Effects of Symbiotic Fungi on Sugars and Soil Fertility and Structure-Mediated Changes in Plant Growth of Vicia villosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Fungal Agents
2.3. Plant Culture
2.4. Determination of Root Fungal Colonization Rate and Plant Growth
2.5. Determination of Chlorophyll and Carbohydrate Concentrations
2.6. Determination of Soil Nutrients, Aggregate Size Distribution, and Aggregate Stability
2.7. Data Analysis
3. Results
3.1. Changes in Root Fungal Colonization Rate
3.2. Changes in Growth Performance
3.3. Changes in Root Morphological Variables
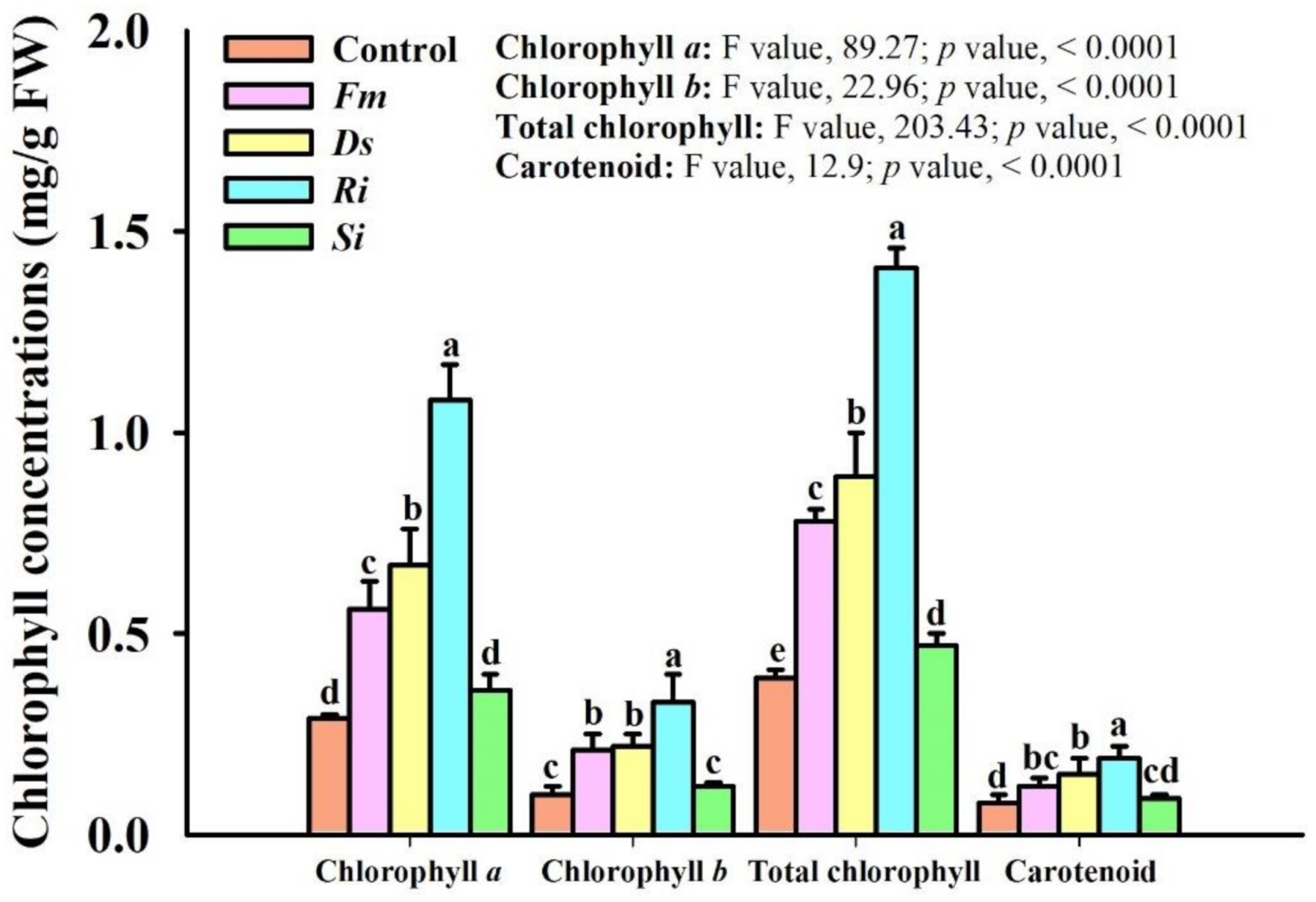
3.4. Changes in Leaf Chlorophyll Concentrations
3.5. Changes in Leaf and Root Sugar Concentrations
3.6. Changes in Soil Nutrients
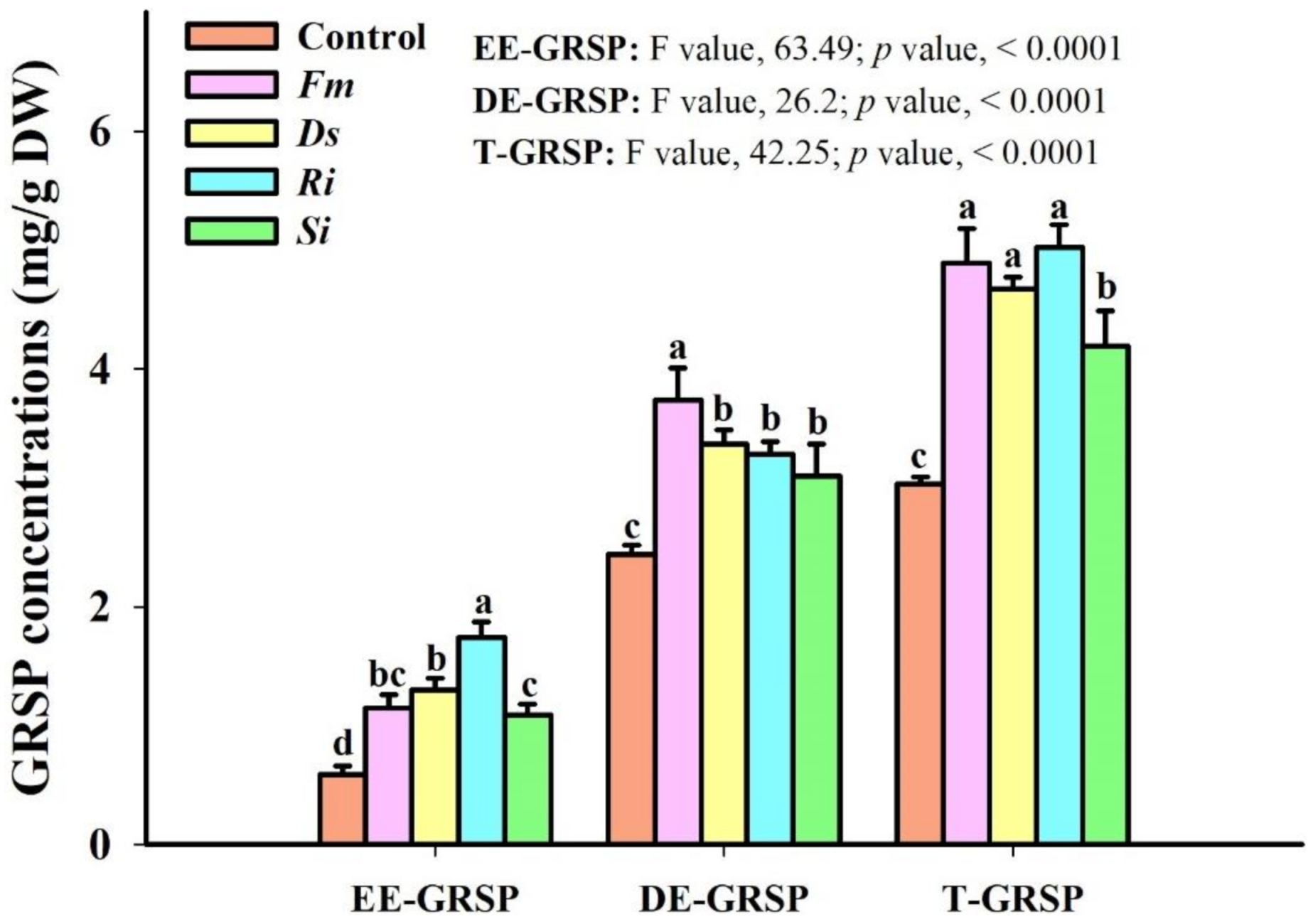
3.7. Changes in Soil GRSP Levels
3.8. Changes in Soil WSA Distribution and Aggregate Stability
3.9. Correlationship Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.F.; Gao, X.L.; Nan, Z.B.; Zhang, Z.X. Potential value of the common vetch (Vicia sativa L.) as an animal feedstuff: A review. J. Anim. Physiol. Anim. Nutr. 2017, 101, 807–823. [Google Scholar] [CrossRef] [PubMed]
- Greveniotis, V.; Bouloumpasi, E.; Zotis, S.; Korkovelos, A.; Ipsilandis, C.G. Assessment of interactions between yield components of common vetch cultivars in both conventional and low-input cultivation systems. Agriculture 2021, 11, 369. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, L.; Duan, Y.; Zhang, J.; Evers, J.B.; Zhang, Y.; Su, Z.; Werf, W. Intercropping potato (Solanum tuberosum L.) with hairy vetch (Vicia villosa) increases water use efficiency in dry conditions. Field Crops Res. 2019, 240, 168–176. [Google Scholar] [CrossRef]
- Kissing Kucek, L.; Riday, H.; Rufener, B.P.; Burke, A.N.; Eagen, S.S.; Ehlke, N.; Krogman, S.; Mirsky, S.B.; Reberg-Horton, C.; Ryan, M.R.; et al. Pod dehiscence in hairy vetch (Vicia villosa Roth). Front. Plant Sci. 2020, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Pál, V.; Zsombik, L. Evaluation of the role of common vetch (Vicia sativa L.) green manure in crop rotations. Acta Agrar. Debr. 2022, 1, 161–171. [Google Scholar] [CrossRef]
