild and Cultivated Opuntia sp.: Chemical, Taxonomic, and Antioxidant Evaluations
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Sampling
2.3. Physico—Chemical Analysis
2.4. Taxonomic Identification
2.4.1. DNA Extraction, Amplification (PCR) and Sequencing
2.4.2. Sequencing, Alignment, and Data Analysis
2.5. Preparation of Extracts
2.6. Chemical Analysis
2.6.1. Total Phenolic Content (TPC)
2.6.2. Total Flavonoid Content (TFC)
2.7. Antioxidant Potential
2.7.1. Trolox Equivalent Antioxidant Capacity (TEAC)
2.7.2. Reducing Power (RP)
2.7.3. Phosphomolybdenum Antioxidant Activity (PMA)
2.7.4. Ferric Reducing Antioxidant Power (FRAP)
2.7.5. Hydrogen Peroxide Scavenging Activity
2.7.6. Hydroxyl Radical (•OH) Assay
2.7.7. Nitric Oxide Radical (•NO) Assay
2.8. Statistical Analysis
3. Results and Discussion
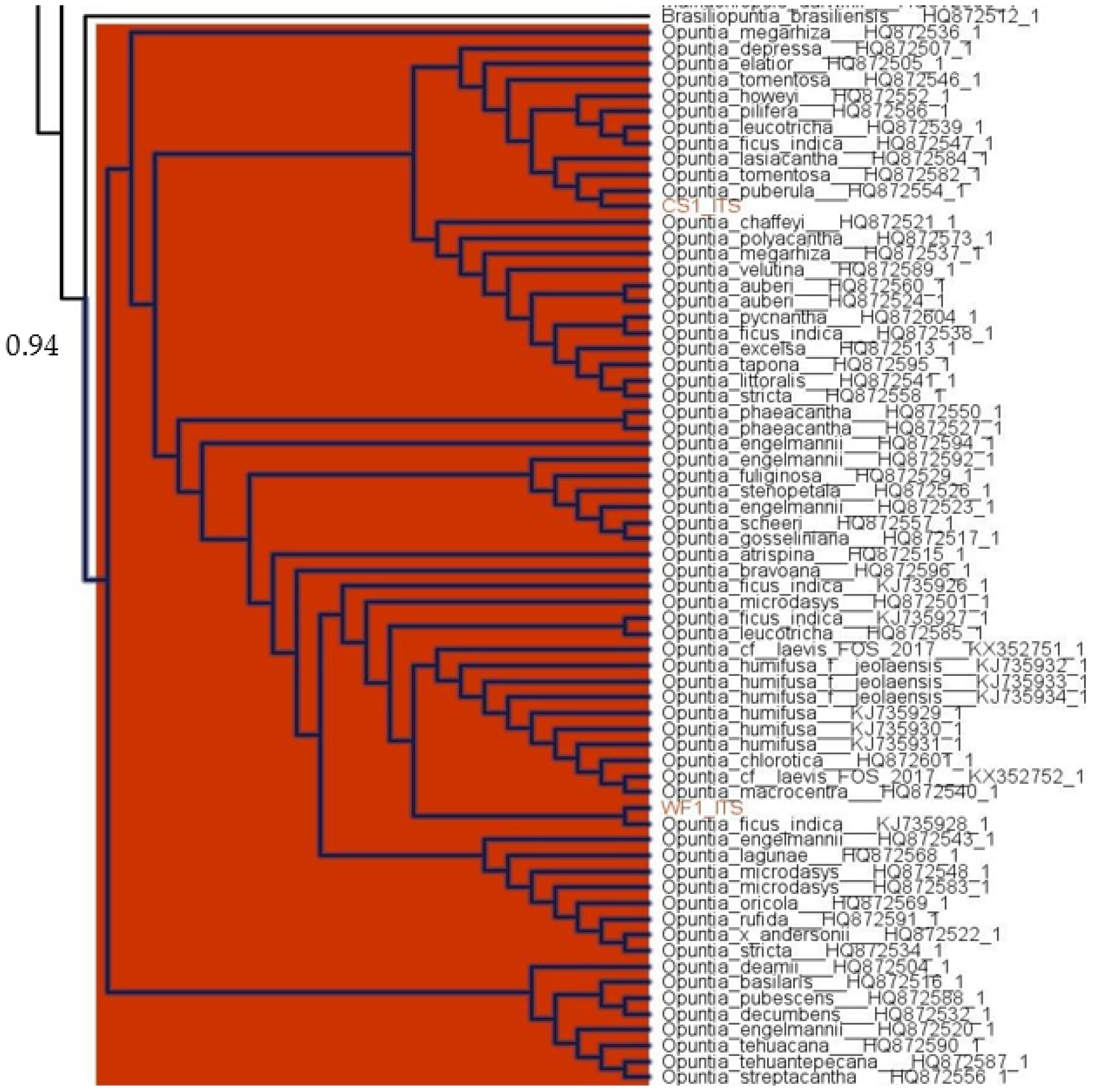
3.1. Taxonomic Identification
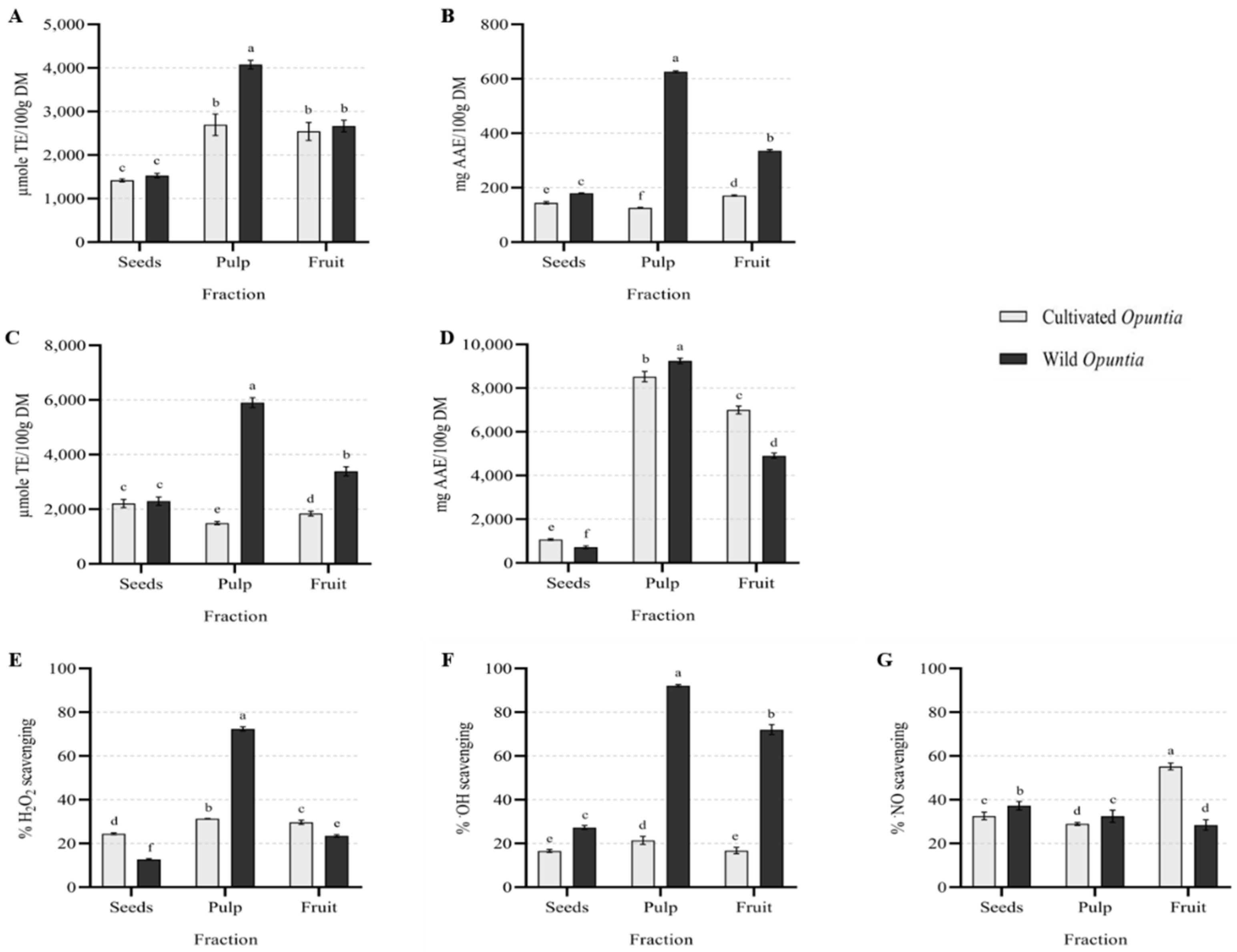
3.2. Total Polyphenols and Total Flavonoids Content
3.3. Antioxidant Activity Evaluation
Effect on Physiological Reactive Species
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Martínez, L.; Reyes Esparza, J.; Rodríguez-Fragoso, L. Cactus (Opuntia ficus-indica): A Review on Its Antioxidants Properties and Potential Pharmacological Use in Chronic Diseases. Nat. Prod. Chem. Res. 2014, 2, 153–160. [Google Scholar] [CrossRef]
- Zeghbib, W.; Boudjouan, F.; Vasconcelos, V.; Lopes, G. Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules 2022, 27, 4763. [Google Scholar] [CrossRef] [PubMed]
- Cota-Sánchez, J.H. Chapter 28—Nutritional Composition of the Prickly Pear (Opuntia ficus-indica) Fruit. In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 691–712. ISBN 978-0-12-408117-8. [Google Scholar]
- El-Mostafa, K.; El-Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El-Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Leem, K.-H.; Kim, M.-G.; Hahm, Y.-T.; Kim, H.K. Hypoglycemic Effect of Opuntia ficus-indica Var. Saboten Is Due to Enhanced Peripheral Glucose Uptake through Activation of AMPK/P38 MAPK Pathway. Nutrients 2016, 8, 800. [Google Scholar] [CrossRef]
- Mazari, A.; Yahiaoui, K.; Fedjer, Z.; Mahdeb, A. Physical Characteristics, Phytochemical Content and Antioxidant Activity of Cactus Pear Fruits Growing in Northeast Algeria. J. Prof. Assoc. Cactus Dev. 2018, 20, 178–188. [Google Scholar] [CrossRef]
- Bouzoubaâ, Z.; Essoukrati, Y.; Tahrouch, S.; Hatimi, A.; Gharby, S.; Harhar, H. Phytochemical Study of Prickly Pear from Southern Morocco. J. Saudi Soc. Agric. Sci. 2016, 15, 155–161. [Google Scholar] [CrossRef]
