1. Introduction
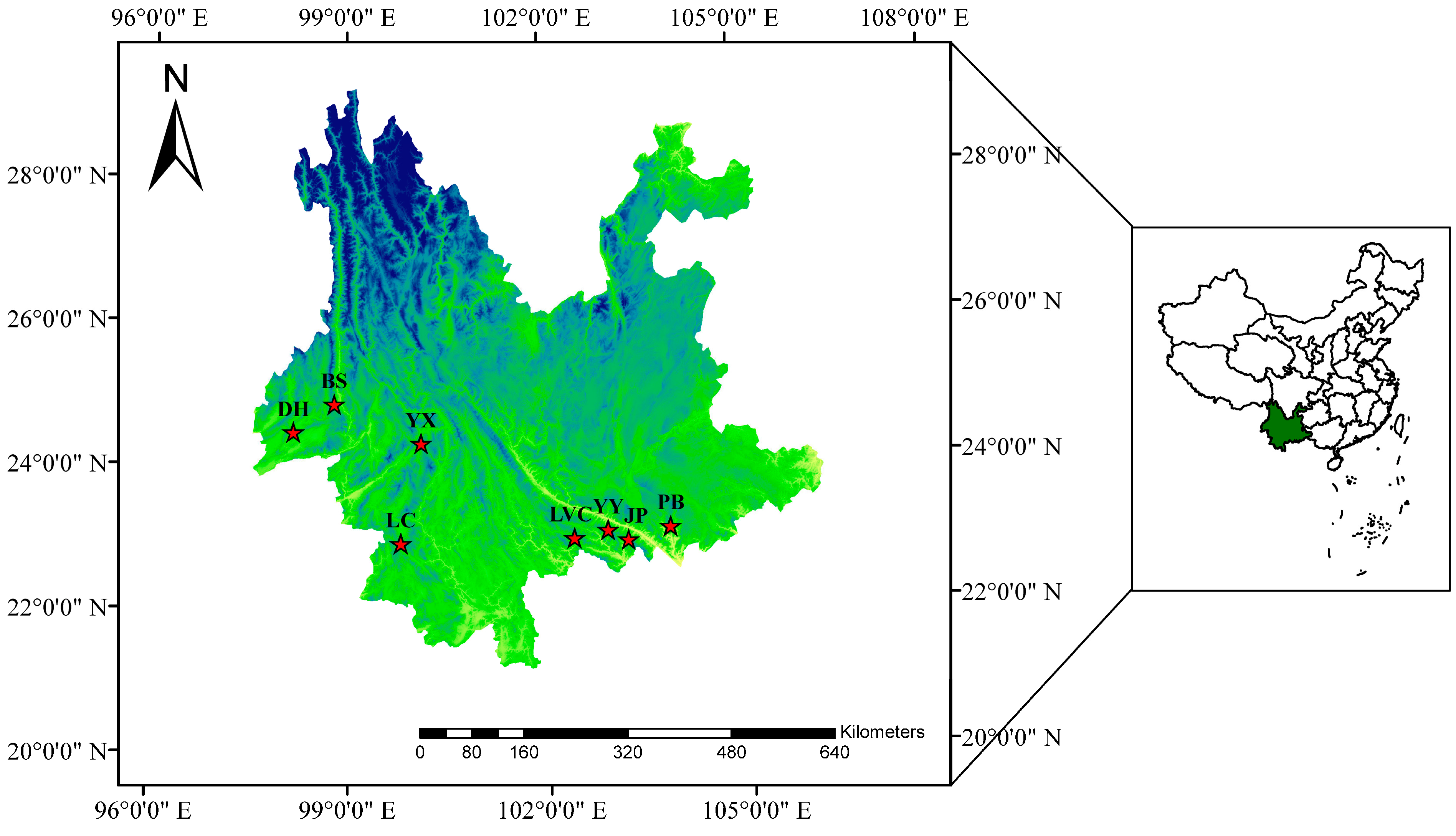
Amomum tsao-ko (2n = 4x = 48) is a medicinal and aromatic plant of the Zingiberaceae family, mainly distributed in the high-altitude mountains (1100 to 1800 m above sea level) of southern Yunnan, China and northern Vietnam and Laos (
Figure 1) [
1]. The ripe fruit of
A. tsao-ko (also called Cao-Guo in China) is a commonly used Chinese herbal medicine for aromatizing dampness in clinical practice [
2]. Pharmacological studies have shown that it has anti-oxidation [
3], antibacterial [
4], anti-inflammation [
5], antidiabetic [
6], anti-tumor [
7], and anticonvulsant properties [
8]. In addition,
A. tsao-ko has a strong spicy and herbal fragrance, which can remove the unpleasant smell of meat ingredients and increase people’s appetite. It is an important condiment in countries such as China, South Korea, and Japan and is known as “one of the five spices” of food seasoning [
9]. As an aromatic Chinese herbal medicine used for both medicinal and edible purposes, essential oil is the most important active ingredient of
