Effect of Various Strains of Lactobacillus buchneri on the Fermentation Quality and Aerobic Stability of Corn Silage
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, M.S.; Coors, J.G.; Roth, G.W. Corn silage. In Madison Silage Science and Technology; Buxton, D.R., Ed.; SSSA: Madison, WI, USA, 2003; pp. 547–608. [Google Scholar]
- Ferraretto, L.; Shaver, R.; Luck, B. Silage review: Recent advances and future technologies for whole-plant and fractionated corn silage harvesting. J. Dairy Sci. 2018, 101, 3937–3951. [Google Scholar] [CrossRef]
- Adesogan, A.T. Corn silage quality in tropical climates. In Proceedings of the 5th Symposium on Strategic Management of Pasture, Vicosa, Brazil, 14–16 November 2010; pp. 311–327. [Google Scholar]
- Pahlow, G.; Muck, R.E.; Driehuis, F. Microbiology of ensiling. In Silage Science and Technology; Buxton, D.R., Ed.; American Society of Agronomy: Madison, WI, USA, 2003. [Google Scholar]
- Jonsson, A.; Pahlow, G. Systematic classification and biochemical characterization of yeasts growing in grass silage inoculated with Lactobacillus cultures. Anim. Res. Dev. 1984, 20, 7–22. [Google Scholar]
- Santos, M.; Golt, C.; Joerger, R.; Mechor, G.; Mourão, G.B.; Kung, L. Identification of the major yeasts isolated from high moisture corn and corn silages in the United States using genetic and biochemical methods. J. Dairy Sci. 2017, 100, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, S.; Oldenburg, E.; Pahlow, G. Influence of microbes and their metabolites on feed and food quality. In Proceedings of the 19th General Meeting of the European Grassland Federation, La Rochelle, France, 27–30 May 2002; pp. 503–511. [Google Scholar]
- Muck, R.E. A lactic acid bacteria strain to improve aerobic stability of silages. In 1996 Research Summaries; US Dairy Forage Research Center: Madison, WI, USA, 1996; pp. 42–43. [Google Scholar]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L., Jr. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Santos, A.; Ávila, C.; Pinto, J.; Carvalho, B.; Dias, D.R.; Schwan, R. Fermentative profile and bacterial diversity of corn silages inoculated with new tropical lactic acid bacteria. J. Appl. Microbiol. 2015, 120, 266–279. [Google Scholar] [CrossRef]
- Gomes, A.; Jacovaci, F.; Bolson, D.; Nussio, L.; Jobim, C.; Daniel, J. Effects of light wilting and heterolactic inoculant on the formation of volatile organic compounds, fermentative losses and aerobic stability of oat silage. Anim. Feed. Sci. Technol. 2019, 247, 194–198. [Google Scholar] [CrossRef]
- Rabelo, C.; Härter, C.J.; da Silva Ávila, C.L.; Reis, R.A. Meta-analysis of the effects of Lactobacillus plantarum and Lactobacillus buchneri on fermentation, chemical composition and aerobic stability of sugarcane silage. Grassl. Sci. 2019, 65, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Arriola, K.G.; Oliveira, A.S.; Jiang, Y.; Kim, D.; Silva, H.M.; Kim, S.C.; Amaro, F.X.; Ogunade, I.M.; Sultana, H.; Cervantes, A.A.P.; et al. Meta-analysis of effects of inoculation with Lactobacillus buchneri, with or without other bacteria, on silage fermentation, aerobic stability, and performance of dairy cows. J. Dairy Sci. 2021, 104, 7653–7670. [Google Scholar] [CrossRef]
- Silva, L.D.; Pereira, O.G.; Silva, T.C.; Leandro, E.S.; Paula, R.A.; Santos, S.; Ribeiro, K.G.; Filho, S.C.V. Effects of Lactobacillus buchneri isolated from tropical maize silage on fermentation and aerobic stability of maize and sugarcane silages. Grass Forage Sci. 2018, 73, 660–670. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, TX, USA, 1990. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Okuda, H.; Fujii, S.; Kawashima, Y. A direct colorimetric determination of blood ammonia. Tokushima J. Exp. Med. 1965, 12, 11–23. [Google Scholar]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Siegfried, V.R.; Ruckemmann, H.; Stumpf, G. Method for the determination of organic acids in silage by high performance liquid chromatography. Landwirtsch Forsch 1984, 37, 298. [Google Scholar]
- Jobim, C.C.; Nussio, L.G.; Reis, R.A.; Schmidt, P. Methodological advances in evaluation of preserved forage quality. Rev. Bras. Zootec. 2007, 36, 101–119. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for STATISTICAL Computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: https://www.R-project.org/ (accessed on 17 February 2020).
