Abstract
Traditional foliar spray and soil drench applications of crop protection compounds have been ineffective at managing huanglongbing (HLB) in citrus. Trunk injection is a technique that delivers crop protection compounds directly into the tree vasculature, which optimizes compound availability while minimizing drift, runoff, and damage to non-target organisms. Five-year-old HLB-affected ‘Valencia’ sweet orange (Citrus sinensis) trees were injected with the insecticide imidacloprid or the antibacterial oxytetracycline in October 2020 and April 2021. Trees were monitored for tree health, psyllid colonization, bacterial titers, fruit quality, fruit drop, and yield for two production seasons. Imidacloprid injection caused 63% mortality of psyllid adults within one week and reduced progeny survival by 80%, though the efficacy waned within two months. Injection with oxytetracycline significantly improved tree health, reduced bacterial titers, and reduced preharvest fruit drop by more than 3-fold with corresponding increases in yield. Residue dynamics varied by injected compound and tissue analyzed. These results suggest trunk injection could be an effective delivery method for existing or novel therapeutics targeting either the insect vector or the pathogen causing HLB.
1. Introduction
Huanglongbing (HLB), or citrus greening, is associated with a phloem limited bacterial pathogen that causes extensive systemic damage to citrus trees resulting in low yields, poor fruit quality, and whole tree decline [1,2]. In Florida citrus production regions, the disease is associated with the putative pathogen Candidatus Liberibacter asiaticus (CLas) and is transmitted by the Asian citrus psyllid (Diaphorini citri) [3,4]. Adult Asian citrus psyllids (ACP) feed on young shoots as well as mature leaves, whilst oviposition and development of immature ACP are confined to young, tender flush shoots [5]. During feeding, the pathogen is transmitted to the phloem, where it causes necrosis and blockage of carbohydrate transport along with other physiological disorders [6,7,8]. Leaves of infected trees display an asymmetrical yellow blotchy mottle and/or chlorosis, often mistaken for nutrient deficiencies, and at an advanced stage, canopy dieback and low-quality fruit that drop prematurely [2].
Although growers have been utilizing a variety of strategies to control the vector and manage HLB, these have not been successful, and citrus production continues to decline. Since its discovery in Florida in 2005, the disease has spread widely and was declared endemic in 2013 [9]. Compared with the pre-HLB era, Florida citrus production has been reduced by more than 70% [10] and HLB is currently the most significant threat to the global citrus industry [11]. The location of the pathogen within the vascular system (phloem) means that any potential disease therapies must be moved systemically throughout the plant; however, the thick cuticle of citrus leaves impedes penetration and uptake of many foliar applied materials [12]. Additionally, the widespread abundance of the psyllid vector is a challenge for growers to manage, and foliar applications and soil drenches for vector control have not substantially reduced psyllid populations nor contained spread of the disease in Florida groves [9]. Short-term management options are needed as long-term control strategies are being developed.
In 2016, citrus growers seeked and were granted emergency use authorization to spray the antibacterials oxytetracycline (OTC) and streptomycin to control the HLB-associated bacteria (https://citrusindustry.net/wp-content/uploads/2017/01/17FL02-and-17FL03-Signed-Auth-Letter-01-10-17.pdf, accessed on 6 September 2022). However, aerial applications of the antibiotics have not shown significant efficacy at reducing bacterial load in citrus [13,14] and although antibiotic sprays are still allowed for use in bearing citrus [15], most growers have ceased to use this management strategy. In contrast, recent trunk injection experiments in both greenhouse and field environments have identified a variety of crop therapeutic compounds with antagonistic effects on CLas, including the antibiotics streptomycin, penicillin, and OTC [16,17,18,19,20,21,22,23,24]. Other materials, including plant defense activators [24], benign Xylella fastidiosa [25], novel molecular compounds [26], and nanoparticles [27,28] have also displayed varying degrees of efficacy against Clas when applied using trunk injection as a delivery method.
Trunk injection is an alternative technique for directly applying crop protection materials into the vasculature of a woody plant [29,30,31]. This method for delivery has been around for centuries but has not been optimized for use in commercial orchards. The expected benefits associated with injections, as opposed to aerial or soil drench applications, include greater product efficacy, a reduced risk of drift and runoff, less impact on non-target organisms, and longer persistence of the applied compound [32,33]. The use of trunk injection for managing citrus pests and diseases began decades ago [34,35,36]. As early as the 1970s, researchers in South Africa were investigating the efficacy of tetracyclines and insecticides to manage citrus greening and psyllid populations [37]. In those studies, injected insecticides effectively reduced psyllid populations, and tetracycline hydrochloride reduced disease symptoms in the season after injection by 20-60 %. Concerns regarding the feasibility of the method, phytotoxicity, and the risk for antibiotic residues in fruits suppressed more extensive research on the topic. However, the rampant spread of HLB in Florida since 2005 and the devastation it has caused to the industry has reignited interest in trunk injection to deliver tetracyclines and other emerging therapies [26,38,39,40].
Injection formulations of OTC have demonstrated efficacy against different bacterial pathogens in both crop and ornamental trees [41]. Notably, OTC is used for management of the phytoplasma associated with lethal-yellowing-type diseases in palms [42]. The lethal-yellows, like HLB, are phloem-limited and are spread by an insect vector [43]. Both curative and preventative injections of OTC have proven highly effective at preventing the development and progression of symptoms associated with the disease [42]. Although efficacy of antibiotic injections against HLB has been demonstrated [23,24], additional studies on the physiological and horticultural effects of OTC injections, especially in young citrus trees, are necessary to better understand the impacts and risks before recommendations for best practices can be established.
Imidacloprid (IMI) is a systemic neonicotinoid insecticide that is commonly used for psyllid management in citrus [44]. IMI is typically applied as a soil drench to young trees and has been shown to effectively control psyllid populations for at least 60 days [45,46] and up to 6 months [44] after application. Soil drenching, however, can negatively impact non-target organisms [47], as well as contribute to run-off and risks for surrounding communities and the environment. Injectable IMI formulations have been successfully used for insect management in multiple other tree species, such as apples and elms [48,49]. However, trunk injection of IMI for psyllid management in HLB-affected citrus has not been extensively evaluated. IMI is one of the products used most regularly by citrus growers; therefore, the potential benefits associated with injection, such as quick mobility to target tissues, longer residual activity of the compound, and reduced dose and volume needed, may prove economically valuable [50].
Trunk injection primarily relies on the application into and distribution of compounds through the vessel elements of the xylem [51,52]; therefore, to be effective, the injected compounds need to be mobile within this tissue. For phloem-limited diseases such as those caused by phytoplasmas or CLas, it is important to also consider the mobility of the injected compounds in the phloem and between the xylem and the phloem [53,54]. In addition, the movement of an injected compound depends on various tree physiological characteristics and environmental conditions such as temperature, vapor pressure deficit, and soil moisture [50,55]. Further studies on uptake, mobility, and distribution of specific formulations are necessary for developing management methods to effectively utilize trunk injection as a technique for managing HLB.
This study was established to determine the physiological and horticultural impacts of trunk injections of IMI and OTC for ACP and HLB management in young citrus trees. The objectives were to (1) monitor the efficacy of trunk-injected IMI and OTC in reducing psyllid abundance and CLas titers over time, (2) assess the impacts of trunk injection of IMI and OTC on tree health, productivity, and physiology of young citrus trees, and (3) determine the distribution and stability of trunk injected IMI and OTC by quantifying residues after injection. Results from this study provide insight into whether trunk injection of therapeutic compounds can be used to effectively manage HLB and to lay the foundation for establishing alternative management options for this destructive disease.
2. Materials and Methods
2.1. Plant Material
Trees used for this study were five-year-old Valencia sweet orange (Citrus sinensis L. Osbeck) trees grafted on Kuharske (C. sinensis × Poncirus trifoliata) rootstock located at the Southwest Florida Research and Education Center (SWFREC) in Immokalee, FL, USA (26°27′49.2″ N 81°26′38.0″ W). The experiment was conducted as a randomized complete block design with four treatments and six single-tree replications. Because of the endemic nature of HLB in Florida since 2013 [9] disease pressure at this location is severe, and all trees were visibly HLB-affected and confirmed CLas positive prior to the start of the experiment using qPCR, as described below. The trees had been fertilized at a rate of 0.5 pounds (0.23 kg) per tree using conventional granular fertilizer (8N-4P-8K; Diamond R, Fort Pierce, FL, USA) every six months and slow-release fertilizer (12N-8P-6K; Diamond R, Fort Pierce, FL, USA), three times per year. Irrigation was by under-tree microjets. Insect management included 3–4 soil drenches of imidacloprid annually during the first 3 years after planting followed by annual dormant and early season foliar applications of broad-spectrum insecticides [56]. No insecticides effective against ACPs were applied during the time of evaluation.
2.2. Tree Injections
Because of COVID-19 pandemic-associated shutdowns in spring 2020, the first trunk injection was performed in fall 2020 (injection 1) and the second injection in spring 2021 (injection 2). Injection 1 was performed on 5 October 2020, approximately 6 months prior to the expected date of harvest. Injection 2 was performed on 5 April 2021, following harvest and flowering.
Four treatments were compared: (1) a non-injected control; (2) a water injected control; (3) oxytetracycline (OTC) injection; and (4) imidacloprid (IMI) injection. The OTC formulation was Arbor OTC (Arborjet, Inc, Woburn, MA, USA; 36.7% oxytetracycline hydrochloride) dissolved in de-ionized water and applied at a concentration of 2 g per tree, or 0.79 g active ingredient. This rate was selected based on the lowest dose recommended by the manufacturer and positive results from previous research that used a similar rate per unit trunk diameter [24] The IMI formulation was 4 mL Xytect as recommended by the manufacturer based on trunk diameter (Rainbow Ecoscience, Minnetonka, MN, USA; 10% infusible imidacloprid), or 0.4 g active ingredient. At the onset of the experiment the average trunk diameter of the trees (measured 5 cm above the graft union) was 6.1 cm.