- Kim, K.; Neuberger, P.; Daly, E.J.; Gorzelak, M.; Hernandez-Ramirez, G. Arbuscular mycorrhizal fungi community linkages to soil nutrient availability across contrasting agroecosystems. Appl. Soil Ecol. 2022, 176, 104464. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Q.; Sun, L.; Yang, X.; Yang, W.; Zhang, H. Stomatal conductance and morphology of arbuscular mycorrhizal wheat plants response to elevated CO2 and NaCl stress. Front. Plant Sci. 2018, 9, 1363. [Google Scholar] [CrossRef]
- Lala, F.; Jasil, Y.; Habeahan, K.; Bayuaji, H.; Wahab, A. The effect of arbuscular mycorrhizal fungus on morphological characters and yield of cayenne pepper (Capsicum frutescens L.). E3S Web Conf. 2021, 306, 01051. [Google Scholar] [CrossRef]
- Basyal, B.; Emery, S.M. An arbuscular mycorrhizal fungus alters switchgrass growth, root architecture, and cell wall chemistry across a soil moisture gradient. Mycorrhiza 2021, 31, 251–258. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Al-Sadi, A.M.; Alharbi, S.A.; Datta, R.; Zuan, A.T.K. Biochar and arbuscular mycorrhizal fungi mediated enhanced drought tolerance in okra (Abelmoschus esculentus) plant growth, root morphological traits and physiological properties. Saudi J. Biol. Sci. 2021, 28, 5490–5499. [Google Scholar] [CrossRef]
- Khan, Y.; Yang, X.; Zhang, X.; Yaseen, T.; Shi, L.; Zhang, T. Arbuscular mycorrhizal fungi promote plant growth of Leymus chinensis (Trin.) Tzvelev by increasing the metabolomics activity under nitrogen addition. Grassl. Sci. 2021, 67, 128–138. [Google Scholar] [CrossRef]
- Kaur, S.; Suseela, V. Unraveling arbuscular mycorrhiza-induced changes in plant primary and secondary metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef]
- Luo, J.; Yan, Q.; Yang, G.; Wang, Y. Impact of the arbuscular mycorrhizal fungus Funneliformis mosseae on the physiological and defence responses of Canna indica to copper oxide nanoparticles stress. J. Fungi 2022, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Boutasknit, A.; Ait-Rahou, Y.; Anli, M.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Meddich, A. Improvement of garlic growth, physiology, biochemical traits, and soil fertility by Rhizophagus irregularis and compost. Gesunde Pflanz. 2021, 73, 149–160. [Google Scholar] [CrossRef]
