Impact of Arbuscular Mycorrhizal Fungi, Phosphate Solubilizing Bacteria and Selected Chemical Phosphorus Fertilizers on Growth and Productivity of Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Chemical Fertilizers
- Nitrogen fertilizer was applied at the rate of 165 kg N ha−1 in the form of urea (46.5% N). Urea was applied in two doses to each plot, with the first 2/3 of dosage being employed as basal application. A total of 30 days after transplanting (DAT), the other 1/3 of dosage was used for top-dressing;
- A chemical phosphorus fertilizer in the form of single super phosphate (SSP) with 15.5% P2O5 was added and incorporated well into the soil at the time of final land preparation as basal application at the rate of 36 kg ha−1;
- Orthophosphoric acid (H3PO4) with 85% phosphoric acid was used. In the booting stage (25 days after transplanting), a liquid solution containing 120 mg L−1 per ha was used for foliar spraying;
- Hydroxyapatite (Ca5 (PO4)3 OH) nanoparticles were used as phosphorus nanoparticles. In the booting stage, a liquid solution of 2400 mg L−1 per ha was sprayed on the leaves as foliar spray. Transmission electron microscopy (TEM) was performed to characterize the size distribution and shape of the synthesized phosphorus nanoparticles (Figure 1) [43].
2.3. Biological Fertilizers
- Phosphate-solubilizing bacteria (PSBs), which included Bacillus megatherium. PSBs were utilized with two different techniques. In the first, at a rate of 2.380 kg ha−1 via powder inoculation, PSBs were used for top-dressing during transplanting in the permanent field 30 days after sowing. Inoculation powder was combined with enough sand to make homogeneous dispersion easier. The other method involved spraying a foliar solution at a rate of 2.975 L ha−1 in the booting stage (25 days after transplanting).
- Arbuscular mycorrhizal fungi (AMFs), which included Glomus sp. AMFs were employed at a rate of 7.200 K ha−1 via powder inoculation and were used for top-dressing upon transplanting in the permanent field 30 days after sowing. Inoculation powder was combined with enough sand to make homogeneous dispersion easier.
2.4. Experiment Treatments
2.5. Studied Characteristics
2.6. Statistical Analyses
3. Results
3.1. Growth Characteristics
3.2. Yield Attributing Characteristics
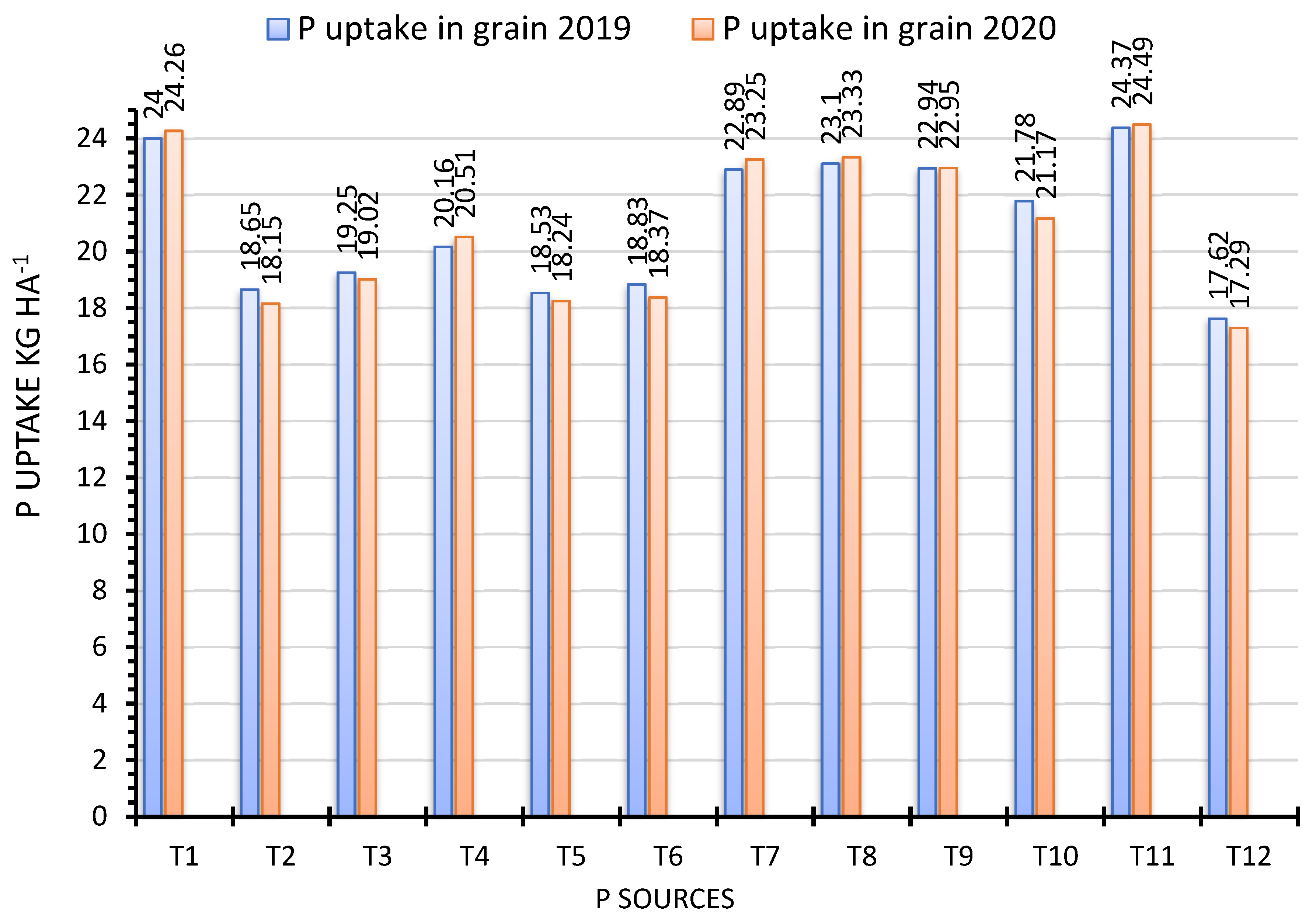
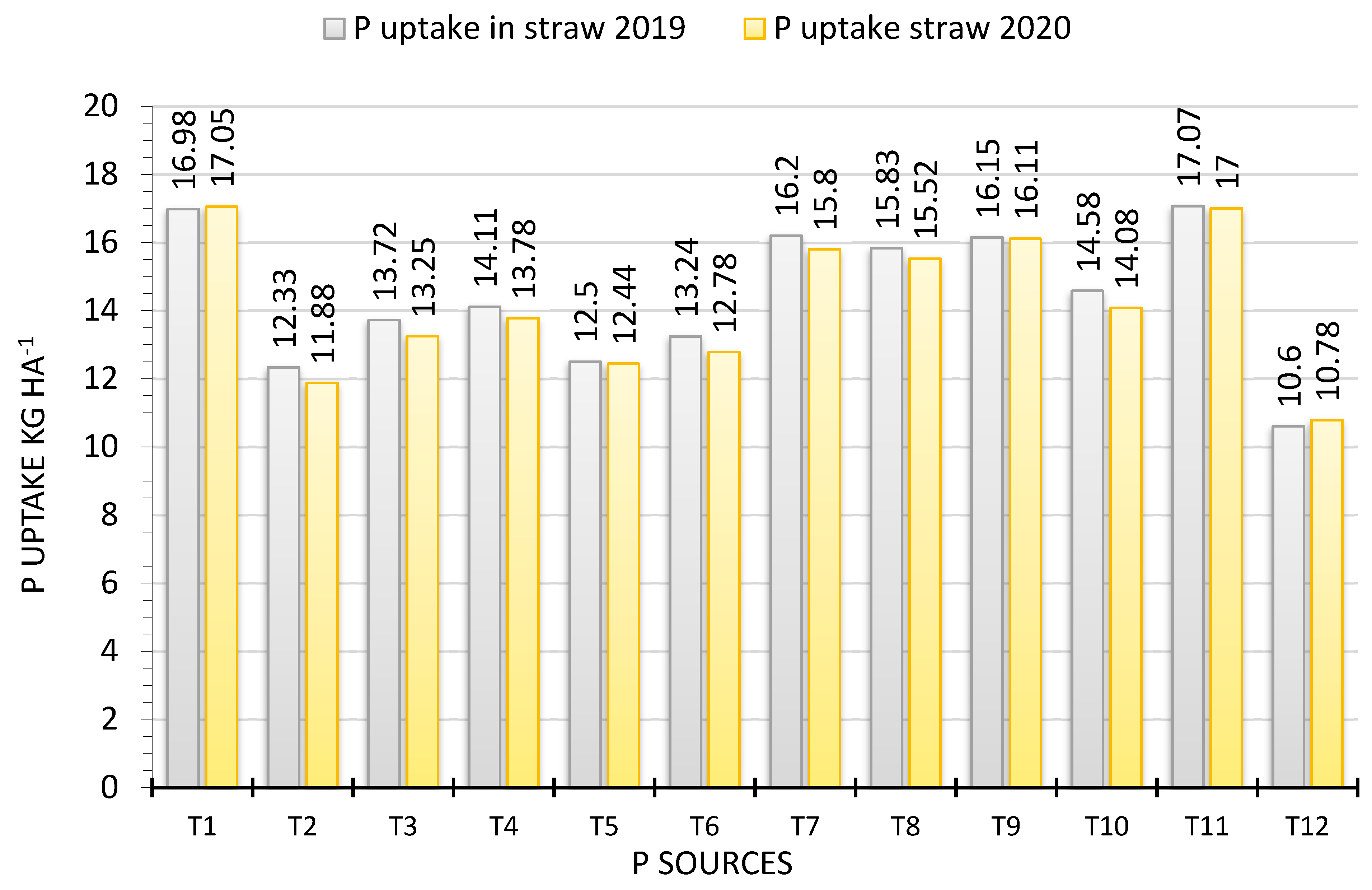
3.3. Phosphorus Uptake in Grain and Straw
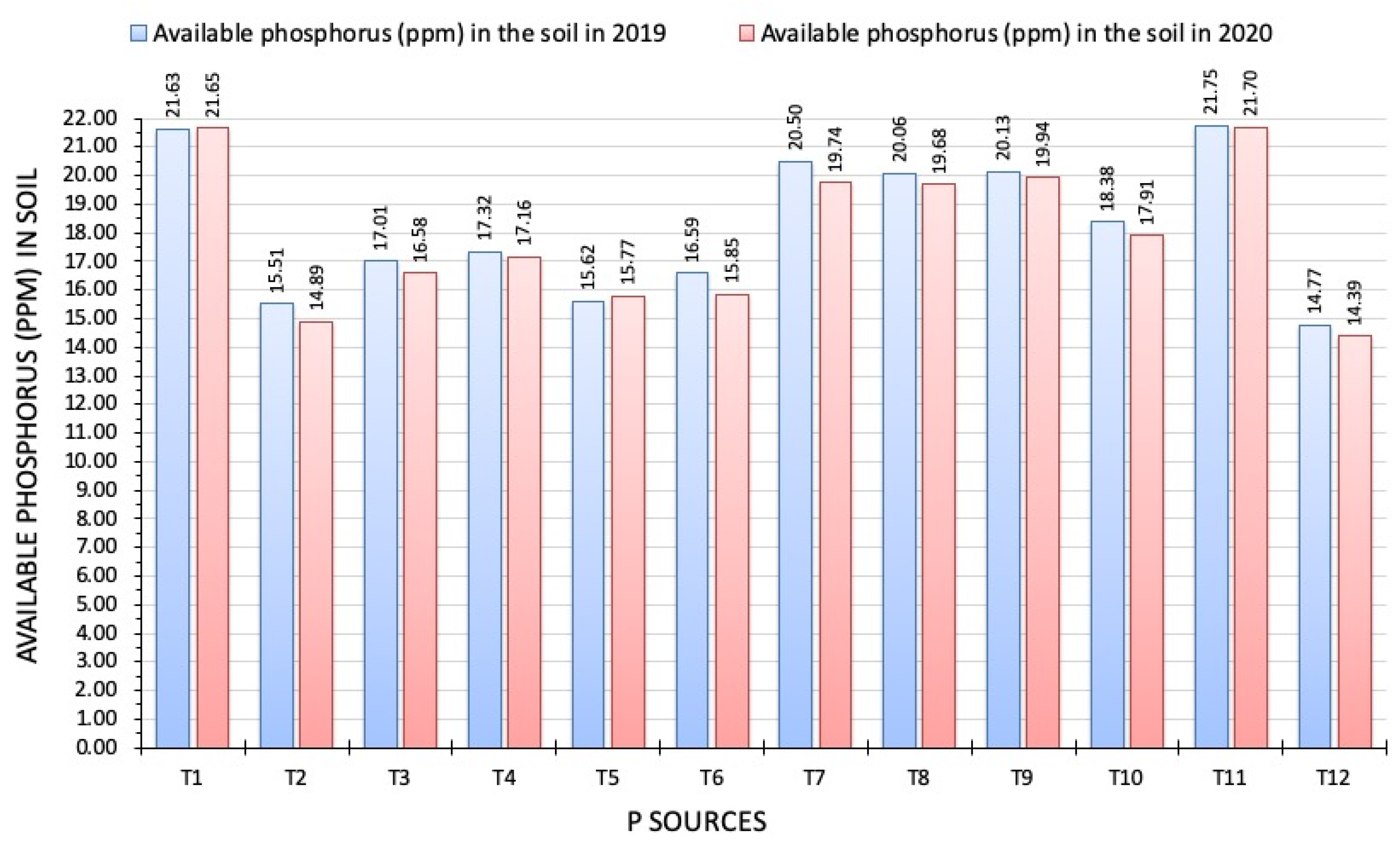
3.4. Available Phosphorus in the Soil after Harvest
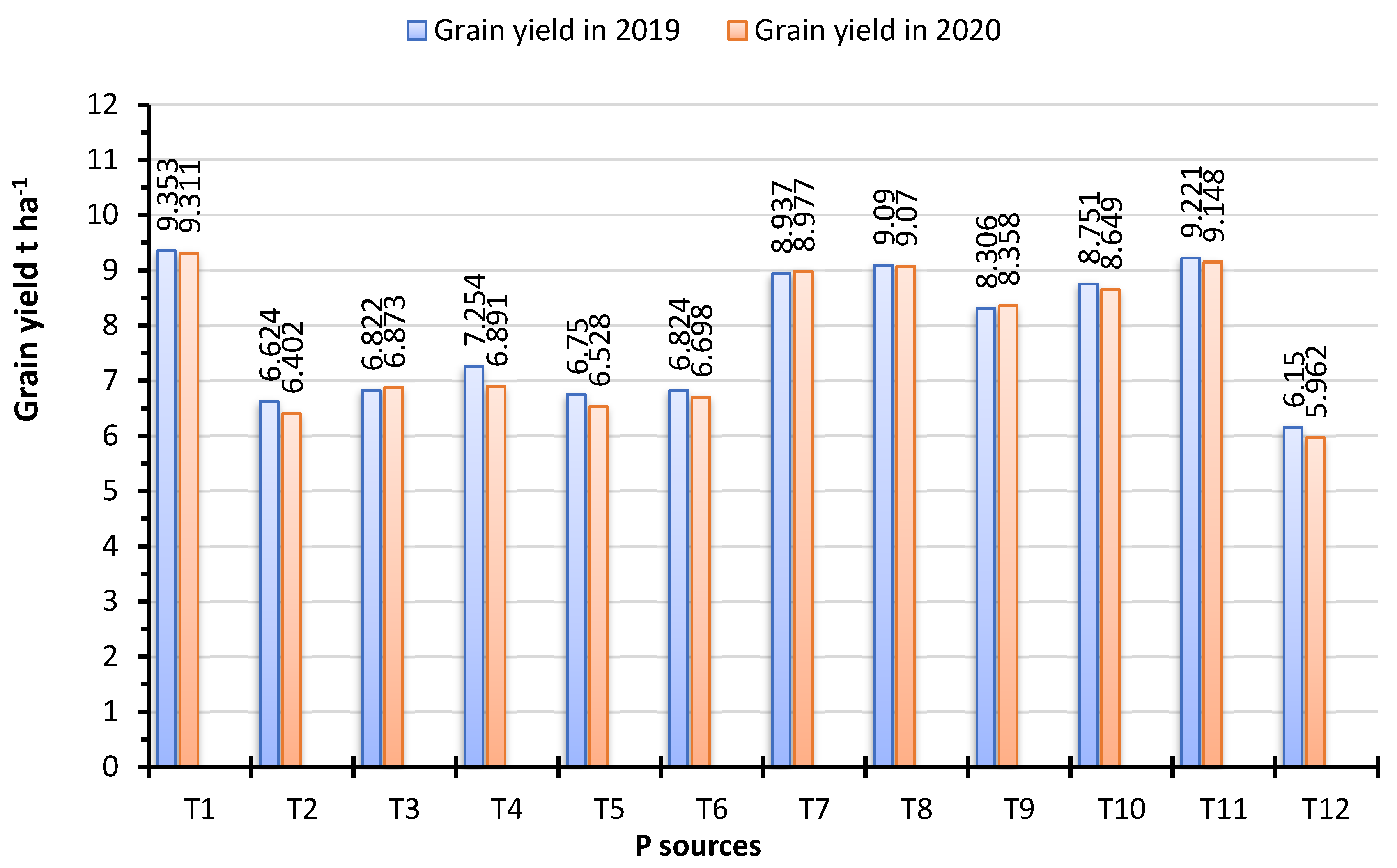
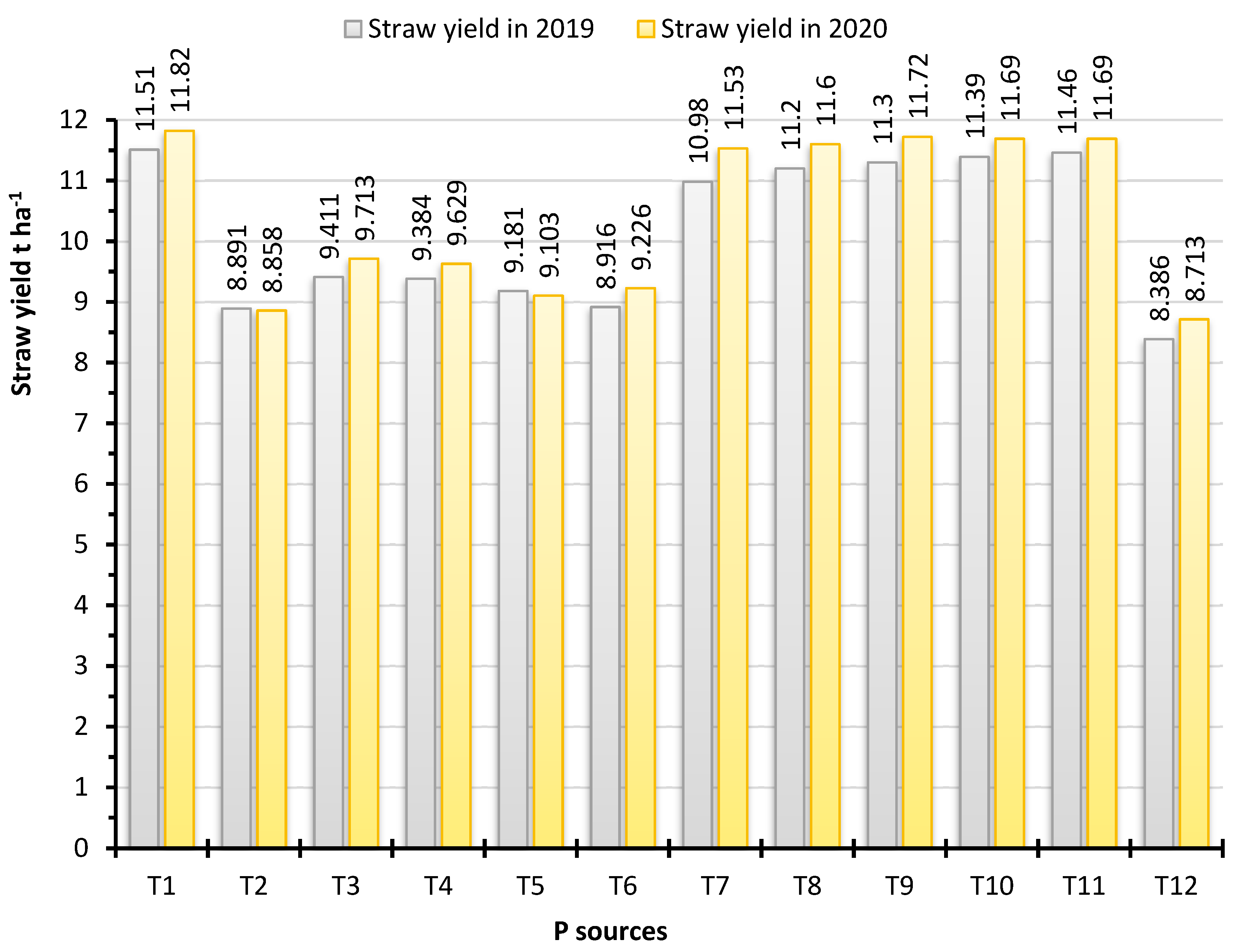
3.5. Grain and Straw Yields
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- RRTC. Rice Research and Training Center (National Rice Research Program): Proceedings of the Rice Workshop, Annual Report; Sakha: Kafrelsheikh, Egypt, 2020. [Google Scholar]
- Feng, K.; Lu, H.M.; Sheng, H.J.; Wang, X.L.; Mao, J. Effect of organic ligands on biological availability of inorganic phosphorus in soils. Pedosphere 2004, 14, 85–92. [Google Scholar]