- Haigh, P.M.; Parker, J.W.G. Effect of silage additives and wilting on silage fermentation, digestibility and intake, and on liveweight change of young cattle. Grass Forage Sci. 1985, 40, 429–436. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Bruning, C.L.; Yokoyama, M.T. Characteristics of Live and Killed Brewer’s Yeast Slurries and Intoxication by Intraruminal Administration to Cattle. J. Anim. Sci. 1988, 66, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Krooneman, J.; Faber, F.; Alderkamp, A.C.; Oude Elferink, S.J.H.W.; Driehuis, F.; Cleenwerck, I.; Swings, J.; Gottschal, J.C.; Vancanneyt, M. Lactobacilllus diolivorans sp. nov., a 1,2 propanediol-degrading bacterium isolated from aerobically stable maize silage. Int. J. Syst. Evol. Microbiol. 2002, 52, 639–646. [Google Scholar] [CrossRef]
- Huhtanen, P.; Rinne, M.; Nousiainen, J. Evaluation of the factors affecting silage intake of dairy cows: A revision of the relative silage dry-matter intake index. Animal 2007, 1, 758–770. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Onishi, H. Aerobic dissimilation of α-rhamnose and the production of α-rhamnnoic acid and 1,2 propanediol by yeast. Agric. Biol. Chem. 1968, 32, 888–893. [Google Scholar]
- Sanchez, R.F.; Cameron, D.C.; Cooney, C.L. Influence of environmental factors in the production of (R)-1,2 propanediol and acetol by Clostridium thermosaccharolyticum. Biotechnol. Lett. 1987, 9, 449–454. [Google Scholar] [CrossRef]