A. tsao-ko, and its content determines the quality of
A. tsao-ko (Pharmacopoeia of the People’s Republic of China, 2020).
Molecular marker technology is widely used in genetic diversity analysis, germplasm resource identification, and molecular marker-assisted breeding [
10]. Random amplified polymorphic DNA (RAPD), simple sequence repeat (SSR), inter-simple sequence repeats (ISSR), and amplified fragment length polymorphism (AFLP) are the four most widely used molecular markers [
11]. However, they are all random DNA molecular markers and are not usually located in gene regions, which limits their application. Targeted molecular marker technology is a new approach that is the result of in-depth development of functional genomics and large-scale sequencing [
12]. Amplification of functional regions or regulatory regions is biased towards generating candidate functional markers, and the resulting markers may be part of a target gene or closely linked to a target gene. This technology can be used across different species and requires little or no genomic sequence information, which has attracted increasing attention from researchers [
13].
Promoter anchored amplified polymorphism (PAAP) based on RAPD markers is a novel dominant DNA marker technology that specifically targets the regulatory regions of a plant genome [
14]. PAAP molecular marker technology uses two primers for PCR amplification, including a 10 nucleotide-long RAPD primer and a 10 nucleotide-long degenerate promoter primer based on conserved core promoter sequences. This marker system can be used to identify and map promoter regions throughout the genome. At present, this approach has been successfully applied in genetic analysis of cotton [
14,
15], foxtail millet [
16], soybean [
17], and other crops. The dominant molecular marker method of conserved DNA-derived polymorphism (CDDP) provides rapid identification of different species by designing primers based on the conserved amino acid sequences of functional genes and gene families in plants [
18]. The amplified fragment from CDDP may be part of the target gene or closely linked to the gene, so CDDP markers have obvious advantages over random markers in the evaluation of plant genetic diversity and quantitative trait locus (QTL) mapping. CDDP markers have been successfully used for genetic diversity analysis of plant germplasm resources such as
Salix taishanensis [
19],
Chrysanthemum morifolium [
20],
Musa [
21], and
Pistacia vera L. [
22].
As an important medicinal and aromatic crop, previous studies of
A. tsao-ko mainly focused on chemical components and pharmacological studies [
2]. However, assessment of the genetic diversity of
A. tsao-ko is lacking, which limits the selection of desirable germplasm and breeding of
A. tsao-ko. Recently, we have conducted several studies of the genetic diversity of
A. tsao-ko, including phenotypic analysis [
23] and RAPD [
24], ISSR [
25], SRAP [
25], and SSR [
1] molecular marker analyses. Compared with dominant markers such as RAPD and ISSR, the primer sequences of PAAP and CDDP markers are specially designed for the regulatory elements and conserved sequences of plant functional genes, and can effectively generate functional molecular markers linked to target traits. It has important application value in genetic studies of plants without reference genomes. However, to our knowledge, the utility of PAAP and CDDP markers for genetic diversity assessment in
A. tsao-ko has not yet been reported. In this study, PAAP and CDDP primers were screened, and 96 germplasms collected from eight populations of
A. tsao-ko were amplified to reveal polymorphisms of these two markers in the germplasm and to determine the genetic diversity and population structure of these germplasm resources. In addition, the utility of the two marker types were compared. Our aims were to provide the theoretical basis for the application of PAAP and CDDP technology in the evaluation of