- Furihata, T.; Suzuki, M.; Sakurai, H.K. Characterization of two phosphate uptake systems with different affinities in suspension-cultured Catharanthus roseus protoplasts. Plant Cell Physiol. 1992, 33, 1151–1157. [Google Scholar]
- Theodorou, M.E.; Plaxton, W.C. Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol. 1993, 101, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Barea, J.; McNeill, A.M.; Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Richardson, A.E.; Hocking, P.J.; Simpson, R.J.; George, T.S. Plant mechanisms to optimize access to soil phosphorus. Crop Pasture Sci. 2009, 60, 124–143. [Google Scholar] [CrossRef]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 7th ed.; Prentice-Hall: Hoboken, NJ, USA, 2004. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Havlin, J.L.; Beaton, J.D.; Nelson, W.L.; Tisdale, S.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2005; p. 515. [Google Scholar]
- Karandashov, V.; Bucher, M. Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci. 2005, 1, 22–29. [Google Scholar] [CrossRef]
- Shen, J.; Li, R.; Zhang, E.; Fan, J.; Tang, C.; Renge, Z. Crop yields, soil fertility and phosphorus fractions in response to long-term fertilization under the rice monoculture system on a calcareous soil. Field Crops Res. 2004, 86, 225–238. [Google Scholar] [CrossRef]
- Xavier, R.A.S.; Oliveira, T.S.; Andrade, E.V.; Mendonca, E.S. Phosphorus fractionation in a sandy soil under organic agriculture in northeastern Brazil. Geoderma 2009, 151, 417–423. [Google Scholar] [CrossRef]
- Elekhtyar, N.M. Influence of different plant growth promoting rhizobacteria (PGPR) strains on rice promising line. Proc. Sixth Field Crops Con. FCRI ARC Egypt 2016, 6, 327–335. [Google Scholar]
- Elekhtyar, N.M. Impact of three strains of Bacillus as bio NPK fertilizers and three levels of mineral NPK fertilizers on growth, chemical compositions and yield of Sakha 106 rice cultivar. Int. J. ChemTech Res. 2015, 8, 2150–2156. [Google Scholar]
- Elekhtyar, N.M. Efficiency of Pseudomonas fluorescns as Plant Growth-Promoting Rhizobacteria (PGPR) for the enhancement of seedling vigor, nitrogen uptake, yield and its attributes of rice (Oryza sativa L.). Int. J. Sci. Res. Agric. Sci. 2015, 2, 57–67. [Google Scholar]
- Lindsay, W.L. Chemical Equilibria in Soil; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Beegle, D.B.; Durst, F.T. Managing Phosphorus for Crop Production. Pa. Nutr. Manag. Program Agron. Facts 2002, 13, 1–6. [Google Scholar]
- Cordier, C.; Lemoine, M.C.; Lemanceau, P.; Pearson, V.; Gianinazzi, S. The beneficial rhizosphere: A necessary strategy for micro plant production. Acta Hortic. 2000, 530, 259–268. [Google Scholar] [CrossRef]
- Naseby, D.C.; Lynch, J.M. Rhizosphere soil enzymes as indicators of perturbation caused by a genetically modified strain of Pseudomonas fluorescens on wheat seed. Soil Biol. Chem. 1997, 29, 1353–1362. [Google Scholar] [CrossRef]