Injections were made using Chemjet® Tree Injectors (Logical Result LLC, Interlochen, MI, USA) following the manufacturer’s instructions. Briefly, a 4.3 mm brad-point drill bit was drilled to a depth of 15 mm. Chemjets are spring-loaded syringes, which release liquid at 25–30 psi after activation. Syringes were inserted directly into the drilled hole at an angle of approximately 20–30 degrees and were removed once all compound was taken up by the tree. Two Chemjets were used per tree for each injection using a volume of 20 mL of de-ionized water or dissolved OTC formulation for a total of 40 mL per tree. For the IMI injections, each tree received 4 mL of formulation, 2 mL in each Chemjet. Chemjets were positioned in the north/south direction for injection 1 and the east/west direction for injection 2. All injections were made on sunny days between 9 am and 10 am into the scion trunk, approximately halfway between the graft union and the lowest scaffold.
2.3. Imidacloprid Effects on Psyllid Mortality
The impact of IMI on psyllid mortality was assessed by monitoring natural psyllid populations and laboratory-reared populations confined on injected trees. To monitor natural psyllid populations, the number of adults on 10 emerging flushes per tree and the number of those shoots infested with eggs and/or nymphs were counted each month from October 2020 until July 2021 to calculate the adult psyllid and oviposition infestation percentage, respectively.
Reared psyllids were collected from a colony maintained on orange jasmine (Murraya paniculata) plants under controlled greenhouse conditions and known to have no IMI resistance. Ten psyllids each were confined on a young shoot (stage 1) inside a 10 × 15 cm nylon organza sleeve cage. Two such cages were placed on each IMI- and water-injected tree. The first cohort of reared psyllids was evaluated on the trees when new flush started to emerge, which was 150 days after injection 1. New cohorts were evaluated on emerging flush 7 days after injection 2 and again 60 days after injection 2. Psyllid mortality was noted after 24 h and every three days thereafter. Cages were emptied of adult psyllids after two weeks, and the number of psyllids in the F1 generation were counted three days after adults began to emerge (8 May 2021).
2.4. Bacterial Titers
Candidatus Liberibacter asiaticus (CLas) titers were monitored in leaf, inner bark, and fine root tissues following injection. Leaves were collected at 0, 15, 30, 60, 90, 120, 150 and 180 days after each injection, and at 240 and 300 days after the second injection. Four leaves from the newest hardened-off flush were randomly collected from around the tree canopy. Only the leaf petioles and midribs were used for detection.
Root tissue was collected at 0, 15, 30, 60, 120, and 180 days after each injection, and at 240 and 300 days after the second injection. Fibrous roots were collected from the upper 20 cm of the soil under the canopy, approximately 1 m from the trunk of each tree in two different directions. Roots were washed and stored at −20 °C until analysis.
Bark tissue was collected at 0, 15, 30, 60, and 120 days after each injection, and at 300 days after the second injection. Bark tissue was collected using a grafting knife to remove a 1 cm2 section of bark (including phloem, and vascular cambium) from three randomly chosen scaffolds in the tree canopy. The outermost layer of the periderm was scraped off to increase the relative proportion of phloem. Leaf and bark tissue was stored at −20 °C until analysis.
All tissues were pulverized in liquid nitrogen with a mortar and pestle. One hundred mg of each tissue was used for DNA extraction. DNA from leaf and bark tissues was extracted using the DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. DNA from root tissue was extracted using the DNeasy Plant Pro Kit (Qiagen). Real-time PCR assays were performed using primers HLBas/HLBr and probe HLBp and normalization with primers COXf/COXr and probe COXp [57]. Amplification was performed over 40 cycles using an Applied Biosystems (Thermo Fisher Scientific, Waltham, MA, USA) QuantStudio 3 Real-Time PCR system and the iTaq Universal Probes Supermix (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. All reactions were conducted in a 20 µL reaction volume using 2 µL extracted DNA.
2.5. Fruit Drop and Yield
In the 2020/21 season, fruit drop was measured every two weeks from 21 December 2020, until harvest on 25 February 2021. In the 2021/22 season, fruit drop was measured monthly, starting in November 2021, by counting and removing fruits on the ground beneath each tree. The total number of fruits on each tree was determined at harvest and the percentage of dropped fruit was calculated for each tree. Yield (kg) was determined for each tree. A random set of 10 fruits from each tree were used to measure the fruit diameter, fruit weight, and fruit quality at the time of harvest. Internal fruit quality was determined by measuring Brix using a digital refractometer (Hanna Instruments, Smithfield, RI, USA) and titratable acidity by titrating sodium hydroxide to a phenolphthalein endpoint; results are presented as the Brix to Acid ratio. Fruit color was determined by using a CR-400 chroma meter (Konica Minolta, Ramsey, NJ, USA) and is presented as the a*/b* color ratio based on the CIE L*a*b* color system. Negative a*/b* ratio values indicate a deeper green color and positive values indicate a deeper red (orange) color [58,59].
2.6. Tree Physiological Response
During the period of spring flush, visual flush ratings were conducted every 10 days, from 3 February 2021, until 4 April 2021. Ratings were based on the percent of the canopy of each tree with branches in flush stage 0 (no flush), stage 1 (feather flush), stage 2 (elongation), stage 3 (leaf expansion), and stage 4 (leaf hardening) (Figure S1). Flowering ratings were conducted every 10 days during peak flowering from March 4 until April 4 by estimating the percent of branches which had flowers at any developmental stage.
Scion and rootstock diameters were measured 5 cm above and 5 cm below the graft union, respectively, and tree height and canopy width were measured in two directions to calculate differences in tree size and growth from the start of the study just before injection 1 (October 2020) to the end of the study in February 2022. Visual ratings of canopy color, HLB severity, and canopy density were conducted prior to injection 1, 6 months after injection 1, 6 months after injection 2 (April 2021), and at the end of the study (February 2022) to assess tree health. Canopy color was rated on a scale of 1 to 5 where 1 = very yellow and 5 = dark green. Canopy density was rated on a scale of 1 to 5 where 1 = very sparse and 5 = very dense. HLB severity was rated on a scale of 1 to 5 where 1 = 0% of branches with HLB symptoms, 2 = 0–25% of branches with HLB symptoms, 3 = 25–50% of branches with HLB symptoms, 4 = 50–75% of branches with HLB symptoms, and 5 = >75% of branches with HLB symptoms. HLB symptoms that were considered included leaf chlorosis, blotchy mottle, and corky veins.
The non-structural carbohydrate content of leaf, root, and bark tissue was determined from the same samples collected for CLas analysis in February 2022. The soluble (sugars and sugar alcohols) and insoluble (starch) carbohydrate content was measured following the protocol described in Leyva et al. [60] with modifications described in Tixier et al. [61] and Davidson et al. [62].
2.7. Oxytetracycline and Imidacloprid Detection
OTC detection was conducted on the same tissues collected for bacterial titer analysis for the first 150 days after each injection following the protocol established in Hijaz et al. [63] with minor modifications. Briefly, the OTC extraction solution (2.2% trichloroacetic acid in 1M HCl) was added to 100 mg of ground tissue followed by incubation on ice for five minutes. The samples were shaken for 5 min with a BioSpec Mini-Beadbeater-96 (Bartlesville, OK, USA), incubated on ice for another 5 min, and shaken for an additional 5 min before proceeding with centrifugation, decanting, and cleanup using Oasis PRiME hydrophilic-lipophilic-balanced cartridges (Waters, Milford, MA, USA) for solid phase extraction. Fluorescence was determined following a europium-based method as described in Hijaz et al. [63] using a multimode reader (BioTek Synergy HTX, Winooski, VT, USA) with the excitation wavelength set to 360 ± 40 nm, the emission wavelength set to 620 ± 20 nm, and gain set to 80.
IMI detection was conducted using the Eurofins Imidacloprid ELISA kit (Eurofins Abraxis, Warminster, PA, USA) following the manufacturer’s instructions for extraction and quantification of IMI residues in leaf tissue and using a spectrophotometer (SpectraMax 190, Molecular Devices, San Jose, CA, USA).
2.8. Statistical Analysis
Statistical analyses were conducted in RStudio Version 1.4.1717 (R Core Team, Vienna, Austria, 2021). Prior to analysis data were checked for assumptions of normality and homogeneity of variance. Bacterial titers, fruit drop, harvest data, psyllid mortality, starch content and visual ratings of tree health were compared among treatments using a one-way analysis of variance. Differences in flush and flower timing were compared among treatments using a repeated measures one-way ANOVA. Where differences were significant (p < 0.05), post hoc comparison of means was calculated using Tukey’s honest significant difference p-value adjustment for multiple pairwise comparisons.
3. Results
3.1. Psyllid Mortality
Natural adult psyllid and oviposition infestation were determined during months when all trees had at least 10 shoots with emerging flush, i.e., November, February, March, April, and June 2021. The adult infestation was highest in April and June (35.4–41.7%) but was not significantly affected by the injected compound (Table 1). Across all months analyzed, both IMI and OTC injection reduced oviposition infestation to 26.7% and 27.0%, respectively, compared to the water-injected trees where 42.1% of branches were infested. However, no treatments were significantly different from the non-injected control (32.1% infestation). The only significant difference among treatments during a single time point was oviposition infestation in April 2021. Treatment with IMI reduced oviposition infestation to 38.3%, compared to 65% and 58% in the non-injected and OTC-injected trees. There were insufficient emerging shoots after injection 1 in October to obtain accurate natural infestation data for the first injection.

