The Detection of Wolbachia in Tea Green Leafhopper (Empoasca onukii Matsuda) and Its Influence on the Host
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Genome Sequencing
2.2.1. DNA Preparation
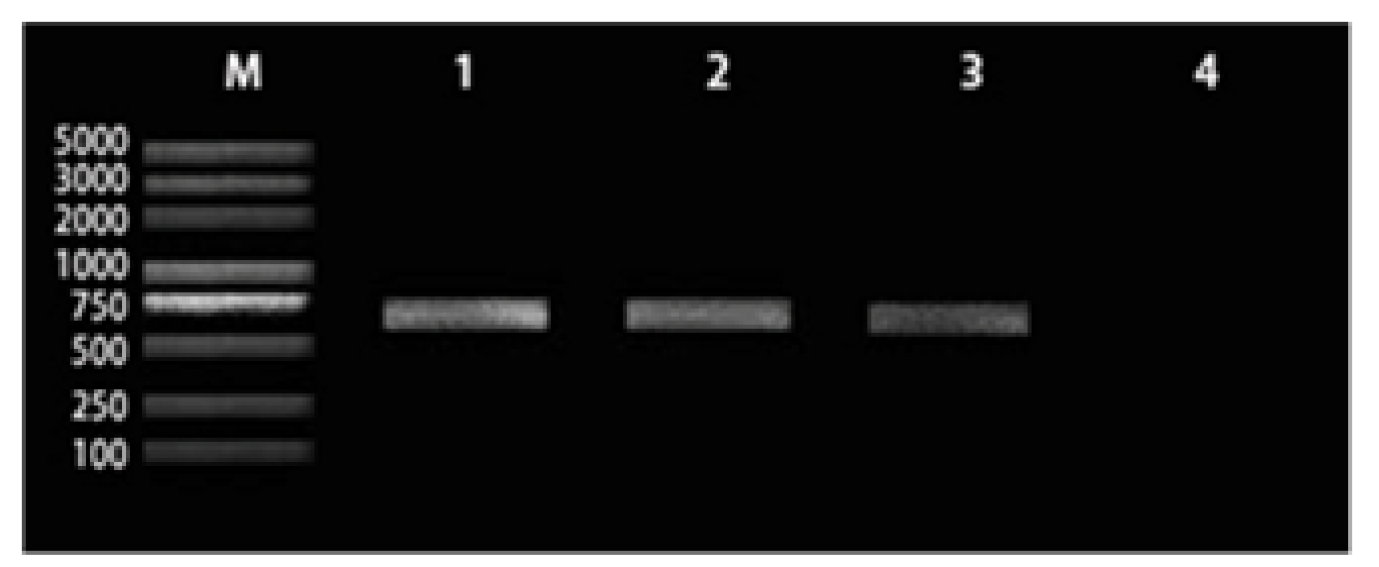
2.2.2. PCR Amplification
2.3. Real-Time PCR
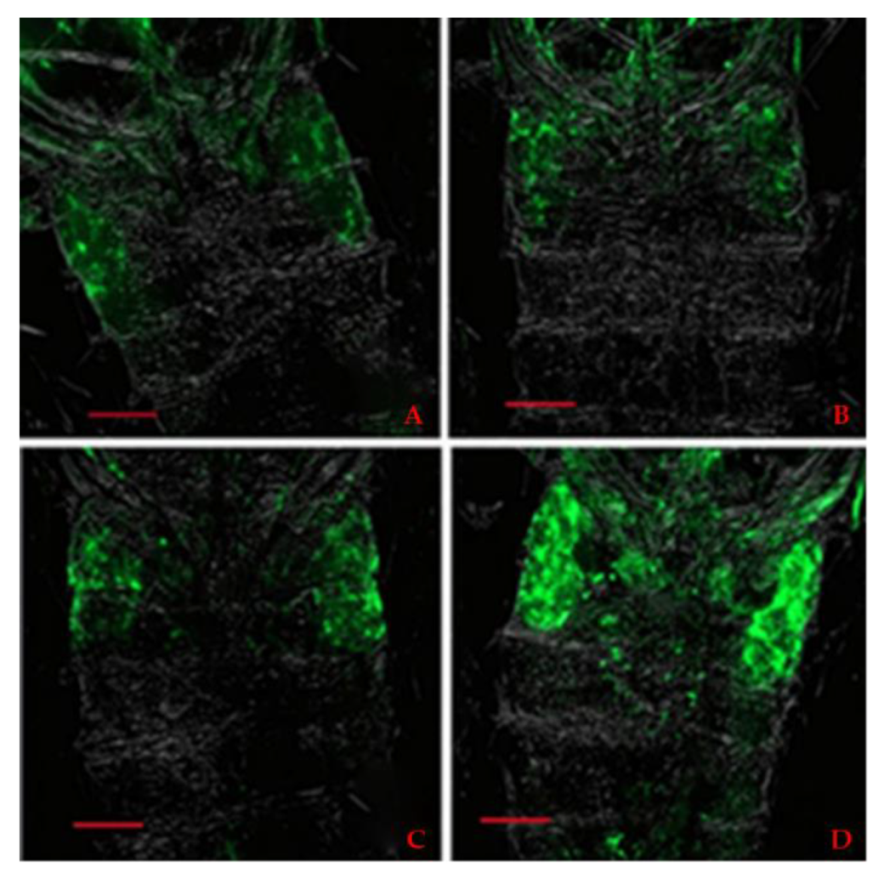
2.4. Fluorescence In Situ Hybridization (FISH)
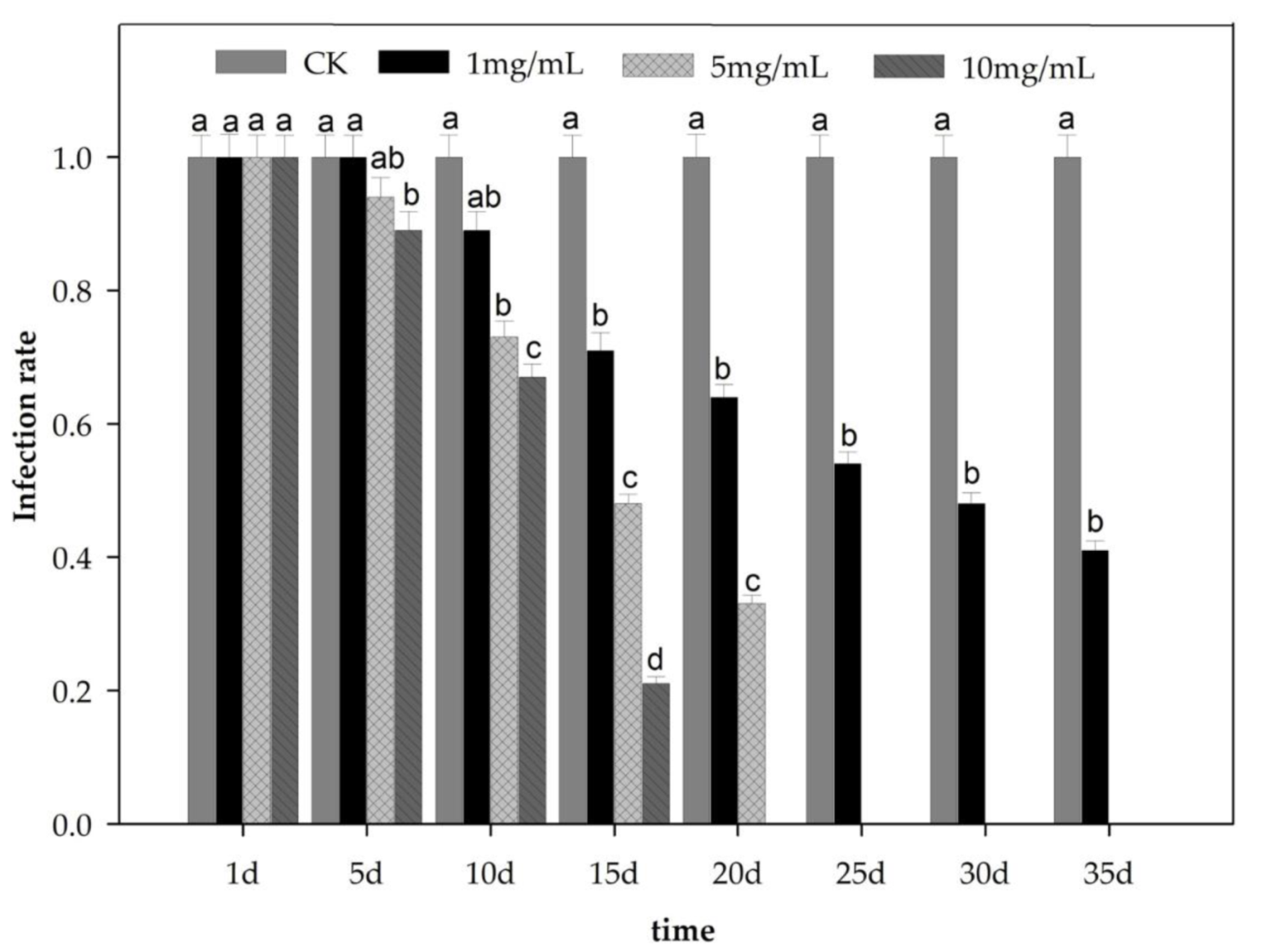
2.5. Antibiotic (Tetracycline) Treatment on Wolbachia Hosted Tea Green Leafhopper
2.6. The Effect of Wolbachia on the Reproductive Development of the Tea Green Leafhopper
2.7. Reproductive Regulation of Wolbachia in Tea Green Leafhopper
2.8. Data Analysis
3. Results
3.1. Identification of Symbiotic Bacterium in Tea Green Leafhopper
3.2. Wsp Content in Tea Green Leafhopper by Real-Time PCR
3.3. Localization of Wolbachia by FISH
3.4. Elimination of Wolbachia in Tea Green Leafhopper
3.5. Effects of Symbiotic Bacteria on the Reproductive Development of Tea Green Leafhopper
3.6. Mating Result between the Wolbachia-Infected Host and Uninfected Tea Green Leafhopper
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pu, X.Y.; Feng, M.G.; Shi, C.H. Impact of three application methods on the field efficacy of a Beauveria bassiana-based mycoinsecticide against the false-eye leafhopper, Empoasca vitis (Homoptera: Cicadellidae) in the tea canopy. Crop Prot. 2005, 24, 167–175. [Google Scholar] [CrossRef]
- Pu, X.; Feng, M. Efficacy of emulsifiable formulations of two entomopathogenic fungi against small green leafhoppers on tea plant. J. Appl. Ecol. 2004, 15, 619–622. [Google Scholar]
- Xu, X.X.; Cai, X.M.; Bian, L.; Luo, Z.X.; Li, Z.Q.; Chen, Z.M. Does background odor in tea gardens mask attractants? Screening and application of attractants for Empoasca onukii Matsuda. J. Econ. Entomol. 2017, 110, 2357–2363. [Google Scholar] [CrossRef]
- Jin, S.; Chen, Z.M.; Backus, E.A.; Sun, X.L.; Xiao, B. Characterization of EPG waveforms for the tea green leafhopper, Empoasca vitis Gothe (Hemiptera: Cicadellidae), on tea plants and their correlation with stylet activities. J. Insect Physiol. 2012, 58, 1235–1244. [Google Scholar] [CrossRef]