- García, F.H.; Coll, L.A.; Cano-Lamadrid, M.; Lluch, D.L.; Barrachina, Á.A.C.; Murcia, P.L. Valorization of Prickly Pear [Opuntia ficus-indica (L.) Mill]: Nutritional Composition, Functional Properties and Economic Aspects; IntechOpen: London, UK, 2020; ISBN 978-1-78985-850-1. [Google Scholar]
- Ćavar Zeljković, S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants 2021, 10, 550. [Google Scholar] [CrossRef]
- Andreu, L.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Antioxidant Properties and Chemical Characterization of Spanish Opuntia ficus-indica Mill. Cladodes and Fruits. J. Sci. Food Agric. 2018, 98, 1566–1573. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Gong, X.; Yang, M.; Zhang, C.; Li, M. Biosynthesis, Chemistry, and Pharmacology of Polyphenols from Chinese Salvia Species: A Review. Molecules 2019, 24, 155. [Google Scholar] [CrossRef]
- Alagumanivasagam, G.; Pasupathy, R.; Kottaimuthu, A.; Manavalan, R. A Review on In-Vitro Antioxidant Methods. Int. J. Pharm. Chem. Sci. 2012, 1, 662–674. [Google Scholar]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Zenteno-Ramírez, G.; Juárez-Flores, B.I.; Aguirre-Rivera, J.R.; Monreal-Montes, M.; García, J.M.; Serratosa, M.P.; Santos, M.Á.V.; Pérez, M.D.O.; Rendón-Huerta, J.A. Juices of Prickly Pear Fruits (Opuntia spp.) As Functional Foods. Ital. J. Food Sci. 2018, 30, 614–627. [Google Scholar] [CrossRef]
- Folgado, A.; Pires, A.S.; Figueiredo, A.C.; Pimentel, C.; Abranches, R. Toward Alternative Sources of Milk Coagulants for Cheese Manufacturing: Establishment of Hairy Roots Culture and Protease Characterization from Cynara cardunculus L. Plant Cell Rep. 2020, 39, 89–100. [Google Scholar] [CrossRef]
- Moon-van der Staay, S.Y.; van der Staay, G.W.M.; Guillou, L.; Vaulot, D.; Claustre, H.; Medlin, L.K. Abundance and Diversity of Prymnesiophytes in the Picoplankton Coμmunity from the Equatorial Pacific Ocean Inferred from 18S RDNA Sequences. Limnol. Oceanogr. 2000, 45, 98–109. [Google Scholar] [CrossRef]
- Katana, A.; Kwiatowski, J.; Spalik, K.; Zakryś, B.; Szalacha, E.; Szymańska, H. Phylogenetic Position of Koliella (Chlorophyta) as Inferred from Nuclear and Chloroplast Small Subunit RDNA. J. Phycol. 2001, 37, 443–451. [Google Scholar] [CrossRef]
- Martínez-González, C.R.; Ramírez-Mendoza, R.; Jiménez-Ramírez, J.; Gallegos-Vázquez, C.; Luna-Vega, I. Improved Method for Genomic DNA Extraction for Opuntia Mill. (Cactaceae). Plant Methods 2017, 13, 82. [Google Scholar] [CrossRef]
