Difference in Accumulation of Five Phthalate Esters in Different Elite Tea Cultivars and Their Correlation with Environment Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Reagents and Standards for PAEs
2.3. Preparation of the Mixed Standard Stock Solution
2.4. Extraction of PAEs from Fresh Tea Leaves
2.5. GC–MS Analysis
2.6. Quality Assurance and Quality Control
2.7. Statistical Data Analysis
3. Results
3.1. Distribution of PAEs in Fresh Tea Leaves of 15 Tea Cultivars
3.2. Seasonal Variation in PAEs Levels of Fresh Tea Leaves from 15 Tea Cultivars
3.3. Correlation Analysis of the five PAEs Content in Fresh Tea Leaves and Environmental Factors
3.4. Systematic Cluster Analysis of PAEs Content from 15 Tea Cultivars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.X.; Wu, Y.; Chen, H.G.; Duan, P.; Wang, L.; Shen, H.Q.; Lu, W.Q.; Sun, B.; Wang, Q.; Zhang, B.; et al. Seminal plasma metabolome in relation to semen quality and urinary phthalate metabolites among Chinese adult men. Environ. Int. 2019, 129, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Chen, L. Research progress in phthalate removal technology. Appl. Chem. Ind. 2020, 49, 426–429. [Google Scholar] [CrossRef]
- Nugegoda, D.; Kibria, G. Effects of environmental chemicals on fish thyroid function: Implications for fisheries and aquaculture in Australia. Gen. Comp. Endocrinol. 2017, 244, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Li, M.K.; Ge, J.; Lin, F.; Han, B.Y. Metabolic kinetics and residues of diethyl phthalate (DEP) in tilapia (Oreochromis niloticus × O. aureus). Oceanol. Limnol. Sin. 2011, 42, 137–141. [Google Scholar]