A. tsao-ko germplasm.
4. Discussion
Genetic variation within a species is necessary for evolutionary adaptation to environmental changes [
35]. Therefore, evaluation of genetic diversity is an important part of germplasm identification and collection [
36]. As a minor crop with homologous medicine and food, the genetic research on
A. tsao-ko is relatively behind. We have used the dominant markers, RAPD, ISSR, and SRAP markers, to identify the genetic diversity of
A. tsao-ko [
24,
25]. However, compared with other major crops, the available marker types and marker numbers are insufficient, which limits the evaluation, identification, and collection of
A. tsao-ko germplasm resources. Therefore, mining and identifying different types of molecular markers can enrich the types and quantities of available molecular markers in
A. tsao-ko, which is of great significance to the research and utilization of this crop. In this study, two novel targeted molecular markers, CDDP and PAAP, were used to analyze the genetic diversity of 96
A. tsao-ko accessions from eight major production areas in China. The number of polymorphic bands (203),
PR (99.48%),
PIC (0.240), and
RP (5.909) of the CDDP markers were higher than those of the PAAP markers (98, 89.09%, 0.214, and 2.870, respectively). The difference in resolution of the two marker techniques may be related to different amplification principles. CDDP generally selects conserved DNA sequences of genes or gene families related to biotic and abiotic stresses and plant development as anchor sites for primer development; most of these genes are multigene families. In contrast, PAAP is a molecular marker specifically developed for the core conserved sequence of regulatory sequence promoters [
14,
18]. The polymorphic ratio (99.48%) generated by CDDP was similar to that of RAPD (98.98%), SRAP (99.29%), and ISSR (99.46%), which indicated that the studied
A. tsao-ko accessions had abundant genetic variations. In comparison with our previous study, CDDP primers showed a higher average
PIC value than the RAPD (0.197) [
24] and ISSR (0.232) [
25] primers but a lower value than the SRAP (0.338) markers [
25]; the mean
PIC value of the PAAP primers was higher than that obtained by the RAPD primers. This result suggests that both PAAP and CDDP markers are useful tools for exploring genetic diversity and determining the genetic relationships of the
A. tsao-ko germplasm.
The average percentage of polymorphic sites, expected heterozygosity, and Shannon′s index at the population level (
Ppop,
Hpop, and
Ipop) are three important parameters used in the study of plant population genetic diversity. Zhang and Yang [
37] presented a statistical analysis of common genetic diversity parameters in multiple plant species. At the population level, the mean percentage of polymorphic loci (
Ppop), gene diversity (
Hpop), and Shannon’s information index (
Ipop) were 40.3%, 0.123, and 0.186, respectively. Our study reveals a relatively high level of
Ppop (60.56% for PAAP, 62.32% for CDDP) in the eight
A. tsao-ko populations;
Hpop (0.177 for PAAP, 0.164 for CDDP) and
Ipop (0.272 for PAAP, 0.259 for CDDP) are higher than the average genetic diversity of other plant populations based on the ISSR markers. In this study, the JP population showed a relatively high level of genetic diversity by the PAAP and CDDP markers, which is consistent with our previous results for RAPD [
24], SRAP, and ISSR [
25] markers. Jinping County has a long history of
A. tsao-ko cultivation, more than 400 years, and it is generally believed that
A. tsao-ko originated in Jinping, which is consistent with the high genetic diversity of germplasm in this area [
25].
Genetic differentiation refers to the distribution of genetic diversity within and between populations [
38]. The degree of genetic differentiation among populations can be evaluated by the genetic differentiation coefficient
Gst; when
Gst > 0.25, the degree of differentiation among populations is very high [
39]. The results of this study showed that only 15.10% (
Gst = 0.151 for PAAP) and 12.8% (
Gst = 0.128 for CDDP) of the total genetic variation was shared among the eight
A. tsao-ko populations, and within population genetic differentiation was 84.90% (PAAP) and 87.2% (CDDP). The results of AMOVA using PAAP and CDDP markers also demonstrated that most of the variability among the accessions was within the populations. Higher genetic diversity within a population is more beneficial for the conservation of a species. Therefore, to manage and protect
A. tsao-ko germplasm resources more effectively, germplasm resource nurseries should be established in areas with high genetic diversity, such as Jinping County. Gene flow is the main factor affecting the genetic structure of the population; when gene flow is greater than one, genetic differentiation among populations caused by genetic drift is avoidable [
40]. In this study, the high gene flow (
Nm = 2.815 for PAAP,
Nm = 3.412 for CDDP) among the populations indicated that gene exchange between different populations was frequent; this is also consistent with the high genetic identity (
GI) revealed by the PAAP (average
GI = 0.957) and CDDP (average
GI = 0.967) markers. The high gene flow may be related to the cross-pollination breeding characteristics and artificial germplasm exchange of
A. tsao-ko [
25,
41,
42].