- Edwards, E.J.; Nyffeler, R.; Donoghue, M.J. Basal Cactus Phylogeny: Implications of Pereskia (Cactaceae) Paraphyly for the Transition to the Cactus Life Form. Am. J. Bot. 2005, 92, 1177–1188. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Carneiro, J.; Pereira, F. A Web-Based Platform of Nucleotide Sequence Alignments of Plants. bioRxiv 2019, bioRxiv:617035. [Google Scholar] [CrossRef]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.Fr: New Generation Phylogenetic Services for Non-Specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Criscuolo, A.; Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): A New Software for Selection of Phylogenetic Informative Regions from Multiple Sequence Alignments. BMC Evol. Biol. 2010, 10, 210. [Google Scholar] [CrossRef]
- Lemoine, F.; Domelevo Entfellner, J.-B.; Wilkinson, E.; Correia, D.; Dávila Felipe, M.; De Oliveira, T.; Gascuel, O. Renewing Felsenstein’s Phylogenetic Bootstrap in the Era of Big Data. Nature 2018, 556, 452–456. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Zeghbib, W.; Boudjouan, F.; Bachir-bey, M. Optimization of Phenolic Compounds Recovery and Antioxidant Activity Evaluation from Opuntia Ficus Indica Using Response Surface Methodology. J. Food Meas. Charact. 2022, 16, 1354–1366. [Google Scholar] [CrossRef]
- Surana, A.; Kumbhare, M.; Wagh, R. Estimation of Total Phenolic and Total Flavonoid Content and Assessment of in Vitro Antioxidant Activity of Extracts of Hamelia Patens Jacq. Stems. Res. J. Phytochem. 2016, 10, 67–74. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction--antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Dasgupta, P.; Roy, D.S.; Palchoudhuri, S.; Chatterjee, I.; Ali, S.; Dastidar, S.G. A Sensitive In Vitro Spectrophotometric Hydrogen Peroxide Scavenging Assay Using 1,10-Phenanthroline. Free Radic. Antioxid. 2016, 6, 124–132. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl Radical Scavenging Activity of Compatible Solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Ganesh, M.; Peng, M.M.; Aziz, A.S.; Jang, H.T. Comparative Antioxidant and Antimycobacterial Activities of Opuntia ficus-indica Fruit Extracts from Summer and Rainy Seasons. Front. Life Sci. 2015, 8, 182–191. [Google Scholar] [CrossRef][Green Version]
- Dehbi, F.; Hasib, A.; Ouatmane, A.; Elbatal, H.; Jaouad, A. Physicochemical Characteristics of Moroccan Prickly Pear Juice (Opuntia ficus-indica L.). Int. J. Emerg. Technol. Adv. Eng. 2014, 4, 300–306. [Google Scholar]
- De Wit, M.; Nel, P.; Osthoff, G.; Labuschagne, M.T. The Effect of Variety and Location on Cactus Pear (Opuntia ficus-indica) Fruit Quality. Plant Foods Hum. Nutr. Dordr. Neth. 2010, 65, 136–145. [Google Scholar] [CrossRef]