- Anli, M.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Ait-Rahou, Y.; Fakhech, A.; Meddich, A. Improving lettuce yield and quality of an agricultural soil using a combination of arbuscular mycorrhizal fungus and phosphate-green wastes compost. Gesunde Pflanz. 2022, 74, 205–217. [Google Scholar] [CrossRef]
- Cissé, G.; Essi, M.; Kedi, B.; Mollier, A.; Staunton, S. Contrasting effects of long term phosphorus fertilization on glomalin-related soil protein (GRSP). Eur. J. Soil Biol. 2021, 107, 103363. [Google Scholar] [CrossRef]
- Bisht, A.; Garg, N. AMF species improve yielding potential of Cd stressed pigeonpea plants by modulating sucrose-starch metabolism, nutrients acquisition and soil microbial enzymatic activities. Plant Growth Regul. 2022, 96, 409–430. [Google Scholar] [CrossRef]
- Selvakumar, G.; Shagol, C.C.; Kang, Y.; Chung, B.N.; Han, S.G.; Sa, T.M. Arbuscular mycorrhizal fungi spore propagation using single spore as starter inoculum and a plant host. J. Appl. Microbiol. 2018, 124, 1556–1565. [Google Scholar] [CrossRef]
- Abdelaziz, M.E.; Abdelsattar, M.; Abdeldaym, E.A.; Atia, M.A.M.; Mahmoud, A.W.M.; Saad, M.M.; Hirt, H. Piriformospora indica alters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Sci. Hortic. 2019, 256, 108532. [Google Scholar] [CrossRef]
- Baghaie, A.H.; Aghili, F. Contribution of Piriformospora indica on improving the nutritional quality of greenhouse tomato and its resistance against cu toxicity after humic acid addition to soil. Environ. Sci. Pollut. Res. 2021, 28, 64572–64585. [Google Scholar]
- Cheng, C.; Li, D.; Wang, B.; Liao, B.; Qu, P.; Liu, W.; Zhang, Y.; Lü, P. Piriformospora indica colonization promotes the root growth of Dimocarpus longan seedlings. Sci. Hortic. 2022, 301, 111137. [Google Scholar] [CrossRef]
- Yang, L.; Zou, Y.N.; Tian, Z.H.; Wu, Q.S.; Kuča, K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci. Hortic. 2021, 277, 109815. [Google Scholar] [CrossRef]