- Heron, S.J.E.; Edwards, R.A.; McDonald, P. Changes in the nitrogenous components of gamma-irradiated and inoculated ensiled ryegrass. J. Sci. Food Agric. 1986, 37, 979–985. [Google Scholar] [CrossRef]
- McKersie, B.D. Effect of pH on Proteolysis in Ensiled Legume Forage1. Agron. J. 1985, 77, 81–86. [Google Scholar] [CrossRef]
- Oude Elferink, S.J.W.H.; Dreiehuis, F.; Gottschal, J.C.; Spoelstra, S.F. Silage fermentation processes and their manipulation. In Silage Making in the Tropics with Particular Emphasis on Smallholders, FAO Electronic Conference on Tropical Silage; Mannetje, L., Ed.; Food and Agriculture Organization (FAO): Rome, Italy, 1999; pp. 17–30. [Google Scholar]
- McDonald, P.; Henderson, A.R.; Heron, S. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow, UK, 1991; p. 340. [Google Scholar]
- Kung, L.; Smith, M.L.; Da Silva, E.B.; Windle, M.C.; Da Silva, T.C.; Polukis, S.A. An evaluation of the effectiveness of a chemical additive based on sodium benzoate, potassium sorbate, and sodium nitrite on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2018, 101, 5949–5960. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Ding, W.; Ke, W.; Li, F.; Zhang, P.; Guo, X. Modulation of metabolome and bacterial community in whole crop corn silage by inoculating homofermentative Lactobacillus plantarum and heterofermentative Lactobacillus buchneri. Front. Microbiol. 2019, 9, 3299. [Google Scholar] [CrossRef] [Green Version]