- Felker, P.; Soulier, C.; Leguizamon, G.; Ochoa, J. A Comparison of the Fruit Parameters of 12 Opuntia Clones Grown in Argentina and the United States. J. Arid Environ. 2002, 52, 361–370. [Google Scholar] [CrossRef]
- Karababa, E.; Coşkuner, Y.; Aksay, S. Some Physical Fruit Properties of Cactus Pear (Opuntia spp.) That Grow Wild in the Eastern Mediterranean Region of Turkey. J. Prof. Assoc. Cactus Dev. 2004, 6, 1–8. [Google Scholar] [CrossRef]
- Bekir, E.A. Cactus Pear (Opuntia ficus-indica Mill.) In Turkey: Growing Regions and Pomological Traits of Cactus Pear Fruits. Acta Hortic. 2006, 728, 51–54. [Google Scholar] [CrossRef]
- Nyffeler, R. Phylogenetic Relationships in the Cactus Family (Cactaceae) Based on Evidence from TrnK/MatK and TrnL-TrnF Sequences. Am. J. Bot. 2002, 89, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Touillaud, M.; Rothwell, J.A.; Romieu, I.; Scalbert, A. Measuring Exposure to the Polyphenol Metabolome in Observational Epidemiologic Studies: Current Tools and Applications and Their Limits123. Am. J. Clin. Nutr. 2014, 100, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martínez-González, M.A.; Medina-Remón, A.; Castañer, O.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.M. Effects of Polyphenol, Measured by a Biomarker of Total Polyphenols in Urine, on Cardiovascular Risk Factors After a Long-Term Follow-Up in the PREDIMED Study. Oxid. Med. Cell. Longev. 2016, 2016, 2572606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, e162750. [Google Scholar] [CrossRef]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as Antiobesity Agents: A Review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef] [PubMed]
- Madoui, S.; Charef, N.; Arrar, L.; Baghianni, A.; Khennouf, S. In Vitro Antioxidant Activities of Various Extracts from Flowers-Leaves Mixture of Algerian Cytisus Triflorus. Annu. Res. Rev. Biol. 2018, 26, 1–13. [Google Scholar] [CrossRef]
- Pabón-Baquero, L.C.; Otálvaro-Álvarez, Á.M.; Fernández, M.R.R.; Chaparro-González, M.P. Plant Extracts as Antioxidant Additives for Food Industry. In Antioxidants in Foods and Its Applications; Shalaby, E., Azzam, G.M., Eds.; IntechOpen: London, UK, 2018; pp. 87–116. ISBN 978-1-78923-379-7. [Google Scholar]
- Chahdoura, H.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Achour, L. Seeds of Opuntia Spp. as a Novel High Potential by-Product: Phytochemical Characterization and Antioxidant Activity. Ind. Crops Prod. 2015, 65, 383–389. [Google Scholar] [CrossRef]