Table 1.
Natural adult psyllid and oviposition infestation (%) during main flushing times after injection in October 2020 and April 2021.
When greenhouse-reared psyllids were placed on emerging flush 7 days after injection 2, adult mortality was 49% for the IMI-injected trees within three days, compared to 9% for the control (water-injected) trees (Figure 1). Six days later, the percentage of adult psyllid mortality was 63% for the IMI-injected trees, compared to 15% for the control trees. There were also significant differences in the F1 progeny survival; on average 17 new adult psyllids emerged from IMI-injected trees, while 68 new adults emerged from the control trees (Figure 1). When reared psyllids were placed on emerging flush 2 months after injection, adult psyllid mortality reached 18% within 9 days, compared to 3.3% mortality for the control trees. There were also no significant differences in reared psyllid mortality 150 days after injection, which was less than 5% on average (data not shown).
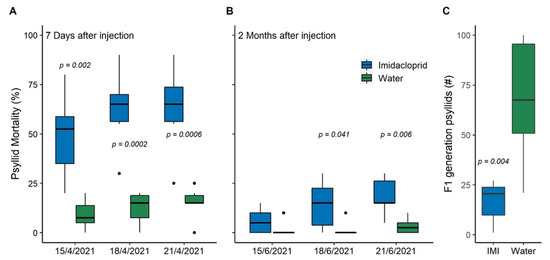
Figure 1.
Mortality and survival of greenhouse-reared psyllids after imidacloprid injection in April 2021. Percent psyllid mortality (A) one week and (B) two months after injection. (C) F1 progeny survival on 8 May 2021. P-values indicate where differences are significant (p < 0.05) for each measurement time.
3.2. Bacterial Titers
CLas titers are expressed as the cycle threshold (Ct) value; low Ct-values indicate a high CLas titer, and high Ct-values indicate a low CLas titer. Leaf Ct-values of OTC-injected trees were higher (26.4) than all other treatments (≤24.2) 15 days after injection 1 (October 2020) (Table S1 and Figure 2), but there were no differences among treatments 30–90 days after injection. From 120 days after injection 1 until 90 days after injection 2 (April 2021), the leaf Ct-values remained higher (and therefore CLas titers lower) in OTC-injected trees compared to all other treatments. The Ct-values of the OTC-injected trees reached a peak at 150 days after injection 1 (32.7) and 15 days after injection 2 (31.2). At the end of the study (Feb 2022), 300 days after injection 2, leaf Ct-values in OTC-injected trees were significantly higher (25.3) than all other treatments (≤22.3).
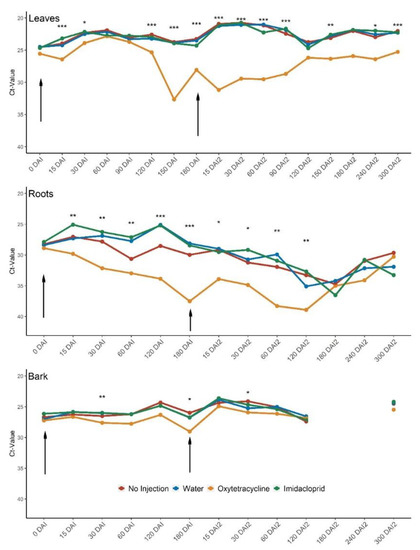
Figure 2.
Root, leaf, and bark Ct-values after injection 1 and 2 (arrows) in October 2020 and April 2021, respectively. Bark tissue was not analyzed at 180 and 240 DAI. Asterisks indicate significant differences among treatments according to Tukey’s honest significant difference test; * p < 0.05; ** p < 0.01; *** p < 0.001. p-values and mean separations are provided in Table S1. Error bars are not shown for clarity. DAI = days after injection. 180 DAI = 0 DAI2.
There were significant differences in root Ct-values between treatments from 15 days after injection 1 to 120 days after injection 2. The OTC-injected trees had significantly higher root Ct-values than all other treatments at 120 and 180 days after injection 1, and 15 and 60 days after injection 2. The root Ct-values for OTC treated trees peaked 180 days after the first injection (37.5) and 120 days after the second injection (38.9).
Ct-values of the inner bark tissue remained relatively consistent across treatments. At 30 days after injection 1 bark Ct-values for OTC-injected trees were significantly higher (27.6) than the water- and IMI- injected trees (26.0), though no different from the non-injected control (26.5). At 180 days after injection 1 the bark Ct-value for the OTC-injected trees was 29.0, significantly higher than all other treatments (≤26.8). At 30 days after injection 2 the OTC-injected trees had higher bark Ct-values (25.9) than the non-injected control (24.1), but there were no differences among the water- or IMI-injected treatments (25.3 and 24.7, respectively).
3.3. Fruit Drop and Yield
The percent fruit drop of OTC-injected trees was 20% and 28% in the 2020/21 and the 2021/22 production season, respectively, while percent drop was 66% and 77% for IMI and 72% and 80% for the no injection treatment in 2020/21 and 2021/22, respectively (Figure 3 and Table S2). Yield at harvest in 2020/21 was 9.4 kg per tree for the OTC-injected trees while all other treatments were less than 4.6 kg per tree (Figure 2). In 2021/22 the yield of the OTC-injected trees was 7.9 kg, while all other treatments were less than 2.2 kg.
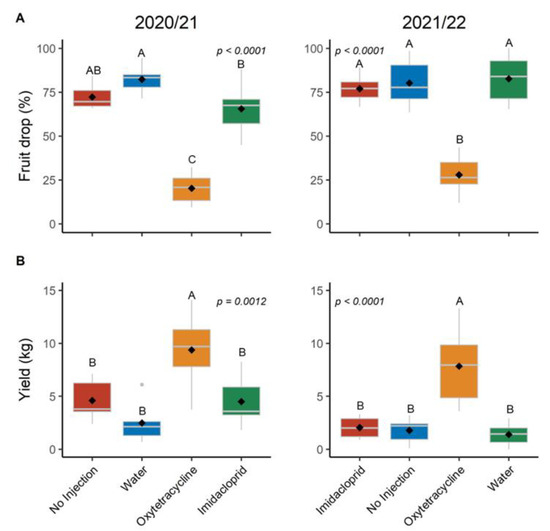
Figure 3.
Fruit drop and yield in production seasons 2020/21 and 2021/22. (A) cumulative percent preharvest fruit drop calculated as the cumulative number of fallen fruit counted monthly divided by the number of fallen plus harvested fruit; (B) fruit yield per tree. Different letters indicate significant differences (p < 0.05) according to Tukey’s honest significant difference test.
The external rind a*/b* ratio of fruits harvested in 2020/21 was significantly higher (more orange) for fruit from OTC-injected trees than all other treatments (Figure 4 and Figure S2), but there were no significant differences among treatments in 2021/22 (Figure 4 and Figure S3). The Brix:acid ratio of fruit juice from the OTC-injected trees was 10.3 in 2020/21, which was significantly higher than that of the control trees (7.8–8.7), though not significantly different from that of the IMI treated trees (8.9). In 2021/22, the Brix:acid ratio of fruit juice from the OTC-treated trees was 11.0, which is more than 3 values higher than all other treatments. There were no differences in fruit size (grams per fruit) for the 2020/21 harvest. However, in 2021/22, OTC-injected trees produced fruits that were more than 70 g heavier than all other treatments.
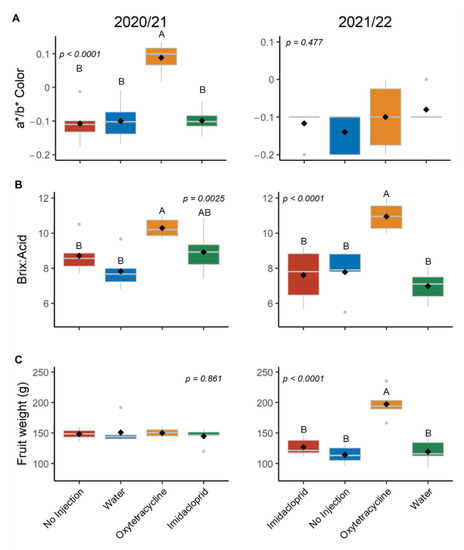
Figure 4.
Fruit quality at harvest in production seasons 2020/21 and 2021/22. (A) External rind a*/b* color ratio; (B) ratio of percent brix to titratable acidity; (C) fruit weight (grams per fruit). Different letters indicate significant differences (p < 0.05) according to Tukey’s honest significant difference test. Letters not shown when differences were not significant.
3.4. Tree Physiological Response
In April 2021, 6 months after injection 1, there were significant differences in visual ratings of tree health (Table 2 and Figure S4). The OTC-injected trees had fewer branches with symptoms of HLB, a denser canopy, and greener leaves. Visual differences in tree health were most apparent in October 2021, 6 months after injection 2, when the HLB disease index of OTC-injected trees was 2.9 while all other treatments were greater than or equal to 4.0 (Figure S5). In February 2022, approximately 10 months after injection 2, OTC-injected trees remained healthier as indicated by a significantly greener and thicker canopy with fewer HLB symptomatic leaves (disease index = 3.7) compared to all other treatments (disease index ≥ 4.2).

Table 2.
Foliar Huanglongbing (HLB) disease index, canopy color, and canopy density of trees injected in October 2020 and April 2021.