- Ge, J.; Li, M.K.; Lin, F.; Zhao, J.; Han, B.Y. Metabolism of phthalic acid esters in Ctenopharyngodon idellus organs and hepatocyte. Oceanol. Limnol. Sin. 2011, 42, 549–553. [Google Scholar]
- Ge, J.; Li, M.K.; Lin, F.; Zhao, J.; Han, B.Y. Study on metabolism of n-butyl benzyl phthalate (BBP) and dibutyl phthalate (DBP) in Ctenopharyngodon idellus by GC and LC-MS/MS. Afr. J. Agric. Res. 2012, 7, 1855–1862. [Google Scholar] [CrossRef]
- Dai, X.J.; Ge, J.; Li, M.K. Metabolic kinetics and residues of dimethyl phthalate (DMP) in shakehead fish. Chin. J. Vet. Sci. 2011, 31, 77–80. [Google Scholar] [CrossRef]
- Liu, P.X.; Chen, H.P.; Gao, G.W.; Hao, Z.X.; Wang, C.; Ma, G.C.; Chai, Y.F.; Liu, X. Occurrence and residue pattern of phthalate esters in fresh tea leaves and during tea manufacturing and brewing. J. Agric. Food Chem. 2016, 64, 8909–8917. [Google Scholar] [CrossRef]
- Du, L.P.; Ma, L.J.; Qiao, Y.; Lu, Y.; Xiao, D.G. Determination of phthalate esters in teas and tea infusions by gas chromatography–mass spectrometry. Food Chem. 2016, 197, 1200–1206. [Google Scholar] [CrossRef]
- Yin, P.; Liu, X.; Chen, H.P.; Pan, R.; Ma, G.C. Determination of 16 phthalate esters in tea samples using a modified QuEChERS sample preparation method combined with GC-MS/MS. Food Addit. Contam. Part A 2014, 31, 1406–1413. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Wang, M.X.; Pan, C.; Mi, S.S.; Han, B.Y. Determination of five phthalate esters in tea and their dynamic characteristics during black tea processing. Foods 2022, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.H.; Bao, M.J.; Xu, Y.; Zhang, L.J.; Tan, F.; Zhao, H.X. Characteristics and risk assessment of organophosphate esters and phthalates in soils and vegetation from Dalian, northeast China. Environ. Pollut. 