- Elferink, S.J.W.H.O.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic conversion of lactic acid to acetic acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, B.F.; Ávila, C.L.S.; Miguel, M.G.C.P.; Pinto, J.C.; Santos, M.C.; Schwan, R. Aerobic stability of sugar-cane silage inoculated with tropical strains of lactic acid bacteria. Grass Forage Sci. 2014, 70, 308–323. [Google Scholar] [CrossRef]
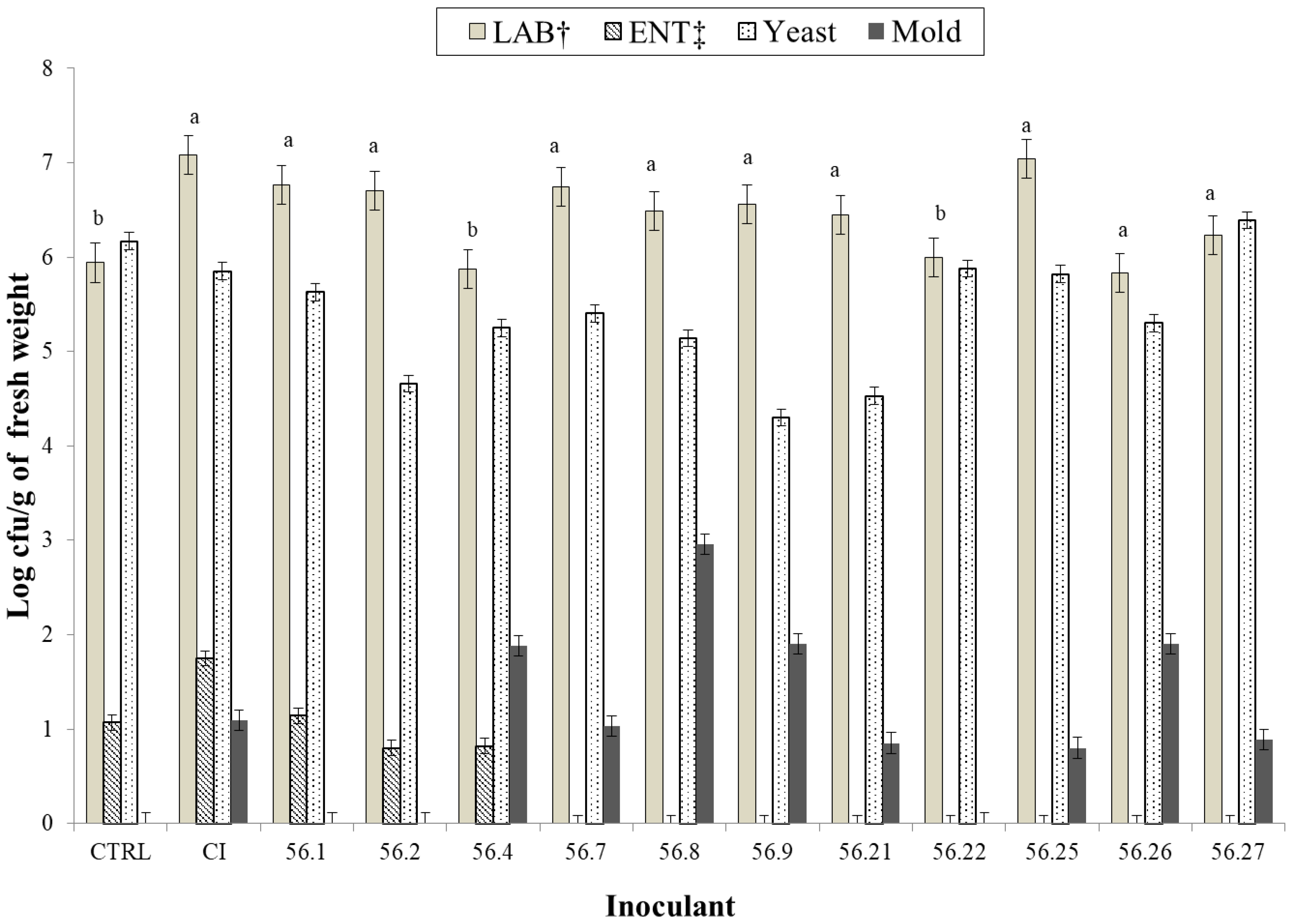
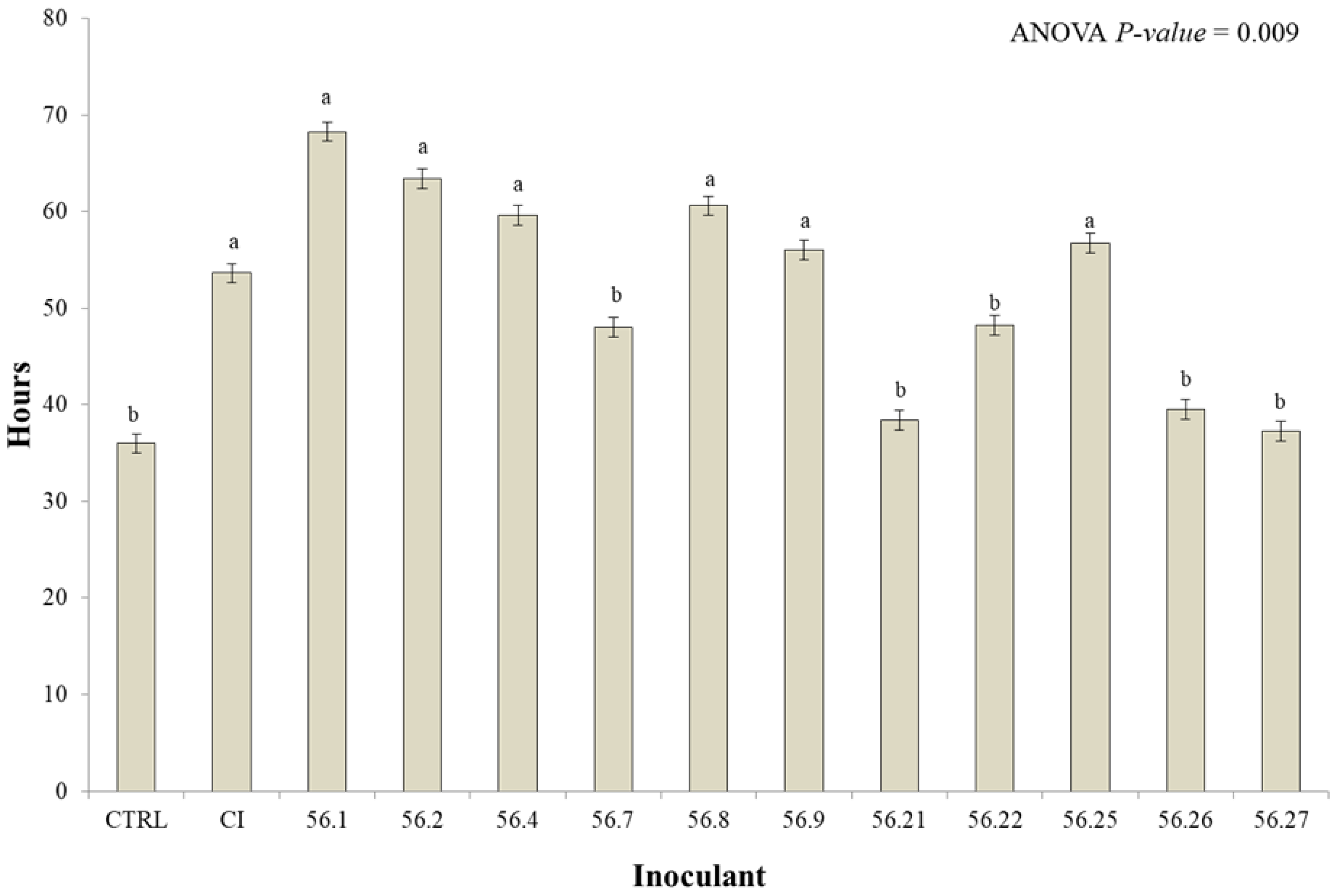
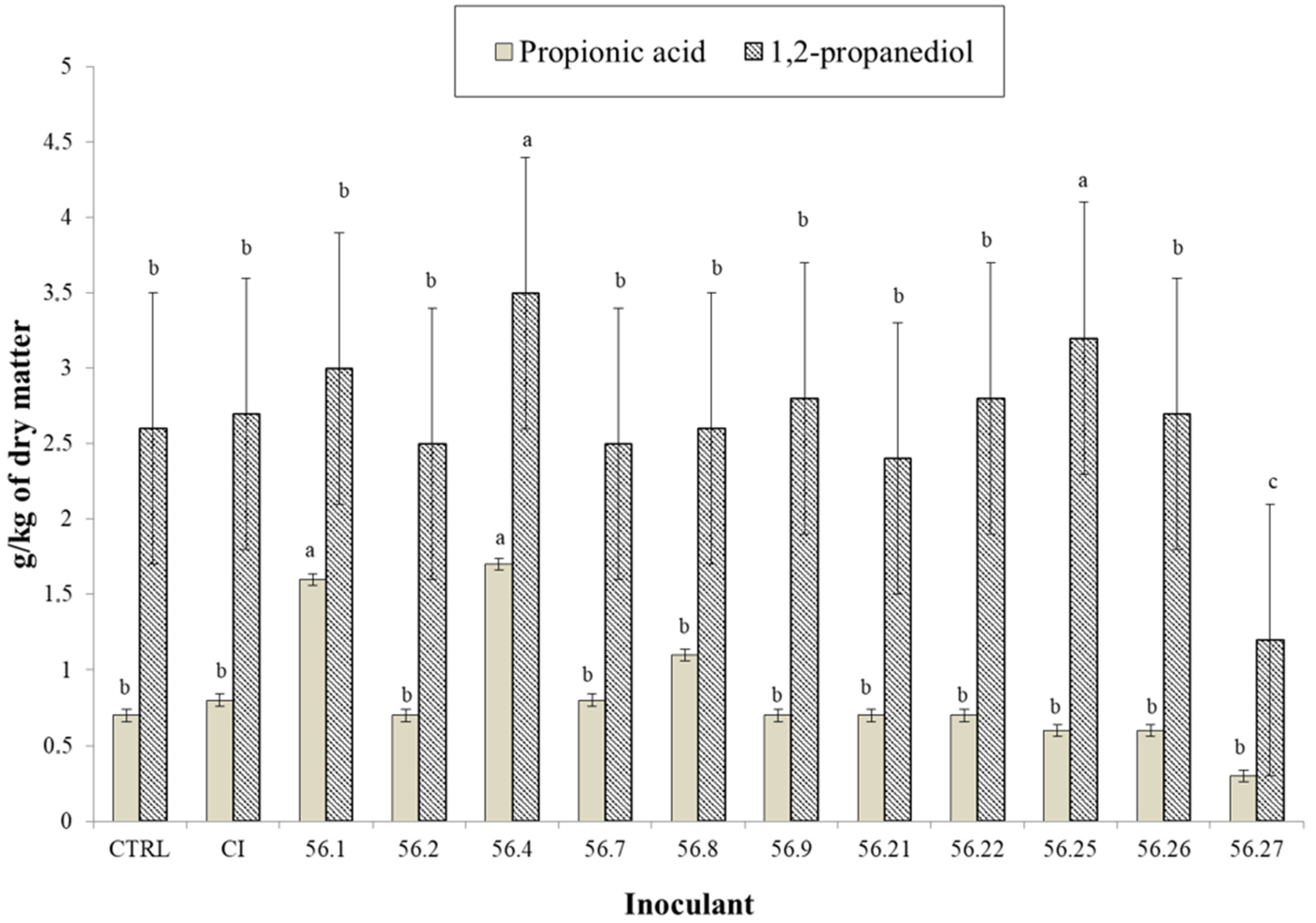
| Treatment | Items | ||||
|---|---|---|---|---|---|
| DM †, g/kg | CP ‡ | NDF § | ADF ¶ | DM rec ¥, % | |
| CTRL | 257 | 54.7 | 549 | 329 | 91.9 |
| CI | 260 | 58.4 | 505 | 333 | 95.9 |
| LB-56.1 | 260 | 56.9 | 515 | 354 | 92.7 |
| LB-56.2 | 268 | 56.7 | 520 | 332 | 95.5 |
| LB-56.4 | 262 | 57.5 | 496 | 330 | 95.0 |
| LB-56.7 | 267 | 55.1 | 505 | 297 | 88.7 |
| LB-56.8 | 268 | 54.7 | 511 | 319 | 97.1 |
| LB-56.9 | 265 | 56.7 | 488 | 341 | 97.0 |
| LB-56.21 | 269 | 51.5 | 510 | 349 | 98.8 |
| LB-56.22 | 272 | 53.9 | 497 | 295 | 99.7 |
| LB-56.25 | 270 | 56.9 | 486 | 280 | 97.8 |
| LB-56.26 | 271 | 55.4 | 497 | 283 | 99.1 |
| LB-56.27 | 276 | 55.1 | 491 | 282 | 97.6 |
| SEM | 2.339 | 0.551 | 5.361 | 5.952 | 4.318 |