- Ceccanti, B.; Pezzarossa, B.; Lancho, F.J.; Masciandaro, G. Bio-tests as markers of soil utilization and fertility. Geomicrobiol. J. 1994, 11, 309–316. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Afzal, A.; Asghari, B. Rhizobium and phosphate solubilizing bacteria improve the yield and phosphorus uptake in wheat (Triticum aestivum). Int. J. Agric. Biol. 2008, 10, 85–88. [Google Scholar]
- Rodriguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Elekhtyar, N.M. Effect of Bio and Mineral Nitrogen Fertilizer on Growth, Yield and Chemical Composition of Rice. Ph.D. Thesis, Faculty of Agriculture, Kafrelsheikh University, Kafr el-Sheikh, Egypt, 2011. [Google Scholar]
- Panneerselvam, P.; Upendra Kumar, T.; Sugitha, C.K.; Parameswaran, C.; Sowarnalisha Sahoo, A.; Binodh, K.; Jahan, A.; Anandan, A. Arbuscular Mycorrhizal Fungi (AMF) for Sustaina-Ble Rice Production; Springer Nature Singapore Pte Ltd.: Singapore, 2017; pp. 99–126. [Google Scholar]
- Shenoy, V.V.; Kalagudi, G.M. Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol. Adv. 2005, 23, 501–513. [Google Scholar] [CrossRef]
- Peterson, R.L.; Massicotte, H.B. Exploring structural definitions of mycorrhizas, with emphasis on nutrient-exchange interfaces. Can. J. Bot. 2004, 82, 1074–1088. [Google Scholar] [CrossRef]
- Azcón, R.; Barea, J.M. Mycorrhizosphere interactions for legume improvement. In Microbes for Legume Improvement; Khanf, M.S., Zaidi, A., Musarrat, J., Eds.; Springer: Vienna, Austria, 2010; pp. 237–271. [Google Scholar]
- Elekhtyar, N.M.; Awad-Allah, M.M.A.; Zidan, A.A. Effect of Plant Growth-Promoting Rhizobacteria and Cyanobacteria on Physico-chemical and cooking characteristics of Giza 179 rice grains. Egypt. J. Agric. Res. 2022, 100, 22–29. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Kumar, G.N.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Alzoubi, M.M.; Gaibore, M. The effect of phosphate solubilizing bacteria and organic fertilization on availability of syrian rock phosphate and increase of triple superphosphate efficiency. World J. Agric. Sci. 2012, 8, 473–478. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Rahimian, H.; Pirdashti, H.; Nematzadeh, G.A. Evaluation of phosphate-solubilizing bacteria on the growth and grain yield of rice (Oryza sativa L.) cropped in northern Iran. J. Appl. Microbiol. 2015, 119, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2015, 21, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 106, 279–297. [Google Scholar] [CrossRef]
- Galbraith, D.W. Nanobiotechnology: Silica breaks through in plants. Nat. Nanotechnol. 2007, 2, 272–273. [Google Scholar] [CrossRef]
- Mahajan, P.; Dhoke, S.K.; Khanna, A.S. Effect of Nano-ZnO Particle Suspension on Growth of Mung (Vigna radiata) and Gram (Cicer arietinum) Seedlings Using Plant Agar Method. J. Nanotechnol. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Boonyanitipong, P.; Kositsup, B.; Kumar, P.; Baruah, S.; Dutta, J. Toxicity of ZnO and TiO2 Nanoparticles on Germinating Rice Seed Oryza sativa L. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 282–285. [Google Scholar] [CrossRef]
- Banfield, J.F.; Zhang, H. Nanoparticles in the Environment; Mineralogical Society of America: Washington, DC, USA, 2001; Chapter 1; pp. 1–58. [Google Scholar] [CrossRef]