2021, 284, 117532. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Gao, Z.Q.; Chen, C.Y.; Wen, S.Y.; Shu, C.; Tang, H.; Yan, L.L. Simulation and analysis of above-ground dry matter and leaf area index of rice based on Logistic model. J. Northeast Agric. Univ. 2022, 53, 10–18. [Google Scholar] [CrossRef]
- GB/T 27417. Comformity Assessment—Guidance on Validation and Verification of Chemical Analytical Methods; [NSPRC] National Standards of the People’s Republic of China: Beijing, China, 2017. [Google Scholar]
- Jia, Y.H.; Sun, X.F.; Zhang, F. Determination of LOD and LOQ with relative standard deviation and relative range or ratio of maximum and minimum. Metall. Anal. 2021, 41, 1–12. [Google Scholar] [CrossRef]
- Li, H.L. Study on Simultaneous Determination of 24 Veterinary Drug Residues Contained in Animal Derived Foods and the Matrix Effect with QuEChERS-UPLC-MS/MS. Master’s Thesis, Southwest University, Chongqing, China, 2022. [Google Scholar]
- Tang, Q.Y. DPS© Data Processing System; Science Press: Beijing, China, 2020; Volume 1, pp. 112–116. [Google Scholar]
- Tang, Q.Y.; Tang, R. DPS© Data Processing System; Science Press: Beijing, China, 2020; Volume 2, pp. 734–749. [Google Scholar]
- He, Y.; Wang, Q.M.; He, W.; Xu, F.L. Phthalate esters (PAEs) in atmospheric particles around a large shallow natural lake (Lake Chaohu, China). Sci. Total Environ. 2019, 687, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sun, Y.Y.; Wang, Z.H.; He, G.; Quan, H.X.; He, H.X. Plastic film mulching increased the accumulation and human health risks of phthalate esters in wheat grains. Environ. Pollut. 2019, 250, 1–7. [Google Scholar] [CrossRef]