| ANOVA p-value | 0.97 | 0.71 | 0.79 | 0.08 | 0.20 |
| Items | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | WSC † | NH3–N ‡ | LA § | AA ¶ | BA ¥ | PA € | ET ƛ | 1,2-PD £ | |
| CTRL | 3.65 c | 10.8 | 80.4 b | 59.1 | 12.4 b | 0.27 | 0.80 e | 17.2 | 3.06 a |
| CI | 3.70 c | 6.90 | 86.3 b | 29.0 | 10.9 c | 0.23 | 7.55 d | 11.7 | 2.56 b |
| LB-56.1 | 3.84 a | 8.10 | 69.9 b | 37.8 | 25.3 a | 0.23 | 30.2 a | 26.2 | 2.75 b |
| LB-56.2 | 3.80 b | 5.40 | 74.9 b | 34.6 | 14.7 b | 0.23 | 15.0 c | 14.9 | 2.46 b |
| LB-56.4 | 3.85 a | 7.90 | 80.1 b | 31.8 | 14.0 b | 0.27 | 16.5 c | 16.2 | 2.40 b |
| LB-56.7 | 3.76 b | 7.00 | 72.3 b | 43.0 | 20.8 a | 0.23 | 15.0 c | 7.10 | 2.36 b |
| LB-56.8 | 3.81 b | 7.10 | 100 a | 39.7 | 15.4 b | 0.27 | 21.1 b | 16.5 | 2.30 b |
| LB-56.9 | 3.83 a | 8.20 | 79.4 b | 36.3 | 14.4 b | 0.25 | 16.2 c | 13.6 | 2.46 b |
| LB-56.21 | 3.71 c | 7.80 | 99.7 a | 53.4 | 13.3 b | 0.23 | 3.50 e | 15.7 | 2.40 b |
| LB-56.22 | 3.70 c | 8.30 | 95.2 a | 38.1 | 8.30 c | 0.25 | 0.80 e | 13.4 | 2.60 b |
| LB-56.25 | 3.71 c | 7.70 | 81.4 b | 41.1 | 6.60 c | 0.23 | 1.25 e | 8.00 | 3.35 a |
| LB-56.26 | 3.67 c | 8.80 | 77.3 b | 35.0 | 8.20 c | 0.23 | 0.70 e | 6.05 | 2.43 b |
| LB-56.27 | 3.68 c | 8.70 | 90.7 a | 50.6 | 11.1 c | 0.27 | 1.25 e | 14.5 | 2.26 b |
| SEM | 0.016 | 0.254 | 2.762 | 3.912 | 1.353 | 0.010 | 1.655 | 1.649 | 0.156 |