- Buffle, J. The key role of environmental colloids/ nanoparticles for the sustainability of life. Environ. Chem. 2006, 3, 155–158. [Google Scholar] [CrossRef]
- Qureshi, A.; Singh, D.K.; Dwivedi, S. Nano-fertilizers: A Novel Way for Enhancing Nutrient Use Efficiency and Crop Productivity. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3325–3335. [Google Scholar] [CrossRef]
- Black, C.A.; Evans, D.D.; Ensminger, L.E.; Clark, F.E. Methods of Soil Analysis. Part 2—Chemical and Microbiological Properties; American Society of Agronomy Inc.: Madison, WI, USA, 1965. [Google Scholar]
- Williams, B.; Carter, C. Transmission Electron Microscopy; Springer: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- Peterpurgski, A.V. Handbook of Agronomic Chemistry; Kolop Publishing House: Moscow, Russia, 1968; pp. 29–86. (In Russian) [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Method of Soil Analysis—Part 2; American Society of Agronomy Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Watson, D.J. Comparative physiological studies on the growth of field crops: I. Variation in net assimilation rate and leaf area between species and varieties and within and between years. Ann. Bot. 1947, 11, 41–76. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; Jahn Wiley Sons: New York, NY, USA, 1984. [Google Scholar]
- Duncan, D.B. Multiple Range and Multiple F Test. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Freed, R. MSTATC Version 1.2; Department of Crop and Soil Sciences, Michigen State University: East Lansing, MI, USA, 1986. [Google Scholar]
- Elekhtyar, N.M. Statistical Analysis of Experiments Using MSTAT-C Computer Software, Arabic ed.; Academy of Scientific Research and Technology (ASRT): Cairo, Egypt, 2018; No.: 3486/2018; ISBN 978-977-268-723-7. [Google Scholar]
- Salimpour, S.; Khavazi, K.; Nadian, H.; Besharati, H.; Miransari, M. Enhancing phosphorous availability to canola (Brassica napus L.) using P solubilizing and sulfur oxidizing bacteria. Aust. J. Crop. Sci. 2010, 4, 330–334. [Google Scholar]
- Ahmed, M.F.; Kennedy, I.R.; Choudhury, A.T.M.A.; Kecskes, M.L.; Deaker, R. Phosphorus adsorption in some Australian soils and influence of bacteria on the-desorption of phosphorus. Commun. Soil Sci. Plant Anal. 2008, 39, 1269–1294. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Chromium reduction, plant growth promoting potentials and metal solubilization by Bacillus sp. isolated from alluvial soil. Curr. Microbiol. 2007, 54, 237–243. [Google Scholar] [CrossRef]
- Rane, M.; Bawskar, M.; Rathod, D.; Nagaonkar, D.; Rai, M. Influence of calcium phosphate nanoparticles, Piriformospora indica and Glomus mosseae on growth of Zea mays. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 5004–5014. [Google Scholar] [CrossRef]
- Azcon, R.; Barea, J.M.; Hayman, D.S. Utilization of rock phosphate in alkaline soils by plants inoculated with mycorrhizal fungi and phosphate solubilizing bacteria. Soil Biol. Biochem. 1976, 8, 135–138. [Google Scholar] [CrossRef]