- Wang, L.Q.; He, L.M.; Zeng, Z.L.; Chen, J.X. Progress in matrix effect of veterinary drug residues analysis by high-performance liquid chromatography tandem mass spectrometry. J. Chin. Mass Spectrom. Soc. 2011, 32, 321–332. [Google Scholar]
- Yarita, T.; Aoyagi, Y.; Otake, T. Evaluation of the impact of matrix effect on quantification of pesticides in foods by gas chromatography-mass spectrometry using isotope-labeled internal standards. J. Chromatogr. A 2015, 1396, 109–116. [Google Scholar] [CrossRef]
- Li, H.L.; Wu, X.Q.; Zhao, B.; Hou, M.L.; Wu, Y.L.; Huang, X.L. Matrix effect in analysis of 24 veterinary drug residues in animal-derived foods by QuEChERS-UPLC-MS/MS. Food Ferment. Ind. 2021, 47, 253–259. [Google Scholar] [CrossRef]
- Qiang, S.J.; Liu, Y.R.; Li, G. Influence and correction of solvent standard and matrix standard on pesticide residue detection results. Chin. Agric. Sci. Bull. 2022, 38, 99–106. [Google Scholar]
- Wang, X.J. Practical application of phytoremediation technology of contaminated soil. J. Xihua Univ.(Nat. Sci. Ed.) 2019, 38, 65–70. [Google Scholar]
- Ma, T.T.; Luo, Y.M.; Christie, P.; Teng, Y.; Liu, W.X. Removal of phthalic esters from contaminated soil using different cropping systems: A field study. Eur. J. Soil Biol. 2012, 50, 76–82. [Google Scholar] [CrossRef]
- Wei, L.Q.; Hu, S.B.; Wang, J.J.; Chai, Q.Q.; Liu, J.B.; Wang, M.K.; Shi, C. Phytoremediation of 4 phthalic acid esters in contaminated soil by beet-grass system. J. Agro-Environ. Sci. 2016, 35, 1097–1102. [Google Scholar]