- Sun, R.T.; Feng, X.C.; Zhang, Z.Z.; Zhou, N.; Feng, H.D.; Liu, Y.M.; Hashem, A.; Al-Arjani, A.F.; Abd_Allah, E.F.; Wu, Q.S. Root endophytic fungi regulate changes in sugar and medicinal compositions of Polygonum cuspidatum. Front. Plant Sci. 2022, 13, 818909. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.C.; Meng, L.L.; Zou, Y.N.; He, X.H.; Wu, Q.S. Introduction of earthworms into mycorrhizosphere of white clover facilitates N storage in glomalin-related soil protein and contribution to soil total N. Appl. Soil Ecol. 2022, 179, 104597. [Google Scholar] [CrossRef]
- Liang, S.M.; Zheng, F.L.; Abd_Allah, E.F.; Muthuramalingam, P.; Wu, Q.S.; Hashem, A. Spatial changes of arbuscular mycorrhizal fungi in peach and their correlation with soil properties. Saudi J. Biol. Sci. 2021, 28, 6495–6499. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Lou, Y.G.; Li, Y. Plant growth and tissue sucrose metabolism in the system of trifoliate orange and arbuscular mycorrhizal fungi. Sci. Hortic. 2015, 181, 189–193. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Muneer, M.A.; Wang, P.; Lin, C.; Ji, B. Potential role of common mycorrhizal networks in improving plant growth and soil physicochemical properties under varying nitrogen levels in a grassland ecosystem. Glob. Ecol. Conserv. 2020, 24, e01352. [Google Scholar] [CrossRef]
- Khaekhum, S.; Ekprasert, J.; Suebrasri, T.; Seemakram, W.; Mongkolthanaruk, W.; Riddech, N.; Jogloy, S.; Boonlue, S. Co-inoculation of an endophytic and arbuscular mycorrhizal fungus improve growth and yield of Helianthus tuberosus L. under field condition. J. Fungi 2021, 7, 976. [Google Scholar] [CrossRef]
- Di Martino, C.; Fioretto, A.; Palmieri, D.; Torino, V.; Giuseppe, P. Influence of tomato plant mycorrhization on nitrogen metabolism, growth and fructification on P-limited soil. J. Plant Growth Regul. 2019, 38, 1183–1195. [Google Scholar] [CrossRef]
- Xie, M.M.; Chen, S.M.; Zou, Y.N.; Srivastava, A.K.; Rahman, M.M.; Wu, Q.S.; Kuča, K. Effects of Rhizophagus intraradices and Rhizobium trifolii on growth and N assimilation of white clover. Plant Growth Regul. 2021, 93, 311–318. [Google Scholar] [CrossRef]
- Huang, G.M.; Zou, Y.N.; Wu, Q.S.; Xu, Y.J.; Kuča, K. Mycorrhizal roles in plant growth, gas exchange, root morphology, and nutrient uptake of walnuts. Plant Soil Environ. 2020, 66, 295–302. [Google Scholar] [CrossRef]