- Elekhtyar, N.M.; Metwally, T.F.; Nour El-Din, M. Evaluation of bio-NPK and compost tea on seedling vigor and yield of rice. In Proceedings of the 1st International Conference of Applied Microbiology, Giza, Egypt, 1–3 March 2016; Volume 1, pp. 8–20. [Google Scholar]
- Osorio, N.W.; Habte, M. Synergistic influence of an arbuscular mycorrhizal fungus and a P solubilizing fungus on growth and P uptake of Leucaena leucocephala in an oxisol. Arid Land Res. Manag. 2001, 15, 263–274. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Carrasco, L.; Roldan, A. Interactions between a plant growth-promoting rhizobacterium, an AM fungus and a phosphate-solubilising fungus in the rhizosphere of Lactuca sativa. Appl. Soil Ecol. 2007, 35, 480–487. [Google Scholar] [CrossRef]
- Matiasa, S.R.; Paganoa, M.C.; Muzzi, F.C. Effect of rhizobia, mycorrhizal fungi and phosphate-solubilizing microorganisms in the rhizosphere of native plants used to recover an iron ore area in Brazil. Eur. J. Soil Biol. 2009, 45, 259–266. [Google Scholar] [CrossRef]
- Subba Rao, N.S.S. Phosphate solubilizing microorganisms. In Biofertilizers in Agriculture, 2nd ed.; Oxford and IBH Publ Co New Delhi, Bombay: Calcutta, India, 1984; pp. 126–132. [Google Scholar]
- Sorour, S.G.R.; Elekhtyar, N.M.; El Rewainy, I.M.; Ibrahim, M.H.; Taha, H.A. Potential use of bio-fertilizer and stimulating growth compounds to promote rice productivity. J. Plant Prod. Mansoura Univ. 2018, 9, 559–565. [Google Scholar] [CrossRef]
- Hafeez, F.Y.; Hameed, S.; Zaidi, A.H.; Malik, K.A. Bio-Fertilizers for Sustainable Agriculture, in Techniques for Sustainable Agriculture Faisalabad; Nuclear Institute of Agriculture and Biology: Faisalabad, Pakistan, 2002; pp. 67–73. [Google Scholar]
- Elekhtyar, N.M.; Elkhoby, W.M.; Zidan, A.A. Prospects of using rhizobium as supplements for mineral nitrogen fertilizer on rice production in Egypt. J. Agric. Research. Kafr El-Sheikh Univ. 2015, 41, 875–884. [Google Scholar]
- Trivedi, P.; Kumar, B.; Pandey, A.; Palni, L.M.S. Growth promotion of rice by phosphate solubilizing bioinoculants in a Himalayan location. In Proceedings Books of First International Meeting on Microbial Phosphate Solubilization; Kluwer: Dordrecht, The Netherlands, 2007; pp. 291–299. [Google Scholar] [CrossRef]
- Sabia, E.; Claps, S.; Morone, G.; Bruno, A.; Sepe, L.; Aleandri, R. Field inoculation of arbuscular mycorrhiza on maize (Zea mays L.) under low inputs: Preliminary study on quantitative and qualitative aspects. Ital. J. Agron. 2015, 10, 30–33. [Google Scholar] [CrossRef]
- Vahed, H.S.; Shahinrokhsar, P.; Heydarnezhad, F. Performance of phosphate solubilizing bacteria for improving growth and yield of rice (Oryza Sativa L.) in the presence of phosphorus fertilizer. Int. J. Agric. Crop Sci. 2012, 4, 1228–1232. [Google Scholar]
- Hedley, M.I.; Kirk, G.J.D.; Santos, M.B. Phosphorus efficiency and the forms of soil phosphorus utilized by upland rice cultivars. Plant Soil 1994, 158, 53–62. [Google Scholar] [CrossRef]