- Lu, L.Q.; Ye, C.M. Bioremediation technology of phthalic acid esters in soil. Anhui Agri. Sci. Bull. 2016, 22, 25–26+42. [Google Scholar] [CrossRef]
- Teng, Y.; Shen, Y.Y.; Luo, Y.M.; Sun, X.H.; Sun, M.M.; Fu, D.Q.; Li, Z.G.; Christie, P. Influence of Rhizobium meliloti on phytoremediation of polycyclic aromatic hydrocarbons by alfalfa in an aged contaminated soil. J. Hazard. Mater. 2011, 186, 1271–1276. [Google Scholar] [CrossRef]
- Wu, Z.H.; Wang, M.X.; Pan, C.; Han, S.J.; Han, B.Y. Research progress and prospect of occurrence regularity and green control of tea green leafhopper. J. Tea Commun. 2021, 48, 200–206+252. [Google Scholar]
- Niu, Y.Q.; Han, S.J.; Wu, Z.H.; Pan, C.; Wang, M.X.; Tang, Y.Y.; Zhang, Q.H.; Tan, G.J.; Han, B.Y. A push-pull strategy for controlling the tea green leafhopper (Empoasca flavescens F.) using semiochemicals from Tagetes erecta and Flemingia macrophylla. Pest Manag. Sci. 2022, 78, 2161–2172. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.L.; Guo, Y.; Wu, Q.; Yang, M.; Wu, M.H.; Kannan, K. Phthalate diesters in airborne PM2.5 and PM10 in a suburban area of Shanghai: Seasonal distribution and risk assessment. Sci. Total Environ. 2014, 497, 467–474. [Google Scholar] [CrossRef]
- Li, P.H.; Jia, H.Y.; Wang, Y.; Li, T.; Wang, L.; Li, Q.Q.; Yang, M.M.; Yue, J.J.; Yi, X.L.; Guo, L.Q. Characterization of PM2.5—bounding phthalic acid esters (PAEs) at regional background site in northern China: Long-range transport and risk assessment. Sci. Total Environ. 2019, 659, 140–149. [Google Scholar] [CrossRef]

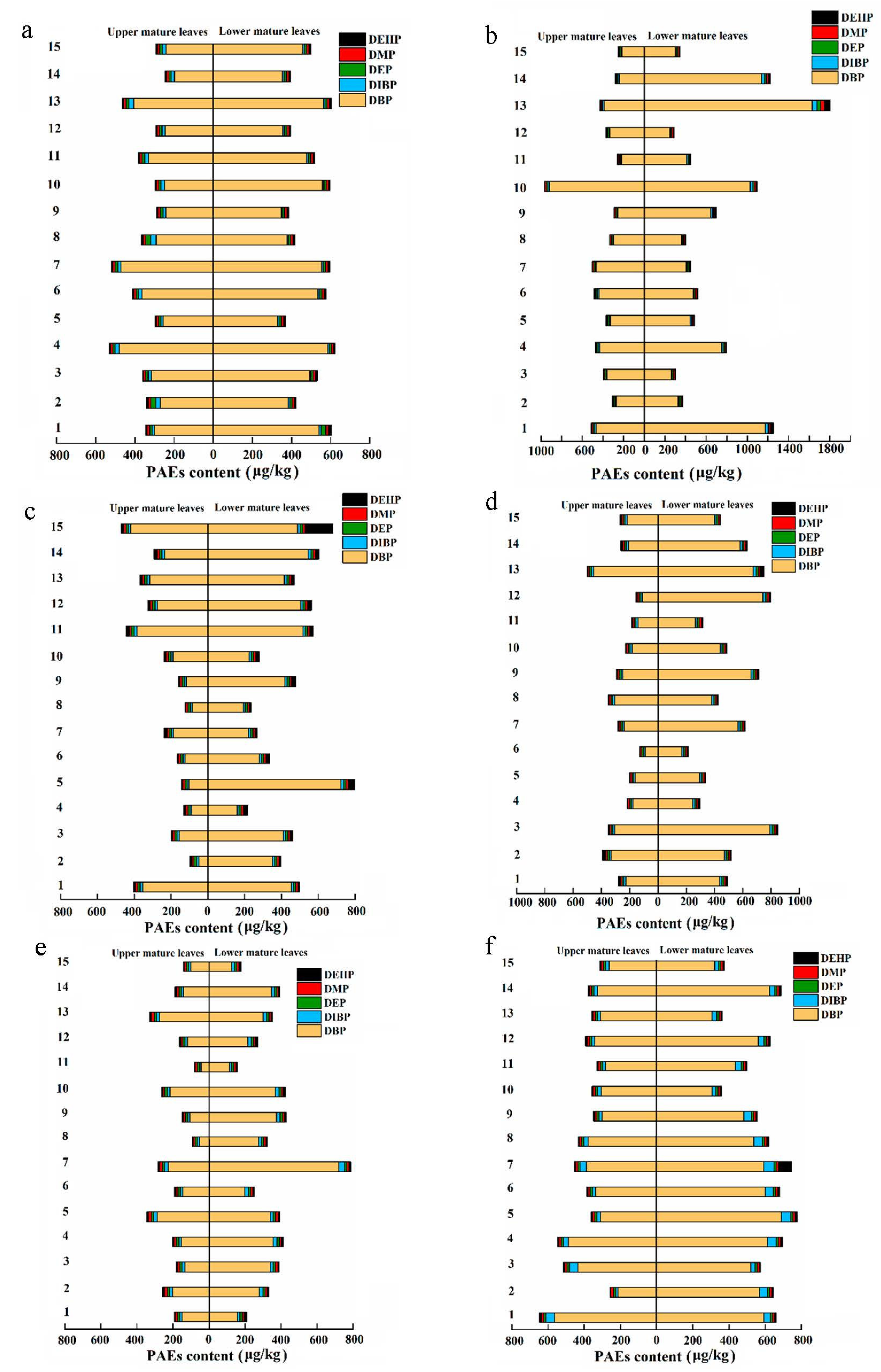
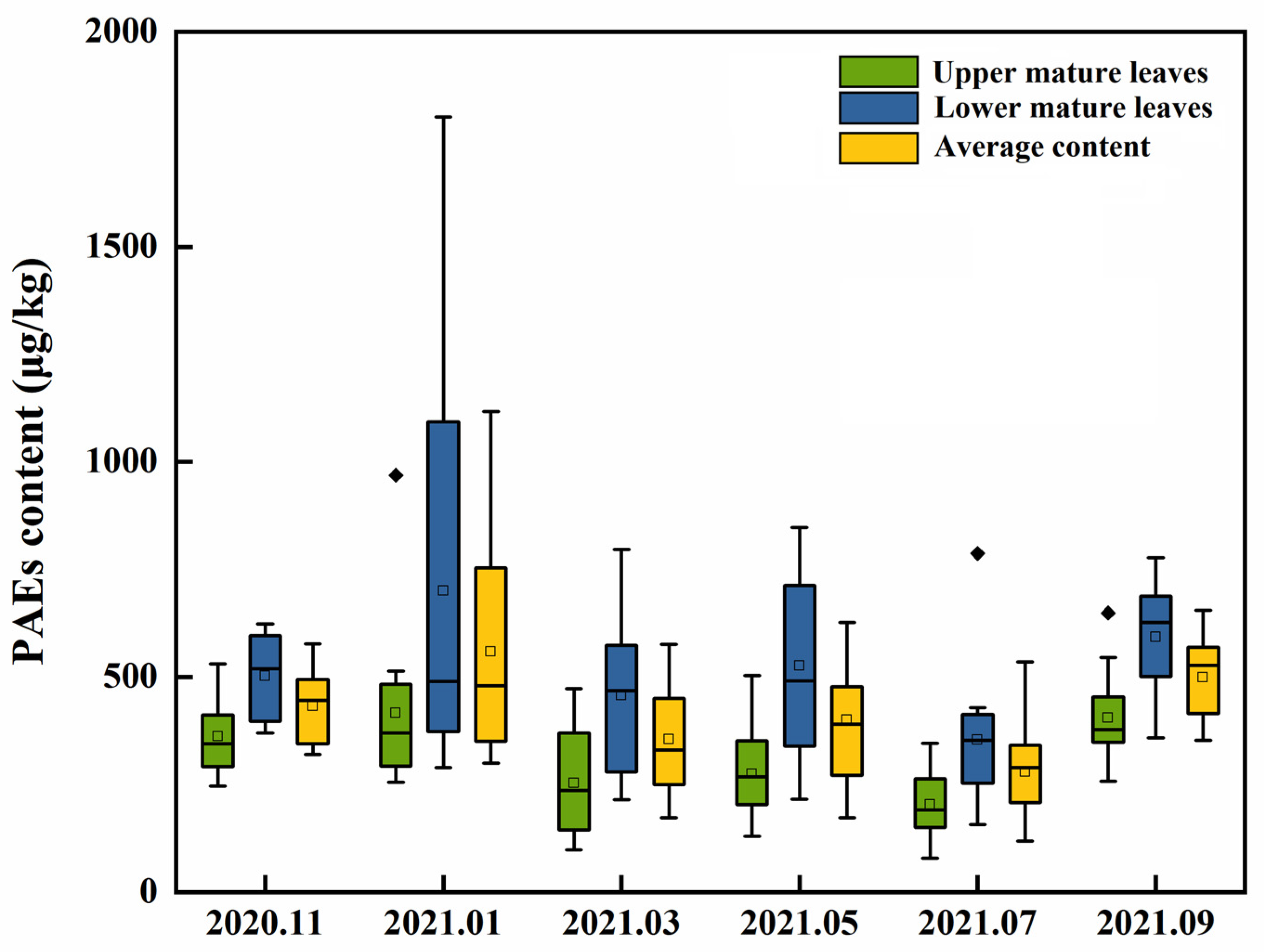
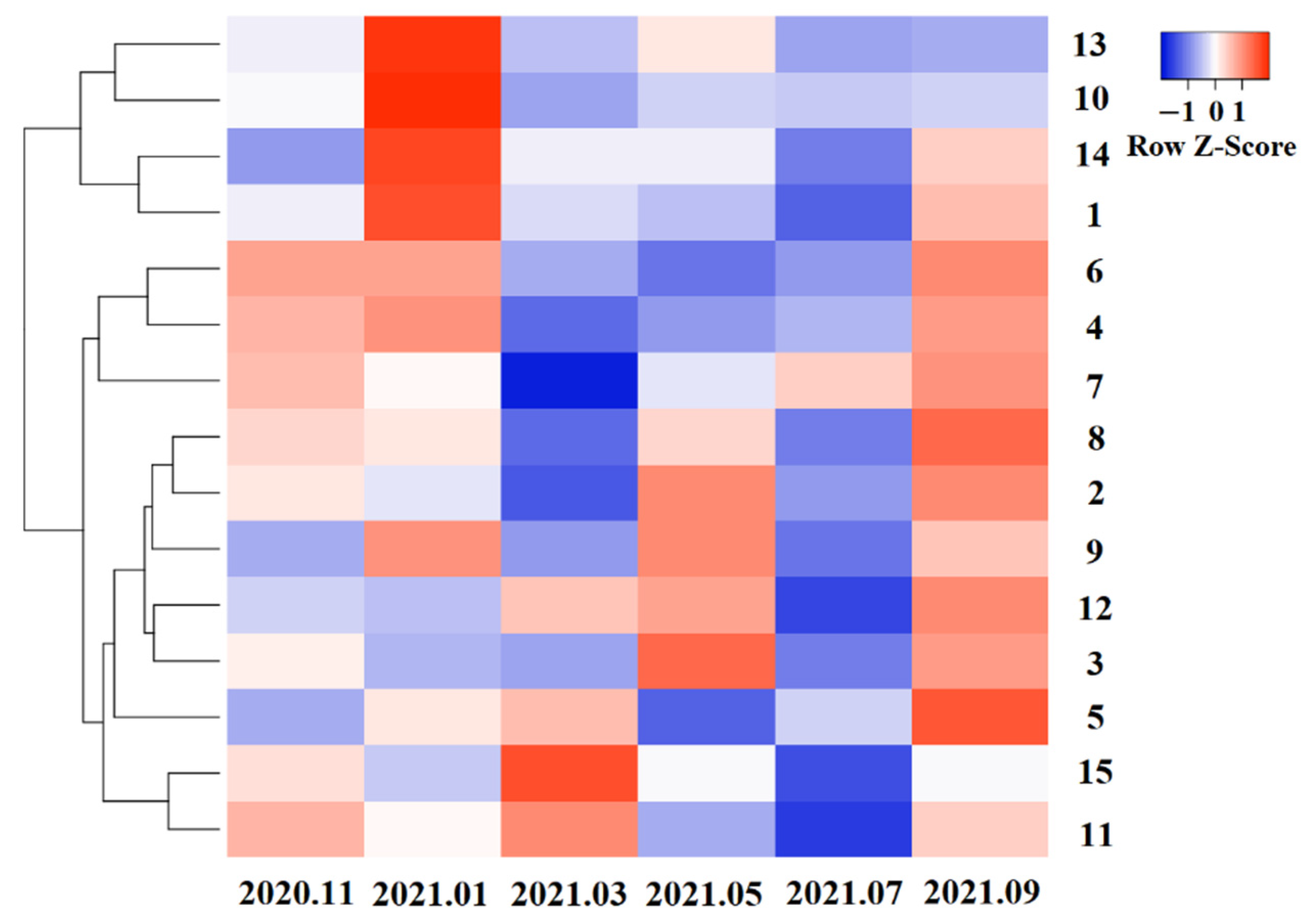
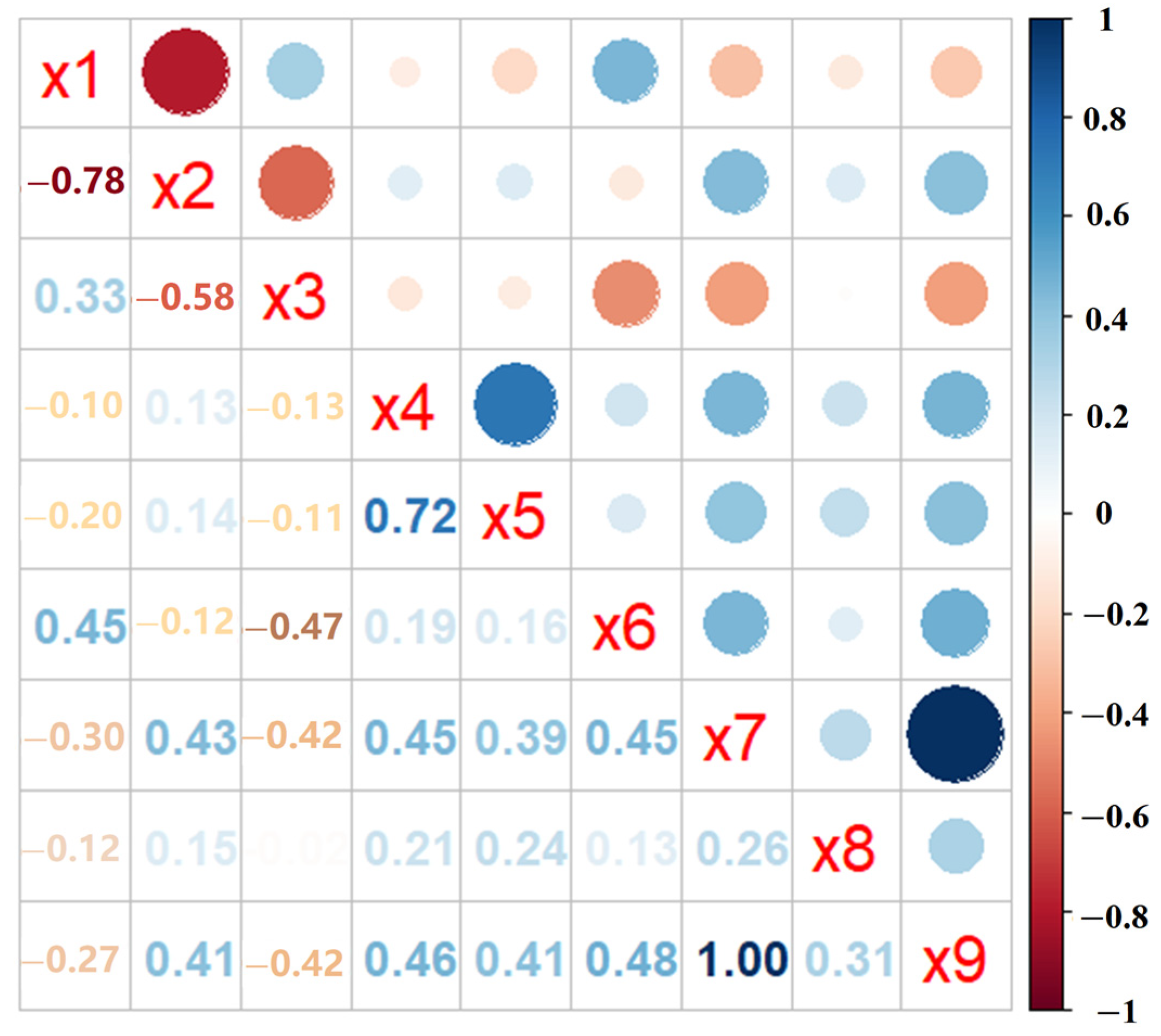