- Feng, J.; Lv, W.; Xu, J.; Huang, Z.; Rui, W.; Lei, X.; Ju, X.; Li, Z. Overlapping root architecture and gene expression of nitrogen transporters for nitrogen acquisition of tomato plants colonized with isolates of Funneliformis mosseae in hydroponic production. Plants 2022, 11, 1176. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wang, P.; Zhang, D.J.; Zou, Y.N.; Kuča, K.; Wu, Q.S. Mycorrhiza-induced change in root hair growth is associated with IAA accumulation and expression of EXPs in trifoliate orange under two P levels. Sci. Hortic. 2018, 234, 227–235. [Google Scholar] [CrossRef]
- Gao, T.; Liu, X.; Shan, L.; Wu, Q.; Liu, Y.; Zhang, Z.; Ma, F.; Li, C. Dopamine and arbuscular mycorrhizal fungi act synergistically to promote apple growth under salt stress. Environ. Exp. Bot. 2020, 178, 104159. [Google Scholar] [CrossRef]
- Winagraski, E.; Kaschuk, G.; Monteiro, P.H.R.; Auer, C.G.; Higa, A.R. Diversity of arbuscular mycorrhizal fungi in forest ecosystems of Brazil: A review. Cerne 2019, 25, 25–35. [Google Scholar] [CrossRef]
- Parihar, M.; Rakshit, A.; Meena, V.S.; Gupta, V.K.; Rana, K.; Choudhary, M.; Jatav, H.S. The potential of arbuscular mycorrhizal fungi in C cycling: A review. Arch. Microbiol. 2020, 202, 1581–1596. [Google Scholar] [CrossRef]
- Salmeron-Santiago, I.A.; Martínez-Trujillo, M.; Valdez-Alarcón, J.J.; Pedraza-Santos, M.E.; Santoyo, G.; Pozo, M.J.; Chávez-Bárcenas, A.T. An updated review on the modulation of carbon partitioning and allocation in arbuscular mycorrhizal plants. Microorganisms 2021, 10, 75. [Google Scholar] [CrossRef]
- Meng, L.L.; Liu, R.C.; Yang, L.; Zou, Y.N.; Srivastava, A.K.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Giri, B.; Wu, Q.S. The change in fatty acids and sugars reveals the association between trifoliate orange and endophytic fungi. J. Fungi 2021, 7, 716. [Google Scholar] [CrossRef]
- Zhu, F.R.; Zhou, N.; Yang, M.; Ding, B.; Pan, X.J.; Qi, J.S.; Guo, D.Q. Effect of different arbuscular mycorrhizal fungi on soil nutrients in rhizosphere soil of Paris polyphylla var. yunnanensis seedlings. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 86–95. [Google Scholar]