- Gulati, A.; Rahi, P.; Vyas, P. Characterization of phosphate solubilizing florescent Pseudomonas from the rhizosphere of sea buckthorn growing in the cold desert of Himalayas. Curr. Microbiol. 2007, 56, 73–79. [Google Scholar] [CrossRef]
- Shaharoona, B.; Naveed, M.; Arshad, M.; Zahir, Z.A. Fertilizer-dependent efficiency of Pseudomonads for improving growth, yield, and nutrient use efficiency of wheat (Triticum aestivum L.). Appl. Microbiol. Biotechnol. 2008, 79, 147–155. [Google Scholar] [CrossRef]
- Elekhtyar, N.M.; Elsharnobi, D.E.; El-Mowafi, H.F. Evaluation of New Egyptian Japonica Green Super Rice Varieties Under Fertilization and Plant Spacing. J. Plant Prod. 2021, 12, 1015–1019. [Google Scholar] [CrossRef]
- Rodriguez, H.; Fraga, R.; Gonzelaz, T.; Bashan, Y. Genetics of phosphate solubilization and its potential application for improving plant growth promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Walpola, B.C.; Yoon, M.H. Prospectus of phosphate solubilizing microorganisms and phosphorus availability in agricultural soils: A review. Afr. J. Microbiol. Res. 2012, 6, 6600–6605. [Google Scholar] [CrossRef]
- Hoberg, E.; Marschner, P.; Lieberei, R. Organic acid exudation and pH changes by Gordonia sp. and Pseudomonas fluorescens grown with P adsorbed to goethite. Microbiol. Res. 2005, 160, 177–187. [Google Scholar] [CrossRef]
- Elekhtyar, N.M. Response of Rice Yield to Application of Nitrogen from Different Sources and Forms. Master’s Thesis, Faculty of Agriculture, Kafrelsheikh University, Kafr el-Sheikh, Egypt, 2007. [Google Scholar]
- Tarafdar, J.C.; Gharu, A. Mobilization of organic and poorly soluble phosphates by Chaetomium globosum. Appl. Soil Ecol. 2006, 32, 273–283. [Google Scholar] [CrossRef]
- Jorquera, M.A.; Hernandez, M.T.; Rengel, Z.; Marschner, P.; Mora, M. Isolation of culturable phosphobacteria with both phytatemineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol. Fertil. Soils 2008, 44, 1025–1034. [Google Scholar] [CrossRef]
- Chung, H.; Park, M.; Madhaiyan, M.; Seshadri, S.; Song, J.; Cho, H.; Sa, T. Isolation and characteri- zation of phosphate-solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol. Biochem. 2005, 37, 1970–1974. [Google Scholar] [CrossRef]
- Jones, D.L.; Darrah, P.R. Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 1994, 166, 247–257. [Google Scholar] [CrossRef]
- Galal, Y.G.; El-Gandaour, J.A.; El-Akel, F.A. Stimulation of the wheat growth and N fixation through Azospirillum and Rhizobium inoculation. A field trial with 15N techniques. In Plant Nutrition-Food Security and Sustainability of Agroecosystems; Horst, W.J., Ed.; Kluwer: Dordrecht, The Netherlands, 2001; pp. 666–667. [Google Scholar] [CrossRef]
- Naderi, M.R.; Sharaki, A. Nanofertilizers and their role in sustainable agriculture. Int. J. Agric. Crop Sci. 2013, 5, 2229–2232. [Google Scholar]