- Amrane-Abider, M.; Nerin, C.; Canellas, E.; Benkerrou, F.; Louaileche, H. Modeling and Optimization of Phenolic Compounds Extraction from Prickly Pear (Opuntia ficus-indica) Seeds via Ultrasound-Assisted Technique. Ann. Univ. Dunarea Jos Galati Fascicle VI-Food Technol. 2018, 42, 109–121. [Google Scholar]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and Quantification of Individual Betalain and Phenolic Compounds in Mexican and Spanish Prickly Pear (Opuntia ficus-indica L. Mill) Tissues: A Comparative Study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Mena, P.; Tassotti, M.; Andreu, L.; Nuncio-Jáuregui, N.; Legua, P.; Del Rio, D.; Hernández, F. Phytochemical Characterization of Different Prickly Pear (Opuntia ficus-indica (L.) Mill.) Cultivars and Botanical Parts: UHPLC-ESI-MSn Metabolomics Profiles and Their Chemometric Analysis. Food Res. Int. 2018, 108, 301–308. [Google Scholar] [CrossRef]
- Missaoui, M.; D’Antuono, I.; D’Imperio, M.; Linsalata, V.; Boukhchina, S.; Logrieco, A.F.; Cardinali, A. Characterization of Micronutrients, Bioaccessibility and Antioxidant Activity of Prickly Pear Cladodes as Functional Ingredient. Molecules 2020, 25, 2176. [Google Scholar] [CrossRef] [PubMed]
- Ben Lataief, S.; Zourgui, M.-N.; Rahmani, R.; Najjaa, H.; Gharsallah, N.; Zourgui, L. Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic Activities of Bioactive Compounds Extracted from Opuntia Dillenii Cladodes. J. Food Meas. Charact. 2021, 15, 782–794. [Google Scholar] [CrossRef]
- Ammar, I.; Ben Salem, M.; Harrabi, B.; Mzid, M.; Bardaa, S.; Sahnoun, Z.; Attia, H.; Ennouri, M. Anti-Inflammatory Activity and Phenolic Composition of Prickly Pear (Opuntia ficus-indica) Flowers. Ind. Crops Prod. 2018, 112, 313–319. [Google Scholar] [CrossRef]
- Ouerghemmi, I.; Harbeoui, H.; Aidi Wannes, W.; Bettaieb Rebey, I.; Hammami, M.; Marzouk, B.; Saidani Tounsi, M. Phytochemical Composition and Antioxidant Activity of Tunisian Cactus Pear (Opuntia ficus-indica L.) Flower. J. Food Biochem. 2017, 41, e12390. [Google Scholar] [CrossRef]
- Chaalal, M.; Louaileche, H.; Touati, N.; Bachir Bey, M. Phytochemicals, in Vitro Antioxidant Capacity and Antiradical Potential of Whole and Ground Seeds of Three Prickly Pear Varieties: A Comparative Study. Ind. Crops Prod. 2013, 49, 386–391. [Google Scholar] [CrossRef]
- Tupec, M.; Hýsková, V.; Bělonožníková, K.; Hraníček, J.; Červený, V.; Ryšlavá, H. Characterization of Some Potential Medicinal Plants from Central Europe by Their Antioxidant Capacity and the Presence of Metal Elements. Food Biosci. 2017, 20, 43–50. [Google Scholar] [CrossRef]