The emergence, development, and maturation patterns of new flush analyzed during the spring flushing period in 2021 (3 February—4 April), were significantly different among treatments (Figure 5). On February 13, 45% of branches from OTC-injected trees had begun flushing, while less than 13% of the branches in the other three treatments were flushing (Stage 0). From 23 February to 14 March the percent of branches in the leaf expansion phase (Stage 3) was significantly higher (32–78%) for OTC treated trees compared to all other treatments (10–41%). By 24 March 65% of branches from OTC trees had reached the leaf hardening phase (Stage 4) while less than 32% of branches from other treatments reached this phase. On 4 April 92% of branches from the OTC trees had fully developed new leaf flush (Stage 4) and less than 2% of branches were in any of the earlier flush stages (Stage 0, 1, 2, or 3). Conversely, less than 50% of branches from the other treatments had fully developed new flush (Stage 4), and more than 10% of branches were in each of the earlier flush stages (Stage 0, 1, 2 and 3).
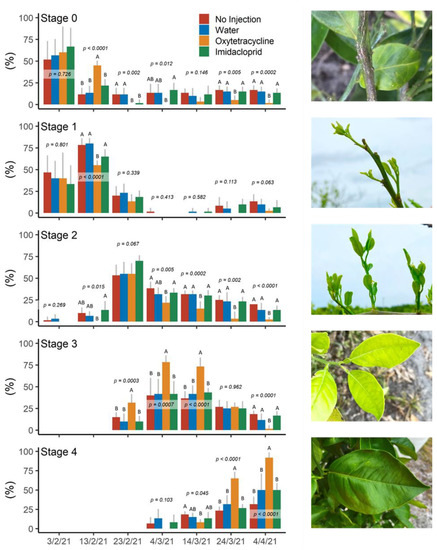
Figure 5.
Emergence and maturation patterns of new flush in spring 2021 after injection in October 2020. S0 = no flush; S1 = feather stage; S2 = elongation stage; S3 = expansion stage; S4 = hardening stage. Different letters within each time point indicate significant differences (p < 0.05) according to Tukey’s honest significant difference test. Letters not shown when differences were not significant.
Significant differences in the flowering intensity among treatments were also found in 2021. On 23 February and 4 March, more than 75% of the OTC-injected trees had branches with flowers, while none of the trees from the other treatments had more than 55% of branches with flowers on 23 February or more than 63% of branches with flowers on 4 March (Figure 6). Flowering intensity decreased overall in the following weeks and no significant differences were found among treatments on 14 March and 24 March.
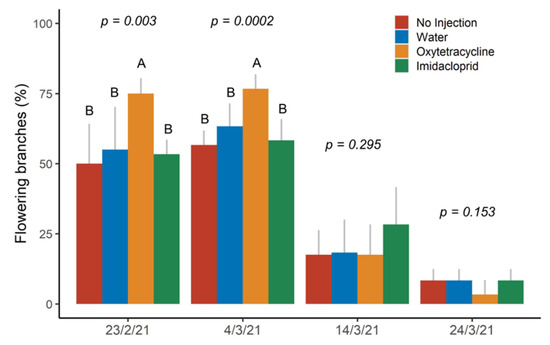
Figure 6.
Percent of branches with flowers in spring 2021 after injection in October 2020. Different letters within each time point indicate significant differences (p < 0.05) among treatments according to Tukey’s honest significant difference test. Letters not shown when differences were not significant.
There were significant differences among treatments in the change in tree size from prior to injection 1 in October 2020 to the end of the study in February 2022 (Figure 7). Trees injected with OTC had an average increase in rootstock trunk circumference of 3.1 cm compared to 0.55 cm for IMI-injected trees, 0.22 cm for water-injected trees, and 0.17 cm for non-injected trees. There was also a greater increase in the scion circumference for the OTC-injected trees (3.6 cm) compared to the non-injected (1.2 cm) and water-injected (1.4 cm) controls, though there was no significant difference compared to the IMI-injected trees (2.5 cm). OTC-injected trees also had the greatest increase in tree height (37 cm) and canopy width (48 cm), but changes were not significantly different from all other treatments.
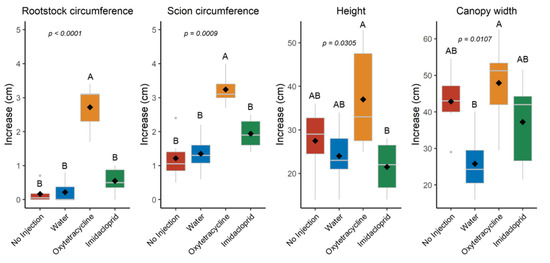
Figure 7.
Increase in rootstock circumference, scion circumference, tree height, and canopy width from October 2020 until February 2022 after injection in October 2020 and April 2021. Different letters indicate significant differences (p < 0.05) according to Tukey’s honest significant difference test. Letters not shown when differences were not significant.
At the end of the study in February 2022, there were measurable differences in the non-soluble carbohydrate (starch) content of the leaves (Figure 8). Leaf starch content of the OTC-injected trees was 63 µg/g, compared to more than 125 µg/g for IMI- and non-injected trees. There were no detectable differences in the starch content of root or bark tissue at the time of analysis, nor were there any differences in the soluble carbohydrate content of any tissue (data not shown).
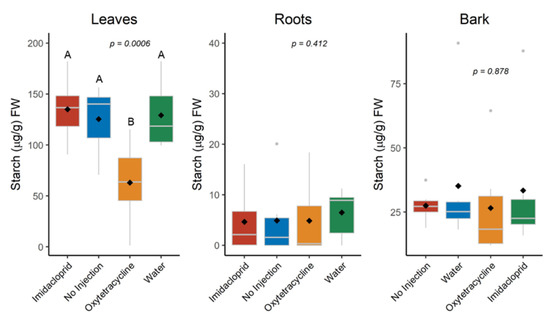
Figure 8.
Starch content in February 2022 of root, leaf, and bark tissue from trees injected in October 2020 and April 2021. Different letters indicate significant differences (p < 0.05) according to Tukey’s honest significant difference test. Letters not shown where differences were not significant.
3.5. Oxytetracycline and Imidacloprid Residue Analysis
Leaf OTC concentrations increased from <0.10 µg/g at 0–30 days after injection 1 to a peak value of 1.76 µg/g at 120 days after injection 1 (Figure 9). Concentrations dropped to 0.33 µg/g immediately prior to injection 2, increased to 0.90 µg/g at 15 days after injection 2 (DAI2), and dropped to 0.25 µg/g by 60 DAI2. Root concentrations were highest 180 days after injection 1 (1.05 µg/g) and 120 days after injection 2 (0.72 µg/g) and were less than 0.39 at the other time points. Concentrations in the bark were highest 60 days after both injections (4.46 and 3.94 µg/g, respectively); and dropped to 0.57 µg/g by 120 DAI2.
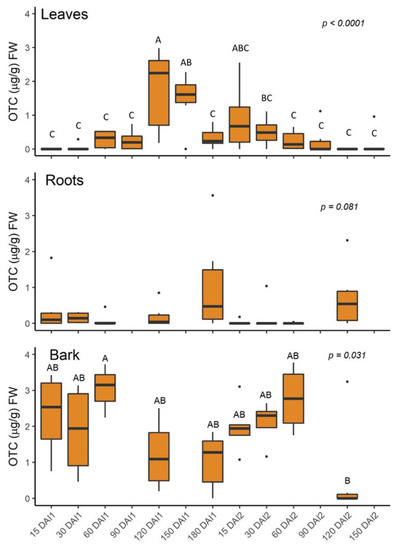
Figure 9.
Leaf oxytetracycline (OTC) concentration after injection 1 in October 2020 and injection 2 in April 2021. DAI1 = days after October injection; DAI2 = days after April injection. 180 DAI = 0 DAI2. Different letters indicate significant differences (p < 0.05) between time points according to Tukey’s honest significant difference test. Letters not shown when differences were not significant.
Leaf IMI concentrations were highest 15 days after each injection (Figure 10); injection in spring (injection 2) resulted in a concentration of 271.4 ng/g, while injection in fall (injection 1) resulted in a concentration of 55.0 ng/g.
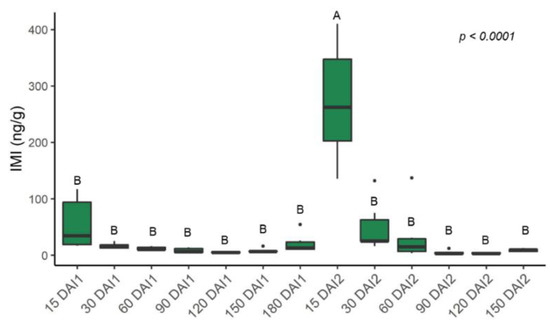
Figure 10.
Leaf imidacloprid (IMI) concentrations after injection 1 in October 2020 and injection 2 in April 2021. DAI1 = days after injection 1; DAI2 = days after injection 2. 180 DAI = 0 DAI2. Different letters indicate significant differences (p < 0.05) between time points according to Tukey’s honest significant difference test.
4. Discussion
In young citrus trees, the Asian citrus psyllid, vector of HLB, is usually managed by frequent soil drench applications of the neonicotinoid imidacloprid (IMI) followed by foliar sprays of insecticides as the trees increase in size [44]. However, these have not been successful in fully controlling the insect and therefore occurrence and spread of HLB [9]. Management strategies that increase the efficacy of either insecticides or other therapeutic compounds would therefore provide significant benefit to growers.
Trunk injections of IMI have been shown to effectively control multiple species of insect pests in apple trees for up to two full growing seasons [33,49], prevent lobate lac scale infestation for more than 22 months after injection in Ficus sp. [64], and significantly increased the mortality of banana thrips and subsequently improve fruit production [65]. However, trunk-injected IMI for control of emerald ash borer in green ash trees [48,66,67] and wooly adelgid in eastern hemlock [68,69] were not consistently effective.