- Backus, E.A.; Serrano, M.S.; Ranger, C.M. Mechanisms of hopper burn: An overview of insect taxonomy, behavior, and physiology. Annu. Rev. Entomol. 2005, 50, 125–151. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.; Cai, X.M.; Luo, Z.X.; Li, Z.Q.; Xin, Z.J.; Chen, Z.M. Design of an attractant for Empoasca onukii (Hemiptera: Cicadellidae) based on the volatile components of fresh tea leaves. J. Econ. Entomol. 2018, 111, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Wu, J.X.; Qin, D.Z.; Liu, X.C.; Lu, Z.C.; Lv, L.Z.; Pan, Z.L.; Chen, H.; Li, G.W. Gene expression profiles of heat shock proteins 70 and 90 from Empoasca onukii (Hemiptera: Cicadellidae) in response to temperature stress. J. Insect Sci. 2015, 15, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, X.; Liu, X.Y.; Zhou, Y.; Wang, X.Q.; Zeng, L.T.; Fu, X.M.; Li, J.L.; Tang, J.C.; Dong, F.; Yang, Z.Y. Formation and emission of linalool in tea (Camellia sinensis) leaves infested by tea green leafhopper (Empoasca (Matsumurasca) onukii Matsuda). Food Chem. 2017, 237, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.J.; Li, R.M. Study on Empoasca vitis gothe egg incubation by non-thermal effect model. J. Chem Pharm. 2014, 6, 47–53. [Google Scholar]
- Liang, L.Y.; Liu, L.Y.; Yu, X.; Han, B.Y. Evaluation of the resistance of different tea cultivars to tea aphids by EPG technique. J. Integr. Agric. 2012, 11, 2028–2034. [Google Scholar] [CrossRef]
- Bian, L.; Sun, X.L.; Cai, X.M.; Chen, Z.M. Slow release of plant volatiles using solgel dispensers. J. Econ. Entomol. 2014, 107, 2023–2029. [Google Scholar] [CrossRef]
- Wiwatanaratanabutr, I. Wolbachia infection in leafhoppers and planthoppers: Diversity, density and geographic distribution in tropical rice agroecosystems. J. Asia Pac. Entomol. 2015, 18, 277–282. [Google Scholar] [CrossRef]
- Wangkeeree, J.; Suwanchaisri, K.; Roddee, J.; Hanboonsong, Y. Effect of Wolbachia infection states on the life history and reproductive traits of the leafhopper Yamatotettix flavovittatus Matsumura. J. Invertebr. Pathol. 2020, 177, 107490. [Google Scholar] [CrossRef]
- Buchner, P. Endosymbiosis of Animals with Plant Microorganisms; Interscience Publishers: New York, NY, USA, 1966; Volume 8, pp. 428–429. [Google Scholar]
- Kikuchi, Y.; Hosokawa, T.; Nikoh, N.; Meng, X.Y.; Kamagata, Y.; Fukatsu, T. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 2009, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, M.A.; Baumann, L.; Munson, M.A.; Baumann, P.; Campbell, B.C.; Duffus, J.E.; Moran, N.A. The eubacterial endosymbionts of whiteflies (Homoptera: Aleyrodoidea) constitute a lineage distinct from the endosymbionts of aphids and mealybugs. Curr. Microbiol. 1992, 25, 119–123. [Google Scholar] [CrossRef]