- Arró-Díaz, D.J.; Castelnaux-Ochoa, N.; Ochoa-Pacheco, A.; Do-Nascimento, Y.M. Antioxidant Activity of Bioactive Compounds Isolated from Leaves and Bark of Gymnanthes Lucida Sw. Rev. Cuba. Quím. 2021, 33, 22–39. [Google Scholar]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in Vivo and in Vitro Methods Evaluation of Antioxidant Activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Kita, A.; Miedzianka, J.; Andreu-Coll, L.; Legua, P.; Hernandez, F. Characterization of Bioactive Compounds of Opuntia ficus-indica (L.) Mill. Seeds from Spanish Cultivars. Molecules 2020, 25, 5734. [Google Scholar] [CrossRef]
- Dontha, S. A Review on Antioxidant Methods. Asian J. Pharm. Clin. Res. 2016, 9, 14–32. [Google Scholar] [CrossRef]
- Chougui, N.; Tamendjari, A.; Hamidj, W.; Hallal, S.; Barras, A.; Richard, T.; Larbat, R. Oil Composition and Characterisation of Phenolic Compounds of Opuntia ficus-indica Seeds. Food Chem. 2013, 139, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Huang, X.; Shah, M.H.; Abbasi, A.M. Evaluation of Polyphenolics Content and Antioxidant Activity in Edible Wild Fruits. BioMed Res. Int. 2019, 2019, e1381989. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-920-1. [Google Scholar]
- Chougui, N.; Louaileche, H.; Mohedeb, S.; Mouloudj, Y.; Hammoui, Y.; Tamendjari, A. Physico-Chemical Characterisation and Antioxidant Activity of Some Opuntia ficus-indica Varieties Grown in North Algeria. Afr. J. Biotechnol. 2013, 12, 299–307. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Lubos, E.; Handy, D.E.; Loscalzo, J. Role of Oxidative Stress and Nitric Oxide in Atherothrombosis. Front. Biosci. J. Virtual Libr. 2008, 13, 5323–5344. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Engelen, M.P.K.J.; Deutz, N.E.P. Regulation of Nitric Oxide Production in Health and Disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef]
- Beevi, S.S.S.; Rasheed, A.M.H.; Geetha, A. Evaluation of Oxidative Stress and Nitric Oxide Levels in Patients with Oral Cavity Cancer. Jpn. J. Clin. Oncol. 2004, 34, 379–385. [Google Scholar] [CrossRef]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of Antioxidant Activity and Total Phenol in Different Varieties of Lantana Camara Leaves. BMC Res. Notes 2014, 7, 560. [Google Scholar] [CrossRef]
- Davies, C.V.; Gerard, L.M.; Ferreyra, M.M.; Schvab, M.D.C.; Solda, C.A. Bioactive Compounds and Antioxidant Activity Analysis during Orange Vinegar Production. Food Sci. Technol. 2017, 37, 449–455. [Google Scholar] [CrossRef]
- Fu, H.; Qiao, Y.; Wang, P.; Mu, X.; Zhang, J.; Fu, B.; Du, J. Changes of Bioactive Components and Antioxidant Potential during Fruit Development of Prunus Humilis Bunge. PLoS ONE 2021, 16, e0251300. [Google Scholar] [CrossRef]