Our results from IMI injection in sweet orange trees showed a maximum concentration of the insecticide in the leaves at 15 days after injection followed by a significant decline by 30 days after injection, which is consistent with results from other species. In ash trees, IMI residues have been found in leaves within 1–7 days after injection, with peak levels occurring 14–60 days after injection [49,66], and low residues in the season after injection [48]. In our trial, the peak leaf IMI concentration 15 days after injection in the fall was lower than the peak concentrations found 15 days after injection in the spring, which is the same trend seen after IMI injection in eastern hemlock [69]. In Florida citrus, the main period for a new vegetative flush is in the early spring (February—April). Newly formed leaves in the spring have higher rates of transpiration compared to the older leaves present in the fall. This typical flush pattern corresponds to an overall reduced metabolic activity during fall compared to spring. Because the rate of compound uptake and distribution after injection increases in rapidly transpiring trees, conditions that contribute to increased transpiration are recommended for quick and well-distributed injections [70]. In our study, injection in the spring coincided with a period of more intense flush and thus a greater abundance of new and rapidly transpiring leaves, which allowed for greater distribution of IMI in leaf tissue after the spring injection compared to after the fall injection.
The IMI concentrations measured in the months after injection align with the observed mortality rates of psyllids exposed to the leaves of injected trees. Psyllids that fed on young flush a week after injection had a higher mortality (63%) and a corresponding decrease in the F1 population size compared to the psyllids that fed on the control trees (15%). However, this effect was much less evident two months after injection when psyllid mortality did not exceed 18% and there was no difference in the population size of the F1 generation. Results from these experiments are similar to findings by Boina et al. [71], who found that a concentration of 0.1 ppm IMI was sublethal but reduced adult psyllid lifespan and fecundity. In our study, the mean peak concentration measured 15 days after the spring injection was 0.27 ppm and was sufficient to cause significant mortality. Two months after injection the IMI concentration in leaves was less than 0.04 ppm, which although resulting in greater mortality (18%) compared to the trees injected with water (3.3%), was likely not effective enough to prove useful for growers. At 5 months after injection, when leaf IMI concentrations were less than 0.01 ppm, no significant difference in psyllid mortality between the treated and untreated trees was found. The costs associated with IMI injection at the currently labeled rates are therefore unlikely to justify injection over soil drenches and foliar sprays considering the short-term efficacy. However, combining insecticides with antibacterial or other therapeutic compounds for injection may provide some benefits [72].
In the absence of effective measures for controlling the vector, methods for directly targeting the putative bacterial pathogen (CLas) associated with HLB are of great interest to citrus growers in Florida. Oxytetracycline (OTC) has demonstrated efficacy against CLas previously [22,23,24], though information on the physiological and horticultural benefits provided by the product has been scant. ArborOTC is an injection-specific formulation that was developed for management of lethal yellowing-type phytoplasmas in palms. The use of trunk injections for widespread containment and management of lethal yellowing phytoplasmas in palms has been effective [73] and can be used as a model for the potential utility of trunk injection techniques to control HLB. It is important to note, however, that the structure and arrangement of the xylem and phloem vessels within the trunk of palms are fundamentally distinct from citrus and trees in general. We found that among the three tissues (leaves, roots, and inner bark) analyzed, OTC concentrations remained the highest in the inner bark where the phloem is concentrated. Compared to the leaf phloem, the phloem beneath the tree bark is less exposed to sunlight or UV radiation, which have been shown to quickly break down OTC [74]. It is likely that OTC concentrations in leaves peaked shortly after injection but had significantly degraded by the first time of sampling 15 days after injection. The increase of leaf OTC concentrations from 15 DAI to 120 DAI concomitant with a decrease of bark OTC concentrations suggests a mobilization of OTC from bark to leaf tissues during the flushing period in spring. OTC concentrations were higher in the roots than in the leaves 15 days after the fall injection but were overall lower, which may have been due to a faster fibrous root regeneration rate and/or limited movement of OTC towards this tissue.
Injection of ArborOTC reduced the amount of CLas present in leaf and root tissue as indicated by higher Ct-values after real time qPCR amplification of bacterial 16S rDNA. Root CLas levels in the OTC-injected trees were lower compared to the other trees as early as 15 days after the fall injection (injection 1) and remained lower for 120 days after the spring injection (injection 2). In the leaves CLas levels were lower in the OTC-injected trees compared to the other trees 15 and 30 days after the fall injection but there were no differences at the other timepoints until 120 days after injection. As noted in Etxeberria et al. [75], bacterial DNA can still be detected for several months after death of the bacteria. Furthermore, OTC is bacteriostatic and not bactericidal, which would suggest that differences in CLas levels would not be measurable until the next leaf flush cycle. Our results suggest that root turnover was more rapid than leaf turnover following the first injection in October as we measured differences in the root CLas levels sooner after injection compared to the leaves. A drastic reduction in leaf CLas levels was evident 150 days after the fall injection, March 2021, at which time new leaves had formed. In these new leaves CLas levels remained significantly lower in the OTC-injected trees compared to the other trees for 300 days after the spring injection. In contrast to leaves and roots, differences in bark bacterial levels over the course of the study were minor. Bark renewal is not as rapid as leaf or root turnover [76]. Therefore, bacterial DNA continues to be detectable, even if the pathogen has been rendered inactive by the treatment [75].
The reduction in bacterial levels in both roots and leaves of the OTC-injected trees contributed to drastic improvements in different measures of tree health, including visual ratings of canopy density, canopy color, and disease index. There was also a notable change in both flush and flowering timing associated with the OTC injection. Uniformity in flower timing leads to uniform fruit maturation at harvest. HLB is known to cause both off-season and prolonged periods of bloom [77,78], resulting in fruits at multiple stages of maturity during the harvest season. OTC injection and the associated improvements in tree health appeared to regulate both flush and flower timing. More than 90% of the branches of the OTC-injected trees had fully developed new flush by 4 April with the remaining branches containing flush in various other stages of development. Comparatively, less than 50% of branches from any of the other treatments had developed new flush with the rest of the branches in various other stages of development. Uniform flush timing may allow for more effective psyllid control because there is a shorter window when the young flush, preferred by the psyllids for feeding and oviposition, is present [79]. Flowering was also more synchronized in OTC-injected trees; the proportion of flowering branches was initially higher and declined faster compared to any of the other treatments. Reduced off-season flowering results in less flowering-related diseases that contribute to fruit drop [80] and can ultimately lead to more synchronized fruit maturation.
Injection of OTC dramatically affected harvest yield and fruit quality. OTC injections significantly decreased fruit drop in the 3 months leading up to harvest. Preharvest fruit drop is the result of mature fruit abscission prior to harvest and has been well documented as a significant factor leading to reduced yields in HLB-affected groves [1,81]. The cause of preharvest fruit drop in HLB-affected trees is still debated. Zhao et al. [82] suggested that infection of fruits with Diplodia, the causal organism of citrus stem end rot, are a contributing factor. Recent research suggests that the tree water status may play a role in the reduced fruit size and increased fruit drop associated with severely infected trees [83], and an increase in the severity of HLB symptoms was closely correlated with an increase in fruit drop [84]. Another possible cause is carbohydrate shortage in mature fruit, although results are not conclusive [83,84]. After injection 1 in fall, OTC-injected trees dropped 20% of their total fruit, compared to the non-injected and water-injected trees which dropped 77% of their fruit before harvest in February. This drastic reduction in fruit drop is likely a function of the decrease in bacterial titer, restoration of phloem function and tree metabolism, and therefore overall improvement of tree health induced by OTC.
The quality of fruits at harvest in February from the OTC-injected trees was significantly improved after the fall injection. Fruits were more orange and had a higher brix to acid ratio. Peel maturation is primarily influenced by the environment, nutrient availability, and hormones [85]. Our results therefore suggest that in addition to visual tree health, OTC injections also restored the hormonal balance and nutrient balance of trees and therefore peel coloration. Internal maturation is largely a function of the availability and distribution of water and carbohydrates, and to a lesser extent organic acids and enzymes [85]. Therefore, OTC injection also appeared to have restored carbohydrate metabolism, which is known to be severely compromised in HLB affected trees [7,8,86], leading to increases in total soluble solids in the fruit. Although we did not measure any differences in soluble sugars, considerably less starch was found to accumulate in leaves of OTC-injected trees than the other treatments, supporting this suggestion.
After the fall injection there was no difference in fruit size associated with any treatment. During the pre-harvest period after the spring injection, the percent fruit drop was still dramatically lower for OTC-injected trees. Most noticeable, however, was the significant increase in fruit size after the spring injection. The spring injection (April) of OTC occurred during stage I of fruit development [85], the period of cell division, and prior to stage II, the period of cell expansion. This suggests that the improved tree health associated with OTC injections, and a resulting improved hydraulic functioning by the time the fruits had reached cell expansion, allowed the fruits to accumulate water more efficiently, in turn leading to larger fruits [83]. Fall injection occurred during the latter part of stage II of fruit development, prior to stage III (maturation). An improvement in vascular functioning by the time the fruits reached the maturation stage appeared to have allowed the fruits to accumulate carbohydrates more efficiently leading to improved fruit quality. The fruits harvested after the spring injection (2021/22), while larger, did not have the same improved fruit quality as the previous season’s harvest after the fall injection. We believe that the fruits harvested from the OTC-injected trees in the 2021/22 season were harvested before they had fully matured. The harvest time in February was chosen to ensure having enough fruit from all treatments available to perform fruit quality analyses; fruits from trees that had not received OTC would have been lost to preharvest fruit drop if harvesting would have been delayed further. For Florida Valencia oranges, however, harvesting in April and May is historically more common and results in fruits with the best quality [59]. We expect the fruits from OTC-injected trees would have matured further and would have had improved juice quality if they had been harvested later in the season.