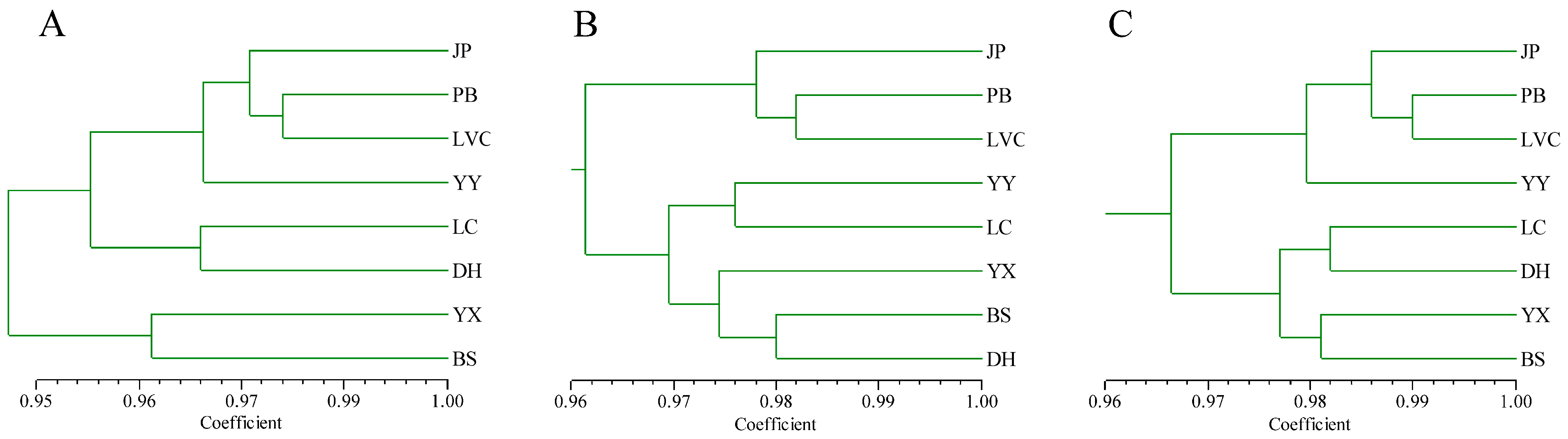
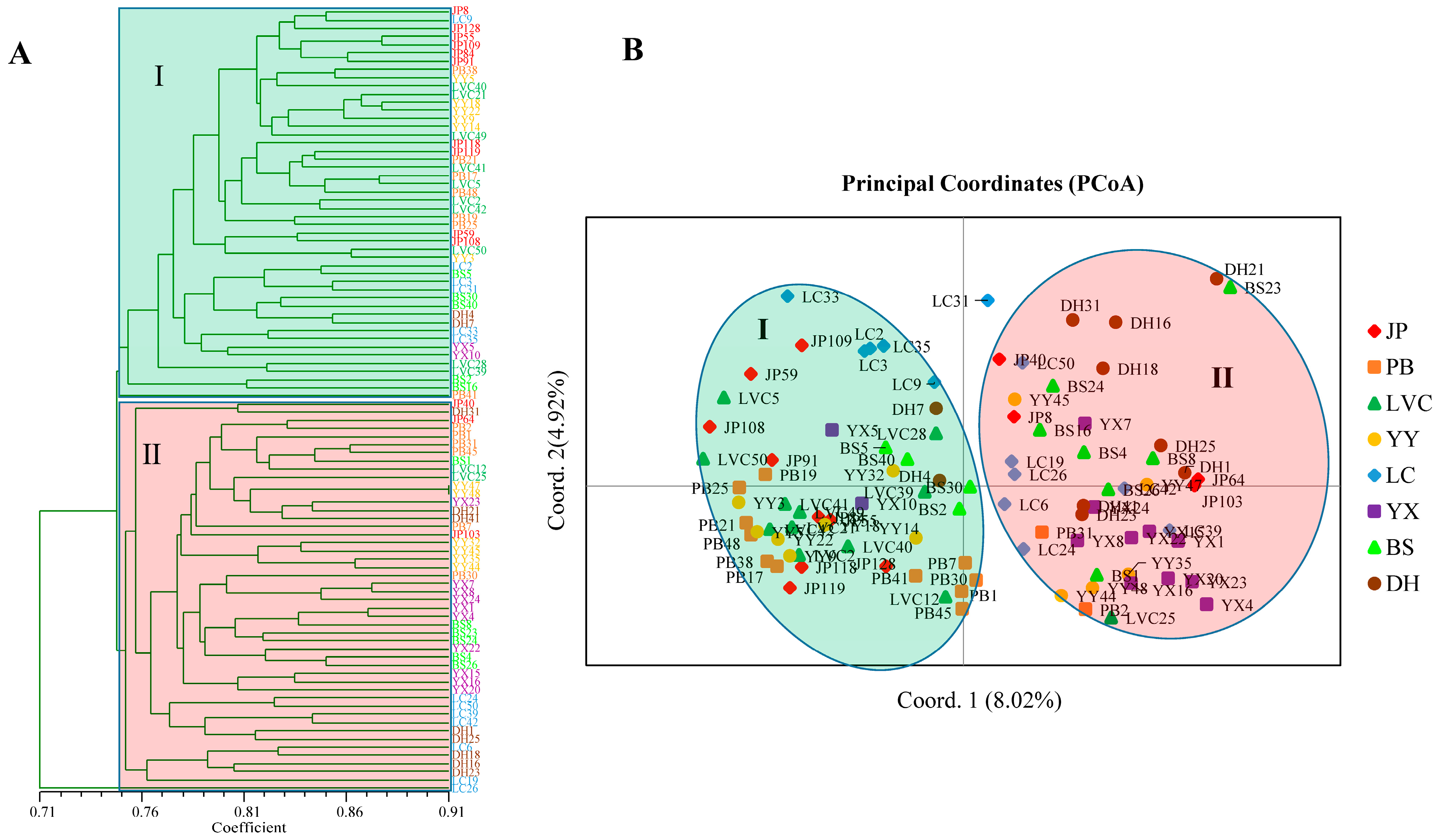
Cluster analysis and PCoA were helpful for understanding the relationships among the 96
A. tsao-ko accessions. Both UPGMA cluster and PCoA analyses divided the 96 accessions into two groups based on the combined PAAP and CDDP marker data. The accessions from different populations were distributed in different groups and were not clustered strictly by geographic origin. This result was consistent with high within-population variation, genetic identity, and gene flow and was found to be in agreement with the results obtained using PAPD, ISSR, and SRAP markers [
24,
25]. Furthermore, we noticed that some accessions from the same population tend to have the highest similarity, such as YY47 and YY48, YX7 and YX8, and JP55 and JP109. Although the linear distance between the collections is not less than 50 m when we sampled in the same population to reduce the possibility of sampling from the same clone, there is still a concern that a small number of accessions have similar genetic backgrounds. It will be necessary to expand the sampling distance between samples within the same population in the future. Population genetic structure is the distribution of each subgroup within the population and reflects the structural characteristics of subgroups. When the Q (the corresponding membership coefficient) value of accessions in a certain group is greater than or equal to 0.6, it is considered that the ancestry of the accession is relatively pure, otherwise the ancestry of the accession is considered to be complex [
43]. In this study, 14.58% (PAAP), 15.63% (CDDP), and 14.58% (PAAP + CDDP) of accessions showed mixed ancestry (Q < 0.6) from the STRUCTURE analysis. This finding further shows that most of the germplasms of
A. tsao-ko are relatively simple in origin, and most of the genetic variation occurs within populations or geographical regions. This result is also consistent with the result of genetic differentiation in
A. tsao-ko populations.