- Williamson, D.L.; Sakaguchi, B.; Hackett, K.J.; Whitcomb, R.F.; Tully, J.G.; Carle, P.; Bove, J.M.; Adams, J.R.; Konai, M.; Henegar, R.B. Spiroplasma poulsonii sp. nov., a new species associated with male-lethality in Drosophila willistoni, a neotropical species of fruit fly. Int. J. Syst. Bacteriol. 1999, 49, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Zchori-Fein, E.; Perlman, S.J. Distribution of the bacterial symbiont Cardinium in arthropods. Mol. Ecol. 2004, 13, 2009–2016. [Google Scholar] [CrossRef]
- Roberts, L.W.; Rapmund, G.; Cadigan, F.C. Sex ratios in rickettsia tsutsugamushi-infected and noninfected colonies of Leptotrombidium (Acari: Trombiculidae). J. Med. Entomol. 1977, 14, 89–92. [Google Scholar] [CrossRef]
- Andreadis, T.G.; Hall, D.W. Development, ultrastructure, and mode of transmission of Amblyospora sp. (Microspora) in the mosquito. J. Protozool. 1979, 26, 444–452. [Google Scholar] [CrossRef]
- Nakanishi, K.; Hoshino, M.; Nakai, M.; Kunimi, Y. Novel RNA sequences associated with late male killing in Homona magnanima. Proc. R. Soc. B 2008, 275, 1249–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stouthamer, R.; Breeuwer, J.A.; Hurst, G.D. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE. 2012, 7, e38544. [Google Scholar] [CrossRef] [Green Version]
- Werren, J.H.; Zhang, W.; Guo, L.R. Evolution and phylogeny of Wolbachia: Reproductive parasites of arthropods. Proc. R. Soc. Lond. B 1995, 261, 55–63. [Google Scholar]
- Beckmann, J.F.; Ronau, J.A.; Hochstrasser, M. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat. Microbiol. 2017, 2, e17007. [Google Scholar] [CrossRef] [PubMed]
- Harumoto, T.; Fukatsu, T.; Lemaitre, B. Common and unique strategies of male killing evolved in two distinct Drosophila symbionts. Proc. R. Soc. B 2018, 285, 20172167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LePage, D.P.; Metcalf, J.A.; Bordenstein, S.R.; Perlmutter, J.I.; Shropshire, J.D.; Layton, E.M.; Funkhouser-Jones, L.J.; Beckmann, J.F.; Bordenstein, S.R.; Prophage, W.O. Genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 2017, 543, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biwot, J.C.; Zhang, H.B.; Liu, C.; Qiao, J.X.; Yu, X.Q.; Wang, Y.F. Wolbachia-induced expression of kenny gene in testes affects male fertility in Drosophila melanogaster. Insect Sci. 2020, 27, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Gebiola, M.; Giorgini, M.; Kelly, S.E.; Doremus, M.R.; Ferree, P.M.; Hunter, M.S. Cytological analysis of cytoplasmic incompatibility induced by Cardinium suggests convergent evolution with its distant cousin Wolbachia. Proc. R. Soc. B 2017, 284, 20171433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, G.D.; Sylvestre, G.; Aguiar, R.; da Costa, G.B.; Martins, A.J.; Lima, J.B.P.; Petersen, M.T.; Lourenco-de-Oliveira, R.; Shadbolt, M.F.; Rasic, G. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Neglect. Trop. Dis. 2019, 13, e000702310. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- Moreira, L.A.; Ye, Y.X.; Turner, K.; Eyles, D.W.; McGraw, E.A.; O’Neill, S.L. The wMelPop strain of Wolbachia interferes with dopamine levels in Aedes aegypti. Parasites Vectors 2011, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.; Nguyen, H.L.; Nguyen, T.Y.; Vu, S.N.; Tran, N.D.; Le, T.N.; Vien, Q.M.; Bui, T.C.; Le, H.T.; Kutcher, S. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasites Vectors 2015, 8, 563–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.F.; Li, Z.X. Establishment of the cytoplasmic incompatibility inducing Wolbachia strain wMel in an important agricultural pest insect. Sci. Rep. 2016, 6, 39200. [Google Scholar] [CrossRef] [PubMed]
- Zchori-Fein, E.; Roush, R.T.; Hunter, M.S. Male production induced by antibiotic treatment in Encarsia formosa (Hymenoptera: Aphelinidae), an asexual species. Experientia 1992, 48, 114–129. [Google Scholar] [CrossRef]
- Stouthamer, R.; Mak, F. Influence of antibiotics on the offspring production of the Wolbachia-infected parthenogenetic parasitoid Encarsia formosa. J. Invertebr. Pathol. 2002, 80, 41–45. [Google Scholar] [CrossRef]
- Zhou, S.X.; Li, Y.; Zhang, F. Influence of Wolbachia on reproduction and the fitness of the parasitoid wasp Encarsia formosa. J. Plant Protec. 2009, 36, 7–10. [Google Scholar]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Beckmann, J.F.; Fallon, A.M. Detection of the Wolbachia protein WPIP0282 in mosquito spermathecae: Implications for cytoplasmic incompatibility. Insect Biochem. Molec. 2013, 43, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.X.; Tan, R.R.; Wang, Y.P.; Chen, X.; Wang, H.J.; Huang, D.J.; Gong, Z.M. Analysis of the bacterial diversity in adults of Empoasca onukii (Matsumurasca) based on 16S rDNA sequences. Plant Prot. 2018, 44, 17–23. [Google Scholar]
- Yang, Y.Y.; Li, X.H.; Ratcliffe, R.G.; Ruan, J.Y. Characterization of ammonium and nitrate uptake and assimilation in roots of tea plants. Russ J. Plant Physiol. 2013, 60, 91–99. [Google Scholar] [CrossRef]