Additional information about trunk injection in citrus is required before recommendations for best practices can be developed. The uptake and distribution of systemic pesticides is a function of the hydraulic characteristics of a tree. A detailed study of water use measured by stem sap flux in eastern hemlock trees allowed Ford et al. [87] to create simple mathematical and graphical models that allow for an estimation of the amount and timing of water use based on tree size and climatic conditions. These models can also be used for improving the efficacy of systemic pesticide applications using trunk injection. Additional information on the hydraulic characteristics of Florida citrus could allow researchers to develop similar models to predict when uptake and distribution of systemic pesticides would be the most efficient. Research on seasonal and hourly sap flow and water use shows that citrus trees have reduced transpiration in winter months [88]. A reduction in tree transpiration and associated metabolic activity may explain results seen in our study which show uptake is less effective after the fall injection compared to the spring injection. Hamido et al. [89] found that water use in HLB-affected trees is significantly lower than in healthy trees; therefore, creating a model that predicts water use and tree hydraulics would need to consider the HLB status of the tree. Additional information on sap flow during specific tree phenological stages would be necessary to develop models that can predict the optimum timing for uptake and distribution of therapeutics with trunk injection. However, the timing of trunk injections must also consider fruit residue levels, which are expected to be lower when the period between injection and harvest is longer, as seen in preliminary trials (data not shown). Lastly, pathogen biology and lifecycle [50], the characteristics of the compound being injected, as well as tree growth and development need to be considered. Injection for HLB management will likely require monitoring seasonal flush periods because flush timing is critical for psyllid management [79], particularly if the compounds being injected include insecticides or combinations of products with insecticidal properties.
Although the costs associated with injection have been discussed [90], projections were preliminary and economic analyses considering whether the potential benefits associated with injection outweigh the costs are in progress. A special local needs label is currently being pursued that would allow growers to utilize the injection method to apply oxytetracycline for mitigating HLB in Florida.
5. Conclusions
The results from this field study demonstrate that trunk injection of antibacterial therapies can drastically improve the health and productivity of HLB-affected trees suggesting its potential for use against this devastating disease or other systemic diseases where foliar sprays are ineffective. In contrast, injection of the insecticide imidacloprid was only temporarily effective against the HLB vector, but not effective in restoring tree health and productivity. The temporary benefit associated with imidacloprid injection suggests the potential for alternative insecticides with longer residual activity to be explored and combined with antibacterial therapies. The long-term effects of injuries associated with trunk injections on tree health remain to be determined.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12101592/s1, Table S1: Leaf, root, and bark Ct-values after trunk injections in October 2020 and April 2021.; Table S2: Harvest and fruit quality data for production seasons 2020/21 and 2021/22; Figure S1: Developmental stages of leaf flush for visual ratings; Figure S2: Fruit from the 2020/21 production season; Figure S3: Fruit from the 2021/22 production season; Figure S4: Phenotype of trees five months after the first injection; Figure S5: Phenotype of trees 12 months after the first injection.
Author Contributions
Conceptualization, L.A. and U.A.; Data curation, L.A.; Formal analysis, L.A.; Funding acquisition, U.A.; Investigation, L.A.; Methodology, L.A., J.Q. and U.A.; Project administration, U.A.; Resources, J.Q. and U.A.; Supervision, U.A.; Visualization, L.A.; Writing—original draft, L.A.; Writing—review and editing, J.Q. and U.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by USDA-NIFA-SCRI, grant #2019-70016-29096 and USDA-NIFA Hatch, grant #1011775.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bove, J. Huanglongbing: A Destructive, Newly-Emerging, Century-Old Disease of Citrus. J. Plant Pathol. 2006, 85, 7–37. [Google Scholar] [CrossRef]
- Gottwald, T.R.; da Graça, J.V.; Bassanezi, R.B. Citrus Huanglongbing: The Pathogen and Its Impact. Plant Health Prog. 2007, 8, 31. [Google Scholar] [CrossRef]
- Halbert, S.E.; Manjunath, K.L. Asian Citrus Psyllids (Sternorrhyncha: Psyllidae) and Greening Disease of Citrus: A Literature Review and Assessment of Risk in Florida. Fla. Entomol. 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Hall, D.G.; Richardson, M.L.; Ammar, E.D.; Halbert, S.E. Asian Citrus Psyllid, Diaphorina citri, Vector of Citrus Huanglongbing Disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Hall, D.G.; Albrigo, L.G. Estimating the Relative Abundance of Flush Shoots in Citrus with Implications on Monitoring Insects Associated with Flush. HortScience 2007, 42, 364–368. [Google Scholar] [CrossRef]
- Ma, W.; Pang, Z.; Huang, X.; Xu, J.; Pandey, S.S.; Li, J.; Achor, D.S.; Vasconcelos, F.N.C.; Hendrich, C.; Huang, Y.; et al. Citrus Huanglongbing Is a Pathogen-Triggered Immune Disease That Can Be Mitigated with Antioxidants and Gibberellin. Nat. Commun. 2022, 13, 529. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U.; Bowman, K.D. Gene Expression in Citrus sinensis (L.) Osbeck Following Infection with the Bacterial Pathogen Candidatus Liberibacter asiaticus Causing Huanglongbing in Florida. Plant Sci. 2008, 175, 291–306. [Google Scholar] [CrossRef]
- Kim, J.S.; Sagaram, U.S.; Burns, J.K.; Li, J.L.; Wang, N. Response of Sweet Orange (Citrus sinensis) to ‘Candidatus Liberibacter asiaticus’ Infection: Microscopy and Microarray Analyses. Phytopathology 2009, 99, 50–57. [Google Scholar] [CrossRef]
- Graham, J.; Gottwald, T.; Setamou, M. Status of Huanglongbing (HLB) Outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- Florida Citrus Statistics 2020–2021. Available online: https://www.nass.usda.gov/Statistics_by_State/Florida/Publications/Citrus/Citrus_Statistics/2020-21/fcs2021b.pdf (accessed on 6 September 2022).
- McCollum, G.; Baldwin, E. Huanglongbing: Devastating Disease of Citrus. In Horticultural Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 44, pp. 315–361. [Google Scholar]
- Killiny, N.; Hijaz, F.; Gonzalez-blanco, P.; Jones, S.E.; Pierre, M.O.; Vincent, C.I. Effect of Adjuvants on Oxytetracycline Uptake upon Foliar Application in Citrus. Antibiotics 2020, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pang, Z.; Duan, S.; Lee, D.; Kolbasov, V.G.; Wang, N. The in Planta Effective Concentration of Oxytetracycline against ‘Candidatus Liberibacter asiaticus’ for Suppression of Citrus Huanglongbing. Phytopathology 2019, 109, 2046–2054. [Google Scholar] [CrossRef]
- Zhang, M.; Karuppaiya, P.; Zheng, D.; Sun, X.; Bai, J.; Ferrarezi, R.S.; Powell, C.A.; Duan, Y. Field Evaluation of Chemotherapy on HLB-Affected Citrus Trees With Emphasis on Fruit Yield and Quality. Front. Plant Sci. 2021, 12, 611287. [Google Scholar] [CrossRef]
- Vincent, C.I.; Hijaz, F.; Pierre, M.; Killiny, N. Systemic Uptake of Oxytetracycline and Streptomycin in Huanglongbing-Affected Citrus Groves after Foliar Application and Trunk Injection. Antibiotics 2022, 11, 1092. [Google Scholar] [CrossRef]
- Zhang, M.; Powell, C.A.; Guo, Y.; Benyon, L.; Duan, Y. Characterization of the Microbial Community Structure in Candidatus Liberibacter asiaticus-Infected Citrus Plants Treated with Antibiotics in the Field. BMC Microbiol. 2013, 13, 112. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.; Powell, C.A.; Avery, P.B.; Wang, J.; Huang, Y.; Duan, Y. Field Evaluation of Integrated Management for Mitigating Citrus Huanglongbing in Florida. Front. Plant Sci. 2019, 9, 1890. [Google Scholar] [CrossRef]