- Elekhtyar, N.M.; Mikhael, B.B.; Wissa, M.T. Utilization of compost and compost tea for improving Egyptian hybrid rice one cultivar. J. Sustain. Agric. Sci. 2017, 43, 141–149.–149. [Google Scholar] [CrossRef][Green Version]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. A review. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Wissa, M.T.; Awad-Allah, M.M.A.; Elekhtyar, N.M. Response of Egyptian hybrid rice one cultivar to times of nitrogen application and foliar spraying of ascobien compound. J. Plant Prod. 2016, 7, 567–574. [Google Scholar] [CrossRef]
- Attia, M.; Ahmed, M.A.; El-Sonbaty, M.R. Use of biotechnologies to increase growth, productivity and fruit of Maghrabi Banana under different rates of phosphorus. World J. Agric. Sci. 2009, 5, 211–220. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Adesemoye, A.O.; Kloepper, J.W. Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 2009, 85, 1–12. [Google Scholar] [CrossRef]
- Jilani, G.; Akram, A.; Ali, R.M.; Hafeez, F.Y.; Shamsi, I.H.; Chaudhry, A.N.; Chaudhry, A.G. Enhancing crop growth, nutrients availability, economics and beneficial rhizosphere microflora through organic and biofertilizers. Ann. Microbiol. 2007, 57, 177–183. [Google Scholar] [CrossRef]
- Yazdani, M.; Bahmanyar, M.A.; Pirdashti, H.; Esmaili, M.A. Effect of phosphate solubilization microorganisms (PSM) and plant growth-promoting rhizobacteria (PGPR) on yield and yield components of corn (Zea mays L.). Proc. World Acad. Sci. Eng. Technol. 2009, 37, 90–92. [Google Scholar]

| 2019 | 2020 | |
|---|---|---|
| Soluble anions (meq. L−1) | ||
| HCO3− | 17.80 | 17.00 |
| Cl− | 17.20 | 16.90 |
| SO4−− | 3.12 | 2.90 |
| Soluble Cations (meq. L−1) | ||
| Ca++ | 9.41 | 8.25 |
| Mg++ | 4.52 | 3.80 |
| K+ | 1.48 | 1.22 |
| Na++ | 12.40 | 13.05 |
| Available micronutrients (ppm) | ||
| Fe++ | 5.95 | 5.30 |
| Mn++ | 3.30 | 3.10 |
| Zn++ | 1.00 | 1.15 |
| Available NH4+ (mg kg−1) | 14.15 | 13.70 |
| Available P (mg kg−1) | 11.92 | 12.00 |
| Available K (mg kg−1) | 375 | 380 |
| Ec (ds.m−1) | 2.55 | 2.25 |
| pH (1:2.5 water suspension) | 8.12 | 8.18 |
| Organic matter (O.M) % | 1.59 | 1.53 |
| Soil texture | Clayey | Clayey |
| Treatment | LAI | DMA (g m2) | Plant Height (cm) | |||
|---|---|---|---|---|---|---|
| Season | ||||||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| Basal application of 100% SSP (P1) | 3.524 ab | 3.324 ab | 394.7 b | 409.2 ab | 95.67 ab | 94.67 b |
| Basal application of 75% SSP (P2) | 2.120 fg | 1.943 f | 199.7 h | 208.8 g | 89.33 d | 89.33 d |
| P2 + top-dressing with PSBs | 2.677 c | 2.520 c | 223.9 fg | 240.2 f | 90.67 c | 90.00 cd |
| P2 + top-dressing with AMFs | 2.914 bc | 3.000 a–d | 239.4 f | 262.3 e | 90.67 c | 91.00 c |
| P2 + foliar spraying of PNPs | 2.160 f | 2.038 e | 214.7 g | 212.0 g | 89.33 d | 89.67 d |
| P2 + foliar spraying of PA | 2.283 d | 2.117 e | 204.7 gh | 216.5 g | 90.00 cd | 90.00 cd |
| P2 + top-dressing with (PSBs + AMFs) | 3.016 b | 2.787 b | 329.4 c | 352.6 b | 95.67 ab | 94.67 b |
| P2 + foliar spraying of (PSBs + PNPs) | 2.927 bc | 2.473 c | 301.1 d | 315.1 cd | 94.33 b | 95.33 a |
| P2 + foliar spraying of (PSBs + PA) | 2.890 b–f | 2.240 d | 299.2 de | 330.4 c | 94.67 b | 95.67 a |
| P2 + foliar spraying of (PNPs + PA) | 2.510 cd | 2.387 cd | 279.8 e | 301.0 d | 90.67 c | 91.00 c |