| ANOVA p-value | <0.01 | 0.009 | 0.01 | 0.04 | <0.01 | 0.99 | <0.01 | <0.01 | 0.0003 |
| Treatment | Items | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DM † | pH | YM ‡ | Tmax ¶ | HTmax ¥ | LA € | AA ƛ | BA £ | Ethanol | |
| CTRL | 261 | 6.36 | 9.16 | 41.7 | 50.1 | 1.80 | 1.53 | 0.266 | 12.5 |
| CI | 252 | 6.61 | 9.11 | 41.8 | 67.9 | 2.25 | 3.26 | 0.233 | 9.23 |
| LB-56.1 | 270 | 6.16 | 8.54 | 38.8 | 86.0 | 4.46 | 3.26 | 0.250 | 13.2 |
| LB-56.2 | 264 | 6.15 | 8.46 | 40.7 | 88.1 | 2.85 | 1.86 | 0.233 | 16.3 |
| LB-56.4 | 266 | 6.08 | 8.67 | 39.7 | 84.7 | 2.25 | 7.83 | 0.300 | 16.8 |
| LB-56.7 | 265 | 6.24 | 8.76 | 40.8 | 69.1 | 1.35 | 2.20 | 0.150 | 18.3 |
| LB-56.8 | 266 | 6.20 | 9.15 | 39.3 | 87.1 | 2.85 | 2.53 | 0.266 | 6.90 |
| LB-56.9 | 269 | 6.00 | 8.90 | 39.7 | 80.4 | 2.60 | 2.40 | 0.200 | 14.8 |
| LB-56.21 | 269 | 6.31 | 9.09 | 41.0 | 55.0 | 2.45 | 2.90 | 0.233 | 5.20 |
| LB-56.22 | 274 | 6.07 | 9.00 | 39.8 | 62.6 | 1.66 | 2.63 | 0.266 | 15.9 |
| LB-56.25 | 265 | 6.26 | 9.19 | 39.8 | 77.2 | 1.03 | 2.20 | 0.266 | 20.8 |
| LB-56.26 | 266 | 6.25 | 8.94 | 41.6 | 57.3 | 1.70 | 2.26 | 0.233 | 22.6 |
| LB-56.27 | 271 | 6.13 | 9.10 | 40.7 | 59.7 | 0.40 | 0.93 | 0.200 | 8.10 |
| SEM | 7.214 | 0.163 | 0.237 | 1.501 | 3.942 | 0.282 | 0.558 | 0.014 | 1.563 |