- Zhou, W.; Rousset, F.; O’Neill, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B 1998, 265, 509–515. [Google Scholar] [CrossRef]
- Saurav, G.K.; Daimei, G.; Rana, V.S.; Popli, S.; Rajagopal, R. Detection and Localization of Wolbachia in Thrips palmi Karny (Thysanoptera: Thripidae). Indian J. Med. Microbiol. 2016, 56, 167–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wangkeeree, J.; Tewaruxsa, P.; Roddee, J.; Hanboonsong, Y. Wolbachia (Rickettsiales: Alphaproteobacteria) Infection in the Leafhopper Vector of Sugarcane White Leaf Disease. J. Insect Sci. 2020, 20, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Nugnes, F.; Gebiola, M.; Monti, M.M.; Gualtieri, L.; Giorgini, M.; Wang, J.; Bernardo, U. Genetic Diversity of the Invasive Gall Wasp Leptocybe invasa (Hymenoptera: Eulophidae) and of its Rickettsia Endosymbiont, and Associated Sex-Ratio Differences. PLoS ONE. 2015, 10, e0124660. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.L.; Barton, N.H.; Rasic, G.; Turley, A.P.; Montgomery, B.L.; Iturbe-Ormaetxe, I.; Cook, P.E.; Ryan, P.A.; Ritchie, S.A.; Hoffmann, A.A. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 2017, 15, e2001894. [Google Scholar] [CrossRef] [PubMed]
- Augustinos, A.A.; Santos-Garcia, D.; Dionyssopoulou, E.; Moreira, M.; Papapanagiotou, A.; Scarvelakis, M.; Doudoumis, V.; Ramos, S.; Aguiar, A.F.; Borges, P.A. Detection and characterization of Wolbachia infections in natural populations of aphids: Is the hidden diversity fully unraveled? PLoS ONE. 2011, 6, e28695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanmohammadi, M.; Falak, R.; Meamar, A.R.; Arshadi, M.; Akhlaghi, L.; Razmjou, E. Molecular detection and phylogenetic analysis of endosymbiont Wolbachia pipientis (Rickettsiales: Anaplasmataceae) Isolated from Dirofilaria immitis in Northwest of Iran. J. Arthropod Borne Dis. 2019, 13, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Altinli, M.; Gunay, F.; Alten, B.; Weill, M.; Sicard, M. Wolbachia diversity and cytoplasmic incompatibility patterns in Culex pipiens populations in Turkey. Parasites Vectors 2018, 11, 198–207. [Google Scholar] [CrossRef]
- Casiraghi, M.; Bordenstein, S.R.; Baldo, L.; Lo, N.; Beninati, T.; Wernegreen, J.J.; Werren, J.H.; Bandi, C. Phylogeny of Wolbachia pipientis based on gltA, groEL and ftsZ gene sequences: Clustering of arthropod and nematode symbionts in the F supergroup, and evidence for further diversity in the Wolbachia tree. Microbiology 2005, 151, 4015–4022. [Google Scholar] [CrossRef] [Green Version]