- Shin, K.; Ascunce, M.S.; Narouei-Khandan, H.A.; Sun, X.; Jones, D.; Kolawole, O.O.; Goss, E.M.; van Bruggen, A.H.C. Effects and Side Effects of Penicillin Injection in Huanglongbing Affected Grapefruit Trees. Crop Prot. 2016, 90, 106–116. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Hijaz, F.; Nehela, Y.; Batuman, O.; Killiny, N. Uptake, Translocation, and Stability of Oxytetracycline and Streptomycin in Citrus Plants. Antibiotics 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- McVay, J.; Sun, X.; Jones, D.; Urbina, H.; Aldeek, F.; Cook, J.M.; Jeyaprakash, A.; Hodges, G.; Smith, T. Limited Persistence of Residues and Metabolites in Fruit and Juice Following Penicillin Trunk Infusion in Citrus Affected by Huanglongbing. Crop Prot. 2019, 125, 104753. [Google Scholar] [CrossRef]
- Li, J.; Kolbasov, V.G.; Lee, D.; Pang, Z.; Huang, Y.; Collins, N.; Wang, N. Residue Dynamics of Streptomycin in Citrus Delivered by Foliar Spray and Trunk Injection and Effect on “Candidatus Liberibacter asiaticus” Titer. Phytopathology 2021, 111, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Powell, C.A.; Zhou, L.; He, Z.; Stover, E.; Duan, Y. Chemical Compounds Effective against the Citrus Huanglongbing Bacterium “Candidatus Liberibacter asiaticus” in Planta. Phytopathology 2011, 101, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, N. Evaluation of the Spatiotemporal Dynamics of Oxytetracycline and Its Control Effect against Citrus Huanglongbing via Trunk Injection. Phytopathology 2016, 106, 1495–1503. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, J.; Wang, N. Control of Citrus Huanglongbing via Trunk Injection of Plant Defense Activators and Antibiotics. Phytopathology 2018, 108, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.L.; Ager, K.L. Biological Control of Citrus Huanglongbing with Eb92-1, a Benign Strain of Xylella fastidiosa. Plant Dis. 2021, 105, 2914–2918. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.L.; da Silva, D.R.; Pagliai, F.A.; Pan, L.; Padgett-Pagliai, K.A.; Blaustein, R.A.; Merli, M.L.; Zhang, D.; Pereira, C.; Teplitski, M.; et al. Assessment of Unconventional Antimicrobial Compounds for the Control of ‘Candidatus Liberibacter asiaticus’, the Causative Agent of Citrus Greening Disease. Sci. Rep. 2020, 10, 5395. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ashworth, V.E.T.M.; Geitner, N.K.; Wiesner, M.R.; Ginnan, N.; Rolshausen, P.; Roper, C.; Jassby, D. Delivery, Fate, and Mobility of Silver Nanoparticles in Citrus Trees. ACS Nano 2020, 14, 2966–2981. [Google Scholar] [CrossRef] [PubMed]
- Stephano-Hornedo, J.L.; Torres-Gutiérrez, O.; Toledano-Magaña, Y.; Gradilla-Martínez, I.; Pestryakov, A.; Sánchez-González, A.; García-Ramos, J.C.; Bogdanchikova, N. ArgovitTM Silver Nanoparticles to Fight Huanglongbing Disease in Mexican Limes (Citrus aurantifolia Swingle). RSC Adv. 2020, 10, 6146–6155. [Google Scholar] [CrossRef]
- Archer, L.; Albrecht, U.; Crane, J. Trunk Injection to Deliver Crop Protection Materials: An Overview of Basic Principles and Practical Considerations: HS1426, 11/2021. UF/IFAS EDIS 2021. pp. 1–7. Available online: https://edis.ifas.ufl.edu/publication/HS1426 (accessed on 6 September 2022). [CrossRef]
- Archer, L.; Crane, J.H.; Albrecht, U. Trunk Injection as a Tool to Deliver Plant Protection Materials—An Overview of Basic Principles and Practical Considerations. Horticulturae 2022, 8, 552. [Google Scholar] [CrossRef]
- Berger, C.; Laurent, F. Trunk Injection of Plant Protection Products to Protect Trees from Pests and Diseases. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Zamora, M.A.S.; Escobar, R.F. Injector-Size and the Time of Application Affects Uptake of Tree Trunk-Injected Solutions. Sci. Hortic. 2000, 84, 163–177. [Google Scholar] [CrossRef]
- Wise, J.C.; VanWoerkom, A.H.; Acimovic, S.G.; Sundin, G.W.; Cregg, B.M.; Vandervoort, C.V. Trunk Injection: A Discriminating Delivering System for Horticulture Crop IPM. Entomol. Ornithol. Herpetol. 2014, 3, 1. [Google Scholar] [CrossRef]
- Tarjan, A. Pressure Injection of Chemicals for Possible Systemic Action against Burrowing Nematodes Infecting Citrus. Plant Dis. Rep. 1959, 43, 451–458. [Google Scholar]
- Van Vuuren, S.P. The Determination of Optimal Concentration and PH of Tetracycline Hydrochloride for Trunk Injection of Greening-Infected Citrus Trees. Phytophylactica 1977, 9, 77–81. [Google Scholar]
- Buitendag, C.; Bronkhorst, G. Injection of Insecticides into Tree Trunks—A Possible New Method for the Control of Citrus Pests? Citrus Subtrop. Fruit J. 1980, 556, 5–7. [Google Scholar]
- Schwarz, R.E.; Moll, J.N.; van Vuuren, S.P. Control of Citrus Greening and Its Psylla Vector by Trunk Injections of Tetracyclines and Insecticides. Int. Organ. Citrus Virol. Conf. Proc. 1957–2010 1974, 6, 26–29. [Google Scholar] [CrossRef]
- Puttamuk, T.; Zhang, S.; Duan, Y.; Jantasorn, A.; Thaveechai, N. Effect of Chemical Treatments on “Candidatus Liberibacter asiaticus” Infected Pomelo (Citrus maxima). Crop Prot. 2014, 65, 114–121. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, Y.; Powell, C.A.; Doud, M.S.; Yang, C.; Duan, Y. Effective Antibiotics against “Candidatus Liberibacter asiaticus” in HLB-Affected Citrus Plants Identified via the Graft-Based Evaluation. PLoS ONE 2014, 9, e111032. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, R.A.; Lorca, G.L.; Teplitski, M. Challenges for Managing Candidatus Liberibacter Spp. (Huanglongbing Disease Pathogen): Current Control Measures and Future Directions. Phytopathology 2018, 108, 425–435. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Duffy, B. Use of Antibiotics in Plant Agriculture. OIE Rev. Sci. Tech. 2012, 31, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Bahder, B.W.; Helmick, E.E.; Chakrabarti, S.; Osorio, S.; Soto, N.; Chouvenc, T.; Harrison, N.A. Disease Progression of a Lethal Decline Caused by the 16SrIV-D Phytoplasma in Florida Palms. Plant Pathol. 2018, 67, 1821–1828. [Google Scholar] [CrossRef]
- Gurr, G.M.; Johnson, A.C.; Ash, G.J.; Wilson, B.A.L.; Ero, M.M.; Pilotti, C.A.; Dewhurst, C.F.; You, M.S. Coconut Lethal Yellowing Diseases: A Phytoplasma Threat to Palms of Global Economic and Social Significance. Front. Plant Sci. 2016, 7, 1521. [Google Scholar] [CrossRef]
- Boina, D.R.; Bloomquist, J.R. Chemical Control of the Asian Citrus Psyllid and of Huanglongbing Disease in Citrus. Pest Manag. Sci. 2015, 71, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.P.; Yamamoto, P.T.; Garcia, R.B.; Lopes, J.P.; Lopes, J.R. Thiamethoxam and Imidacloprid Drench Applications on Sweet Orange Nursery Trees Disrupt the Feeding and Settling Behaviour of Diaphorina citri (Hemiptera: Liviidae). Pest Manag. Sci. 2016, 72, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, J.A.; Kostyk, B.C.; Stansly, P.A. Insecticidal Suppression of Asian Citrus Psyllid Diaphorina citri (Hemiptera: Liviidae) Vector of Huanglongbing Pathogens. PLoS ONE 2014, 9, e112331. [Google Scholar] [CrossRef]
- Chen, X.D.; Gill, T.A.; Pelz-Stelinski, K.S.; Stelinski, L.L. Risk Assessment of Various Insecticides Used for Management of Asian Citrus Psyllid, Diaphorina citri in Florida Citrus, against Honey Bee, Apis Mellifera. Ecotoxicology 2017, 26, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Mota-Sanchez, D.; Cregg, B.M.; McCullough, D.G.; Poland, T.M.; Hollingworth, R.M. Distribution of Trunk-Injected 14C-Imidacloprid in Ash Trees and Effects on Emerald Ash Borer (Coleoptera: Buprestidae) Adults. Crop Prot. 2009, 28, 655–661. [Google Scholar] [CrossRef]
- VanWoerkom, A.H.; Aćimović, S.G.; Sundin, G.W.; Cregg, B.M.; Mota-Sanchez, D.; Vandervoort, C.; Wise, J.C. Trunk Injection: An Alternative Technique for Pesticide Delivery in Apples. Crop Prot. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Martin, D.K.H.; Turcotte, R.M.; Meredith, C.L.; Munck, I.A. Choosing an Adequate Pesticide Delivery System for Managing Pathogens with Difficult Biologies: Case Studies on Diplodia corticola, Venturia inaequalis and Erwinia amylovora. In Plant Diseases—Current Threats and Management Trends; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Byrne, F.J.; Almanzor, J.; Tellez, I.; Eskalen, A.; Grosman, D.M.; Morse, J.G. Evaluation of Trunk-Injected Emamectin Benzoate as a Potential Management Strategy for Kuroshio Shot Hole Borer in Avocado Trees. Crop Prot. 2020, 132, 105136. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Vanwoerkom, A.H.; Reeb, P.D.; Vandervoort, C.; Garavaglia, T.; Cregg, B.M.; Wise, J.C. Spatial and Temporal Distribution of Trunk-Injected Imidacloprid in Apple Tree Canopies. Pest Manag. Sci. 2014, 70, 1751–1760. [Google Scholar] [CrossRef]