| Number | Tea Cultivars | Upper Mature Leaves | Lower Mature Leaves | Average Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximum | Minimum | Median | Geometric Mean | Maximum | Minimum | Median | Geometric Mean | Maximum | Minimum | Median | Geometric Mean | ||
| 1 | Jiukengzao | 650.96 | 163.26 | 346.84 | 396.99 | 1320.75 | 159.96 | 545.71 | 619.35 | 883.75 | 200.58 | 462.25 | 508.17 |
| 2 | Zimudan | 393.92 | 94.27 | 283.57 | 276.24 | 662.94 | 317.42 | 418.11 | 447.31 | 453.75 | 247.27 | 362.18 | 361.78 |
| 3 | Mingke 1 | 522.58 | 100.71 | 350.02 | 334.34 | 867.54 | 115.16 | 462.01 | 518.36 | 599.73 | 286.28 | 398.25 | 426.35 |
| 4 | Huangguanyin | 580.60 | 127.43 | 349.05 | 350.13 | 803.37 | 207.34 | 514.20 | 507.40 | 636.88 | 173.31 | 441.73 | 428.76 |
| 5 | Jiaming 1 | 444.19 | 140.79 | 282.26 | 369.43 | 799.55 | 312.23 | 422.12 | 527.78 | 569.30 | 271.59 | 399.30 | 407.27 |
| 6 | Maolv | 499.81 | 80.24 | 251.33 | 482.97 | 690.07 | 210.40 | 415.92 | 429.52 | 532.34 | 172.95 | 372.08 | 362.08 |
| 7 | Tieguanyin | 547.87 | 140.71 | 396.73 | 380.44 | 801.99 | 177.30 | 589.79 | 578.01 | 599.28 | 253.96 | 507.39 | 479.22 |
| 8 | Zijuan | 436.65 | 88.09 | 328.93 | 284.39 | 636.67 | 230.58 | 415.00 | 404.67 | 527.24 | 179.44 | 379.82 | 344.53 |
| 9 | Huangmudan | 360.56 | 119.18 | 273.37 | 255.08 | 749.60 | 165.65 | 476.89 | 543.32 | 502.82 | 288.95 | 394.44 | 399.20 |
| 10 | Pingyang Tezao | 1077.59 | 165.29 | 275.65 | 392.05 | 1117.46 | 154.20 | 449.53 | 539.76 | 1031.06 | 257.82 | 358.11 | 465.90 |
| 11 | Longjing Changye | 445.67 | 78.98 | 275.27 | 280.47 | 583.24 | 117.89 | 501.13 | 420.40 | 508.22 | 118.03 | 386.28 | 350.44 |