| ANOVA p-value | 0.98 | 0.19 | 0.34 | 0.87 | 0.09 | 0.09 | 0.60 | 0.85 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento Agarussi, M.C.; Pereira, O.G.; da Silva, L.D.; da Silva, V.P.; de Paula, R.A.; Fonseca e Silva, F.; Guimarães Ribeiro, K. Effect of Various Strains of Lactobacillus buchneri on the Fermentation Quality and Aerobic Stability of Corn Silage. Agriculture 2022, 12, 95. https://doi.org/10.3390/agriculture12010095
Nascimento Agarussi MC, Pereira OG, da Silva LD, da Silva VP, de Paula RA, Fonseca e Silva F, Guimarães Ribeiro K. Effect of Various Strains of Lactobacillus buchneri on the Fermentation Quality and Aerobic Stability of Corn Silage. Agriculture. 2022; 12(1):95. https://doi.org/10.3390/agriculture12010095
Chicago/Turabian StyleNascimento Agarussi, Mariele Cristina, Odilon Gomes Pereira, Leandro Diego da Silva, Vanessa Paula da Silva, Rosinea Aparecida de Paula, Fabyano Fonseca e Silva, and Karina Guimarães Ribeiro. 2022. "Effect of Various Strains of Lactobacillus buchneri on the Fermentation Quality and Aerobic Stability of Corn Silage" Agriculture 12, no. 1: 95. https://doi.org/10.3390/agriculture12010095
APA StyleNascimento Agarussi, M. C., Pereira, O. G., da Silva, L. D., da Silva, V. P., de Paula, R. A., Fonseca e Silva, F., & Guimarães Ribeiro, K. (2022). Effect of Various Strains of Lactobacillus buchneri on the Fermentation Quality and Aerobic Stability of Corn Silage. Agriculture, 12(1), 95. https://doi.org/10.3390/agriculture12010095