| P2 + foliar spraying of (PSBs + PNPs + PA) | 3.706 a | 3.527 a | 464.3 a | 462.8 a | 96.33 a | 95.00 ab |
| Zero P (Control) | 1.960 e | 1.667 g | 179.3 i | 194.6 h | 88.67 d | 89.00 d |
| F. Test | ** | ** | ** | ** | ** | ** |
| Treatment | No. of Panicles (m2) | Filled Grain Weight (g Panicle−1) | Filled Grains (%) | |||
|---|---|---|---|---|---|---|
| Season | ||||||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| Basal application of 100% SSP (P1) | 521.1 a | 547.1 a | 3.534 ab | 3.767 a | 96.47 a | 96.24 a |
| Basal application of 75% SSP (P2) | 312.5 e | 318.6 f | 2.763 de | 2.979 c | 94.92 b | 94.38 b |
| P2 + top-dressing with PSBs | 374.7 d | 398.0 e | 2.997 d | 3.334 b | 94.74 b | 94.09 b |
| P2 + top-dressing with AMFs | 395.7 c | 420.7 d | 3.173 c | 3.249 b | 95.36 ab | 96.01 a |
| P2 + foliar spraying of PNPs | 328.2 e | 322.0 f | 2.763 de | 2.934 c | 94.93 b | 94.44 b |
| P2 + foliar spraying of PA | 366.9 d | 376.8 ef | 3.154 c | 3.239 b | 95.35 ab | 95.25 ab |
| P2 + top-dressing with (PSBs + AMFs) | 445.8 b | 456.5 c | 3.369 bc | 3.546 ab | 96.39 a | 95.89 ab |
| P2 + foliar spraying of (PSBs + PNPs) | 453.8 b | 473.8 b | 3.429 b | 3.405 ab | 95.42 ab | 95.56 ab |
| P2 + foliar spraying of (PSBs + PA) | 444.4 b | 444.5 c | 2.991 d | 3.315 b | 95.61 ab | 96.37 a |
| P2 + foliar spraying of (PNPs + PA) | 416.3 bc | 450.1 c | 3.597 a | 3.739 a | 95.36 ab | 94.96 b |
| P2 + foliar spraying of (PSBs + PNPs + PA) | 506.3 ab | 521.9 ab | 3.549 ab | 3.627 a | 96.19 a | 95.43 ab |
| Zero P (Control) | 271.2 f | 311.3 fg | 2.964 d | 3.045 c | 94.66 b | 93.75 b |
| F. Test | ** | ** | ** | ** | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elekhtyar, N.M.; Awad-Allah, M.M.A.; Alshallash, K.S.; Alatawi, A.; Alshegaihi, R.M.; Alsalmi, R.A. Impact of Arbuscular Mycorrhizal Fungi, Phosphate Solubilizing Bacteria and Selected Chemical Phosphorus Fertilizers on Growth and Productivity of Rice. Agriculture 2022, 12, 1596. https://doi.org/10.3390/agriculture12101596
Elekhtyar NM, Awad-Allah MMA, Alshallash KS, Alatawi A, Alshegaihi RM, Alsalmi RA. Impact of Arbuscular Mycorrhizal Fungi, Phosphate Solubilizing Bacteria and Selected Chemical Phosphorus Fertilizers on Growth and Productivity of Rice. Agriculture. 2022; 12(10):1596. https://doi.org/10.3390/agriculture12101596
Chicago/Turabian StyleElekhtyar, Nehal M., Mamdouh M. A. Awad-Allah, Khalid S. Alshallash, Aishah Alatawi, Rana M. Alshegaihi, and Reem A. Alsalmi. 2022. "Impact of Arbuscular Mycorrhizal Fungi, Phosphate Solubilizing Bacteria and Selected Chemical Phosphorus Fertilizers on Growth and Productivity of Rice" Agriculture 12, no. 10: 1596. https://doi.org/10.3390/agriculture12101596
APA StyleElekhtyar, N. M., Awad-Allah, M. M. A., Alshallash, K. S., Alatawi, A., Alshegaihi, R. M., & Alsalmi, R. A. (2022). Impact of Arbuscular Mycorrhizal Fungi, Phosphate Solubilizing Bacteria and Selected Chemical Phosphorus Fertilizers on Growth and Productivity of Rice. Agriculture, 12(10), 1596. https://doi.org/10.3390/agriculture12101596