| 12 | Zhongcha102 | 397.80 | 110.39 | 292.81 | 283.49 | 806.93 | 165.20 | 391.67 | 490.84 | 509.86 | 217.13 | 394.44 | 387.17 |
| 13 | Zhongcha108 | 508.84 | 239.79 | 374.86 | 409.24 | 1915.01 | 268.96 | 529.95 | 723.66 | 1116.84 | 341.38 | 476.22 | 566.45 |
| 14 | Longjing 43 | 383.25 | 138.84 | 259.79 | 274.63 | 1298.33 | 104.79 | 602.61 | 655.81 | 753.00 | 291.05 | 447.08 | 465.22 |
| 15 | Baiye 1 | 480.04 | 140.33 | 270.36 | 290.03 | 685.06 | 167.23 | 423.43 | 422.48 | 575.49 | 158.79 | 349.08 | 356.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Tang, Y.; Mi, S.; Pan, J.; Guo, C.; Li, J.; Han, B. Difference in Accumulation of Five Phthalate Esters in Different Elite Tea Cultivars and Their Correlation with Environment Factors. Agriculture 2022, 12, 1516. https://doi.org/10.3390/agriculture12101516
Wang M, Tang Y, Mi S, Pan J, Guo C, Li J, Han B. Difference in Accumulation of Five Phthalate Esters in Different Elite Tea Cultivars and Their Correlation with Environment Factors. Agriculture. 2022; 12(10):1516. https://doi.org/10.3390/agriculture12101516
Chicago/Turabian StyleWang, Mengxin, Yanyan Tang, Shuishan Mi, Jingen Pan, Chunwei Guo, Jie Li, and Baoyu Han. 2022. "Difference in Accumulation of Five Phthalate Esters in Different Elite Tea Cultivars and Their Correlation with Environment Factors" Agriculture 12, no. 10: 1516. https://doi.org/10.3390/agriculture12101516
APA StyleWang, M., Tang, Y., Mi, S., Pan, J., Guo, C., Li, J., & Han, B. (2022). Difference in Accumulation of Five Phthalate Esters in Different Elite Tea Cultivars and Their Correlation with Environment Factors. Agriculture, 12(10), 1516. https://doi.org/10.3390/agriculture12101516