- Boudjouan, F.; Zeghbib, W.; Bachir Bey, M.; Djaoudene, O.; Elothmani, D.; Bououdina, M.; Louaileche, H. Extraction Methods Comparison for Polyphenols Recovery and Antioxidant Activity from Opuntia Ficus Indica’s Seeds. Stud. Univ. Vasile Goldis Arad Ser. Stiintele Vietii 2021, 31, 164–171. [Google Scholar]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat Phenolic Compounds Regulate Metabolic Syndrome in High Fat Diet-Fed Mice via Gut Microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The Positive Correlation of Antioxidant Activity and Prebiotic Effect about Oat Phenolic Compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Wiczkowski, W.; Sawicki, T.; Bartoszek, A. The Comparison of Betalain Composition and Chosen Biological Activities for Differently Pigmented Prickly Pear (Opuntia ficus-indica) and Beetroot (Beta Vulgaris) Varieties. Int. J. Food Sci. Nutr. 2019, 70, 442–452. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołębiewska, E.; Świderski, G.; Męczyńska-Wielgosz, S.; Lewandowska, H.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Świsłocka, R.; Samsonowicz, M.; et al. Plant-Derived and Dietary Hydroxybenzoic Acids—A Comprehensive Study of Structural, Anti-/Pro-Oxidant, Lipophilic, Antimicrobial, and Cytotoxic Activity in MDA-MB-231 and MCF-7 Cell Lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef]



| Region | Sequences | Annealing Temperature | Reference |
|---|---|---|---|
| COX-3 | COX-3f: CCGTAGGAGGTGTGATGT COX-3r: CTCCCCACCAATAGATAGAG | 51 °C | [19] |
| 18S | 18S-f: ACCTGGTTGATCCTGCCAG 18S-r: TGATCCTTCYGCAGGTTCAC 18S402-f: GCTACCACATCCAAGGAAGGCA 18S895-f: GTCAGAGGTGAAATTCTTGGAT 18S919-r: TAAATCCAAGAATTTCACCTCT 18S1339-r: CTCGTTCGTTAACGGAATTAACC | 55 °C | [17] |
| rbcL | 1f: ATGTCACCACAAACAGAAAC 724r: TCGCATGTACCTGCAGTAGC | 48 °C | [20] |
| matk | matkx: TAATTTACGATCAATTCATTC matk5: GTTCTAGCACCAGAAAGTCG | 48 °C | [19] |
| ITS | ITS5: GGAAGTAAAAGTCGTAACAAGG ITS4: TCCTCCGCTTATTGATATGC | 57 °C | [19] |
| Opuntia Species | Weight (g) | Moisture (%) | °Brix | Ash (%) |
|---|---|---|---|---|
| Cultivated fruit | 65.62 ± 18.09 a | 86.48 ± 0.10 a | 11.48 ± 0.03 a | 0.32 ± 0.05 b |
| Wild fruit | 21.93 ± 1.93 b | 83.14 ± 0.14 b | 10.10 ± 0.10 b | 0.53 ± 0.09 a |
| Match Rank | Phylum | Class | Order | Family | Genus | Species | Subspecies | Score | Similarity | E-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | ficusindica | 874 | 100 | 0 | |
| 2 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | ellisiana | 874 | 100 | 0 | |
| 3 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | engelmannii var. lindheimeri | 874 | 100 | 0 | |