- Kleier, D.A. Phloem Mobility of Xenobiotics I. Mathematical Model Unifying the Weak Acid and Intermediate Permeability Theories. Plant Physiol. 1988, 86, 803–810. [Google Scholar] [CrossRef]
- Riederer, M. Uptake and Transport of Xenobiotics. In Plant Toxicology; Hock, B., Elstner, E.F., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 131–150. [Google Scholar]
- Tyree, M.T.; Peterson, C.A.; Edgington, L.V. A Simple Theory Regarding Ambimobility of Xenobiotics with Special Reference to the Nematicide, Oxamyl. Plant Physiol. 1979, 63, 367–374. [Google Scholar] [CrossRef]
- Qureshi, J.A.; Stansly, P.A. Dormant Season Foliar Sprays of Broad-Spectrum Insecticides: An Effective Component of Integrated Management for Diaphorina citri (Hemiptera: Psyllidae) in Citrus Orchards. Crop Prot. 2010, 28, 860–866. [Google Scholar] [CrossRef]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative Real-Time PCR for Detection and Identification of Candidatus Liberibacter Species Associated with Citrus Huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Ayers, J.E.; Tomes, M.L. Effect of 2 Uniform Ripening Genes on Chlorophyll and Carotenoid Contents of Tomato Fruit. J. Am. Soc. Hortic. Sci. 1966, 88, 550–556. [Google Scholar]
- Bai, J.; Baldwin, E.; Plotto, A.; Manthey, J.; McCollum, G.; Irey, M.; Luzio, G. Influence of Harvest Time on Quality of ‘Valencia’ Oranges and Juice. Proc. Fla. State Hortic. Soc. 2009, 122, 308–315. [Google Scholar]
- Leyva, A.; Quintana, A.; Sánchez, M.; Rodríguez, E.N.; Cremata, J.; Sánchez, J.C. Rapid and Sensitive Anthrone-Sulfuric Acid Assay in Microplate Format to Quantify Carbohydrate in Biopharmaceutical Products: Method Development and Validation. Biologicals 2008, 36, 134–141. [Google Scholar] [CrossRef]
- Tixier, A.; Sperling, O.; Orozco, J.; Lampinen, B.; Amico Roxas, A.; Saa, S.; Earles, J.M.; Zwieniecki, M.A. Spring Bud Growth Depends on Sugar Delivery by Xylem and Water Recirculation by Phloem Münch Flow in Juglans regia. Planta 2017, 246, 495–508. [Google Scholar] [CrossRef]
- Davidson, A.M.; Le, S.T.; Cooper, K.B.; Lange, E.; Zwieniecki, M.A. No Time to Rest: Seasonal Dynamics of Non-Structural Carbohydrates in Twigs of Three Mediterranean Tree Species Suggest Year-Round Activity. Sci. Rep. 2021, 11, 5181. [Google Scholar] [CrossRef]
- Hijaz, F.; Nehela, Y.; Batuman, O.; Killiny, N. Detection of Oxytetracycline in Citrus Phloem and Xylem Saps Using Europium-Based Method. Antibiotics 2021, 10, 1036. [Google Scholar] [CrossRef]
- Bhandari, B.P.; Cheng, Z. Lobate Lac Scale, Paratachardina pseudolobata (Hemiptera: Keriidae), in Hawaii’s Urban Landscape: Hosts and Management. Int. J. Trop. Insect Sci. 2018, 38, 71–76. [Google Scholar] [CrossRef]
- Fu, B.; Qiu, H.; Li, Q.; Tang, L.; Zeng, D.; Liu, K.; Gao, Y. Flower Injection of Imidacloprid and Spirotetramat: A Novel Tool for the Management of Banana Thrips Thrips hawaiiensis. J. Pest Sci. 2020, 93, 1073–1084. [Google Scholar] [CrossRef]
- Harrell, M. Imidacloprid Concentrations in Green Ash (Fraxinus pennsylvanica) Following Treatments with Two Trunk-Injection Methods. Arboric. Urban For. 2006, 32, 126–129. [Google Scholar] [CrossRef]
- McCullough, D.G.; Poland, T.M.; Tluczek, A.R.; Anulewicz, A.; Wieferich, J.; Siegert, N.W. Emerald Ash Borer (Coleoptera: Buprestidae) Densities over a 6-Yr Period on Untreated Trees and Trees Treated with Systemic Insecticides at 1-, 2-, and 3-Yr Intervals in a Central Michigan Forest. J. Econ. Entomol. 2019, 112, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Cowles, R.S.; Montgomery, M.E.; Cheah, C.A.S.-J. Activity and Residues of Imidacloprid Applied to Soil and Tree Trunks to Control Hemlock Woolly Adelgid (Hemiptera: Adelgidae) in Forests. J. Econ. Entomol. 2009, 99, 1258–1267. [Google Scholar] [CrossRef]
- Turcotte, R.M.; Lagalante, A.; Jones, J.; Cook, F.; Elliott, T.; Billings, A.A.; Park, Y.L. Spatial and Temporal Distribution of Imidacloprid within the Crown of Eastern Hemlock. J. Insect Sci. 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tattar, T.; Tattar, S. Evidence for the Downward Movement of Materials Injected into Trees. Arboric. Urban For. 1999, 25, 325–332. [Google Scholar] [CrossRef]
- Boina, D.R.; Onagbola, E.O.; Salyani, M.; Stelinski, L.L. Antifeedant and Sublethal Effects of Imidacloprid on Asian Citrus Psyllid, Diaphorina citri. Pest Manag. Sci. 2009, 65, 870–877. [Google Scholar] [CrossRef]
- Grosman, D.M.; Eskalen, A.; Brownie, C. Evaluation of Emamectin Benzoate and Propiconazole for Management of a New Invasive Shot Hole Borer (Euwallacea Nr. fornicatus, Coleoptera: Curculionidae) and Symbiotic Fungi in California Sycamores. J. Econ. Entomol. 2019, 112, 1267–1273. [Google Scholar] [CrossRef]
- Soto, N.; Humphries, A.R.; Mou, D.F.; Helmick, E.E.; Glover, J.P.; Bahder, B.W. Effect of Oxytetracycline-Hydrochloride on Phytoplasma Titer and Symptom Progression of the 16SrIV-D Phytoplasma in Cabbage Palms from Florida. Plant Dis. 2020, 104, 2330–2337. [Google Scholar] [CrossRef]
- Christiano, R.S.C.; Reilly, C.C.; Miller, W.P.; Scherm, H. Oxytetracycline Dynamics on Peach Leaves in Relation to Temperature, Sunlight, and Simulated Rain. Plant Dis. 2010, 94, 1213–1218. [Google Scholar] [CrossRef]
- Etxeberria, E.; Gonzalez, P.; Singerman, A.; Ebert, T. An Improved Method to Track Changes of Candidatus Liberibacter asiaticus Titer in HLB-Affected Citrus Trees. HortScience 2019, 54, 1357–1360. [Google Scholar] [CrossRef]
- Romero, C. Bark Structure and Functional Ecology. Bark: Use, Management and Commerce in Africa; New York Botanical Garden Press: New York, NY, USA, 2014; p. 17. [Google Scholar]
- Peres, N.A.; Dewdney, M.M. 2021–2022 Florida Citrus Production Guide: Postbloom Fruit Drop: CG007/PP-45, Rev. 4/2021; UF/IFASEDIS 2021; University of Florida George A Smathers Libraries: Gainesville, FL, USA, 2022. [Google Scholar]
- Stover, E.; Lin, Y.; Yang, X.; Vashisth, T. Hydrogen Cyanamide on Citrus: Preliminary Data on Phytotoxicity and Influence on Flush in Potted and Field Trees. Horttechnology 2016, 26, 839–845. [Google Scholar] [CrossRef]
- Hall, D.G.; Albrecht, U.; Bowman, K.D. Transmission Rates of “Ca. Liberibacter asiaticus” by Asian Citrus Psyllid Are Enhanced by the Presence and Developmental Stage of Citrus Flush. J. Econ. Entomol. 2016, 109, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Singh, G.; Dewdney, M.; Vashisth, T. Effects of Exogenous Gibberellic Acid in Huanglongbing-Affected Sweet Orange Trees under Florida Conditions—I. Flower Bud Emergence and Flower Formation. HortScience 2021, 56, 1531–1541. [Google Scholar] [CrossRef]
- Albrigo, L.G.; Stover, E.W. Effect of Plant Growth Regulators and Fungicides on Huanglongbing-Related Preharvest Fruit Drop of Citrus. Horttechnology 2015, 25, 785–790. [Google Scholar] [CrossRef]
- Zhao, W.; Gottwald, T.; Bai, J.; McCollum, G.; Irey, M.; Plotto, A.; Baldwin, E. Correlation of Diplodia (Lasiodiplodia theobromae) Infection, Huanglongbing, Ethylene Production, Fruit Removal Force and Pre-Harvest Fruit Drop. Sci. Hortic. 2016, 212, 162–170. [Google Scholar] [CrossRef]
- Tang, L.; Singh, S.; Vashisth, T. Association between Fruit Development and Mature Fruit Drop in Huanglongbing-Affected Sweet Orange. HortScience 2020, 55, 851–857. [Google Scholar] [CrossRef]
- Tang, L.; Chhajed, S.; Vashisth, T. Preharvest Fruit Drop in Huanglongbing-Affected ‘Valencia’ Sweet Orange. J. Am. Soc. Hortic. Sci. 2019, 144, 107–117. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R.; et al. Physiology of Citrus Fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.; Brlansky, R.H.; Gmitter, F.G.; Li, Z.G. Changes in Carbohydrate Metabolism in Citrus sinensis Infected with ‘Candidatus Liberibacter asiaticus’. Plant Pathol. 2010, 59, 1037–1043. [Google Scholar] [CrossRef]
- Ford, C.R.; Vose, J.M.; Daley, M.; Phillips, N. Use of Water by Eastern Hemlock: Implications for Systemic Insecticide Application. Arboric. Urban For. 2007, 33, 421–427. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W.; Nkedi-Kizza, P.; Obreza, T.A. Water Use in Drip- and Microsprinkler-Irrigated Citrus Trees. Soil Sci. Soc. Am. J. 2014, 78, 1351–1361. [Google Scholar] [CrossRef]
- Hamido, S.A.; Morgan, K.T.; Kadyampakeni, D.M. The Effect of Huanglongbing on Young Citrus Tree Water Use. Horttechnology 2017, 27, 659–665. [Google Scholar] [CrossRef]
- Li, S.; Wu, F.; Duan, Y.; Singerman, A.; Guan, Z. Citrus Greening: Management Strategies and Their Economic Impact. HortScience 2020, 55, 604–612. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).