- Liu, R.C.; Gao, W.Q.; Srivastava, A.K.; Zou, Y.N.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Differential effects of exogenous glomalin-related soil proteins on plant growth of trifoliate orange through regulating auxin changes. Front. Plant Sci. 2021, 12, 745402. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.F.; Xie, M.M.; Li, Y.; Liu, B.Y.; Liu, C.Y.; Wu, Q.S.; Kuča, K. Effects of field inoculation with arbuscular mycorrhizal fungi and endophytic fungi on fruit quality and soil properties of Newhall navel orange. Appl. Soil Ecol. 2022, 170, 104308. [Google Scholar] [CrossRef]


| Treatments | Height (cm) | Diameter (mm) | Leaf Number (num./Plant) | Biomass (g/Plant) | ||
|---|---|---|---|---|---|---|
| Leaf | Stem | Root | ||||
| Control | 13.6 ± 1.5 d | 0.474 ± 0.048 c | 15.9 ± 2.1 c | 0.248 ± 0.042 d | 0.116 ± 0.017 b | 0.550 ± 0.035 a |
| Fm | 24.1 ± 1.9 b | 0.641 ± 0.074 ab | 31.8 ± 3.1 a | 0.698 ± 0.120 b | 0.345 ± 0.048 a | 0.663 ± 0.078 a |
| Ds | 25.8 ± 2.9 ab | 0.698 ± 0.035 a | 33.6 ± 4.9 a | 0.705 ± 0.133 b | 0.362 ± 0.059 a | 0.634 ± 0.078 a |
| Ri | 27.6 ± 1.9 a | 0.694 ± 0.051 a | 36.0 ± 2.4 a | 0.838 ± 0.090 a | 0.378 ± 0.071 a | 0.678 ± 0.052 a |
| Si | 16.6 ± 1.5 c | 0.584 ± 0.025 b | 22.0 ± 1.3 b | 0.404 ± 0.059 c | 0.168 ± 0.032 b | 0.614 ± 0.065 a |
| F value | 46.96 | 17.62 | 40.08 | 32.59 | 30.89 | 2.52 |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0739 |
| Treatments | Total Length (cm) | Projected Area (cm2) | Surface Area (cm2) | Diameter (mm) | Volume (cm3) |
|---|---|---|---|---|---|
| Control | 131.2 ± 25.7 b | 9.4 ± 1.0 a | 13.9 ± 1.5 b | 0.461 ± 0.026 b | 0.47 ± 0.11 b |
| Fm | 178.2 ± 9.3 a | 10.9 ± 1.0 a | 16.4 ± 0.8 a | 0.530 ± 0.049 a | 1.36 ± 0.24 a |
| Ds | 171.0 ± 28.3 a | 10.8 ± 1.3 a | 15.8 ± 1.0 a | 0.465 ± 0.025 b | 0.78 ± 0.23 b |
| Ri | 172.6 ± 6.1 a | 11.2 ± 0.4 a | 15.4 ± 1.1 ab | 0.526 ± 0.059 a | 1.34 ± 0.31 a |
| Si | 183.0 ± 18.1 a | 11.4 ± 1.5 a | 16.1 ± 0.7 a | 0.438 ± 0.011 b | 0.59 ± 0.18 b |
| F value | 4.49 | 2.02 | 3.54 | 4.70 | 14.11 |
| p value | 0.0139 | 0.1438 | 0.0317 | 0.0117 | <0.0001 |