| 4 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | engelmannii var. linguiformis | 874 | 100 | 0 | |
| 5 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | orbiculata | 874 | 100 | 0 | |
| 6 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | dillenii | 874 | 100 | 0 | |
| 7 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | ficusindica | 874 | 100 | 0 | |
| 8 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | magnifica | 874 | 100 | 0 | |
| 9 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | phaeacantha | 874 | 100 | 0 | |
| 10 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | setispina | 874 | 100 | 0 | |
| 11 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | stricta | 874 | 100 | 0 | |
| 12 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | dillenii | 874 | 100 | 0 | |
| 13 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | ficusindica | 874 | 100 | 0 | |
| 14 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | engelmannii | var. engelmannii | 874 | 100 | 0 |
| 15 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | martiniana | 874 | 100 | 0 | |
| 16 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | pailana | 874 | 100 | 0 | |
| 17 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | ellisiana | 873 | 100 | 0 | |
| 18 | Magnoliophyta | Magnoliopsida | Caryophyllales | Cactaceae | Opuntia | engelmannii | var. engelmannii | 873 | 100 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudjouan, F.; Zeghbib, W.; Carneiro, J.; Silva, R.; Morais, J.; Vasconcelos, V.; Lopes, G. Comparison Study on Wild and Cultivated Opuntia sp.: Chemical, Taxonomic, and Antioxidant Evaluations. Agriculture 2022, 12, 1755. https://doi.org/10.3390/agriculture12111755
Boudjouan F, Zeghbib W, Carneiro J, Silva R, Morais J, Vasconcelos V, Lopes G. Comparison Study on Wild and Cultivated Opuntia sp.: Chemical, Taxonomic, and Antioxidant Evaluations. Agriculture. 2022; 12(11):1755. https://doi.org/10.3390/agriculture12111755
Chicago/Turabian StyleBoudjouan, Fares, Walid Zeghbib, João Carneiro, Raquel Silva, João Morais, Vitor Vasconcelos, and Graciliana Lopes. 2022. "Comparison Study on Wild and Cultivated Opuntia sp.: Chemical, Taxonomic, and Antioxidant Evaluations" Agriculture 12, no. 11: 1755. https://doi.org/10.3390/agriculture12111755
APA StyleBoudjouan, F., Zeghbib, W., Carneiro, J., Silva, R., Morais, J., Vasconcelos, V., & Lopes, G. (2022). Comparison Study on Wild and Cultivated Opuntia sp.: Chemical, Taxonomic, and Antioxidant Evaluations. Agriculture, 12(11), 1755. https://doi.org/10.3390/agriculture12111755