| Treatments | Sucrose (mg/g DW) | Fructose (mg/g DW) | Glucose (mg/g DW) | |||
|---|---|---|---|---|---|---|
| Leaves | Roots | Leaves | Roots | Leaves | Roots | |
| Control | 26.55 ± 4.91 c | 84.64 ± 9.92 b | 32.60 ± 1.74 c | 62.89 ± 5.47 c | 87.05 ± 5.63 b | 102.87 ± 4.58 b |
| Fm | 37.66 ± 1.91 ab | 102.55 ± 8.79 a | 44.93 ± 4.17 ab | 99.53 ± 5.02 a | 85.42 ± 8.93 b | 128.51 ± 9.79 a |
| Ds | 41.27 ± 8.14 ab | 80.59 ± 11.05 b | 44.10 ± 3.50 ab | 86.68 ± 6.87 b | 114.33 ± 7.59 a | 126.67 ± 7.81 a |
| Ri | 47.06 ± 7.72 a | 63.81 ± 3.93 c | 46.34 ± 6.15 a | 85.17 ± 4.85 b | 86.36 ± 1.31 b | 129.45 ± 3.19 a |
| Si | 34.58 ± 6.54 bc | 80.23 ± 7.81 b | 39.31 ± 1.86 b | 69.10 ± 5.72 c | 112.00 ± 6.69 a | 106.76 ± 16.27 b |
| F value | 5.7 | 10.22 | 8.52 | 27.19 | 20.22 | 7.39 |
| p value | 0.0054 | 0.0003 | 0.0009 | <0.0001 | <0.0001 | 0.0017 |
| Treatments | NH4-N (mg/kg) | NO3-N (mg/kg) | Olsen-P (mg/kg) | Available K (mg/kg) |
|---|---|---|---|---|
| Control | 23.3 ± 3.9 c | 1.5 ± 0.3 e | 254.8 ± 32.7 a | 29.9 ± 1.7 a |
| Fm | 29.2 ± 4.1 b | 4.0 ± 0.8 d | 176.4 ± 17.1 b | 25.2 ± 1.2 c |
| Ds | 51.9 ± 1.1 a | 13.4 ± 2.0 b | 164.5 ± 7.6 b | 25.1 ± 1.5 c |
| Ri | 22.5 ± 2.1 c | 15.8 ± 0.6 a | 177.2 ± 6.9 b | 26.7 ± 2.0 bc |
| Si | 27.1 ± 4.0 bc | 10.1 ± 0.8 c | 180.8 ± 10.2 b | 28.3 ± 0.6 ab |
| F value | 55.27 | 126.08 | 16.79 | 7.45 |
| p value | <0.0001 | <0.0001 | <0.0001 | 0.0016 |
| Treatments | Distribution of WSA Fraction (%) | MWD (mm) | |||
|---|---|---|---|---|---|
| 2–4 mm | 1–2 mm | 0.5–1 mm | 0.25–0.5 mm | ||
| Control | 0.42 ± 0.08 d | 0.36 ± 0.07 b | 1.38 ± 0.20 d | 8.82 ± 1.21 a | 0.061 ± 0.004 e |
| Fm | 1.09 ± 0.18 b | 1.16 ± 0.15 a | 3.43 ± 0.35 ab | 11.31 ± 2.38 a | 0.118 ± 0.004 b |
| Ds | 0.76 ± 0.07 c | 1.19 ± 0.19 a | 3.26 ± 0.43 b | 10.81 ± 1.25 a | 0.105 ± 0.011 c |
| Ri | 1.44 ± 0.25 a | 1.24 ± 0.16 a | 3.94 ± 0.42 a | 10.90 ± 1.86 a | 0.132 ± 0.008 a |
| Si | 0.53 ± 0.12 cd | 0.35 ± 0.07 b | 1.92 ± 0.34 c | 12.38 ± 1.35 a | 0.082 ± 0.003 d |
| F value | 29.45 | 45.19 | 36.77 | 2.4 | 71.94 |
| p value | <0.0001 | <0.0001 | <0.0001 | 0.0966 | <0.0001 |
| Root Colonization | NH4-N | NO3-N | Olsen-P | Available K | Distribution of WSA Fraction | MWD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2–4 mm | 1–2 mm | 0.5–1 mm | 0.25–0.5 mm | |||||||
| Root colonization | 1 | 0.36 | 0.66 | −0.64 ** | −0.65 ** | 0.81 ** | 0.91 ** | 0.92 ** | 0.09 | 0.89 ** |
| EE-GRSP | 0.83 ** | 0.12 | 0.83 | −0.69 ** | −0.52 * | 0.79 ** | 0.72 ** | 0.80 ** | 0.36 | 0.87 ** |
| DE-GRSP | 0.66 ** | 0.31 | 0.34 | −0.74 ** | −0.64 ** | 0.61 ** | 0.67 ** | 0.74 ** | 0.41 | 0.75 ** |
| T-GRSP | 0.82 ** | 0.25 | 0.63 ** | −0.79 ** | −0.65 ** | 0.77 ** | 0.77 ** | 0.85 ** | 0.43 | 0.91 ** |
| Chlorophyll a | Chlorophyll b | Total Chlorophyll | Carotenoid | Leaf Sucrose | Root Sucrose | Leaf Fructose | Root Fructose | Leaf Glucose | Root Glucose | |
|---|---|---|---|---|---|---|---|---|---|---|
| Root colonization | 0.88 ** | 0.87 ** | 0.90 ** | 0.78 ** | 0.73 ** | −0.21 | −0.21 | −0.21 | −0.06 | 0.76 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.-X.; Wu, Q.-S.; Hashem, A.; Abd_Allah, E.F.; Muthuramalingam, P.; Al-Arjani, A.-B.F.; Zou, Y.-N. Effects of Symbiotic Fungi on Sugars and Soil Fertility and Structure-Mediated Changes in Plant Growth of Vicia villosa. Agriculture 2022, 12, 1523. https://doi.org/10.3390/agriculture12101523
He W-X, Wu Q-S, Hashem A, Abd_Allah EF, Muthuramalingam P, Al-Arjani A-BF, Zou Y-N. Effects of Symbiotic Fungi on Sugars and Soil Fertility and Structure-Mediated Changes in Plant Growth of Vicia villosa. Agriculture. 2022; 12(10):1523. https://doi.org/10.3390/agriculture12101523
Chicago/Turabian StyleHe, Wan-Xia, Qiang-Sheng Wu, Abeer Hashem, Elsayed Fathi Abd_Allah, Pandiyan Muthuramalingam, Al-Bandari Fahad Al-Arjani, and Ying-Ning Zou. 2022. "Effects of Symbiotic Fungi on Sugars and Soil Fertility and Structure-Mediated Changes in Plant Growth of Vicia villosa" Agriculture 12, no. 10: 1523. https://doi.org/10.3390/agriculture12101523
APA StyleHe, W.-X., Wu, Q.-S., Hashem, A., Abd_Allah, E. F., Muthuramalingam, P., Al-Arjani, A.-B. F., & Zou, Y.-N. (2022). Effects of Symbiotic Fungi on Sugars and Soil Fertility and Structure-Mediated Changes in Plant Growth of Vicia villosa. Agriculture, 12(10), 1523. https://doi.org/10.3390/agriculture12101523