- Yen, J.H.; Barr, A.R. The etiological agent of cytoplasmic incompatibility in Culex pipiens. J. Invertebr. Pathol. 1973, 22, 242–250. [Google Scholar] [CrossRef]
- Rasgon, J.L.; Scott, T.W. Wolbachia and cytoplasmic incompatibility in the California Culex pipiens mosquito species complex: Parameter estimates and infection dynamics in natural populations. Genetics 2003, 165, 2029–2038. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhao, D.X.; Hong, X.Y. Establishment of singly Wolbachia- and singly Cardinium-infected white backed planthopper (Sogatella furcifera) lines by microinjecting penicillin G. Acta. Entomol. Sin. 2012, 55, 782–789. [Google Scholar]
- Qi, S.U. A comparative study of the removal of endosymbionts in Bemisia tabaci biotypes B and Q using three antibiotics. Chinese J. Appl. Entomol. 2012, 49, 190–196. [Google Scholar]
- Bi, J.; Wang, Y.F. The effect of the endosymbiont Wolbachia on the behavior of insect hosts. Insect Sci. 2020, 27, 846–858. [Google Scholar] [CrossRef]
- Crespigny, F.; Pitt, T.D.; Wedell, N. Increased male mating rate in Drosophila is associated with Wolbachia infection. J. Evolution Biol. 2010, 19, 1964–1972. [Google Scholar] [CrossRef]
- Goodacre, S.L.; Martin, O.Y. Mhodification of insect and arachnid behaviours by vertically transmitted endosymbionts: Infections as drivers of behavioural change and evolutionary novelty. Insects 2012, 3, 246–261. [Google Scholar] [CrossRef] [Green Version]
- Panteleev, D.; Goriacheva, I.I.; Andrianov, B.V.; Reznik, N.L.; Lazebnyi, O.E.; Kulikov, A.M. The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Genetika 2007, 43, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Shen, Z.R.; Hong, L.Z. Wolbachia endosymbionts and their manipulation of reproduction of arthropod hosts. Acta Entomol. Sin. 2002, 22, 241–252. [Google Scholar]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature. 2019, 572, 56–61. [Google Scholar] [CrossRef] [PubMed]



| Mating Type | No. of Observe Mates | No. of Nymph from per Mate | Average Sex-Ratio (Female♀: Male♂) | Average Adult Period of Offspring | |
|---|---|---|---|---|---|
| Female (♀)/day | Male (♂)/day | ||||
| W−♀×W♂ | 17 | 16 d | 1.7 (10:6) | 13.0 a | 9.8 c |
| W♀×W♂ | 19 | 18 c | 1.6 (11:7) | 9.8 a | 9.5 b |
| W♀×W−♂ | 21 | 20 b | 1.5 (12:8) | 16.2 a | 11.3 b |
| W−♀×W−♂ | 16 | 25 a | 1.3 (14:11) | 14.1 a | 12.7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Lan, R.; Ji, D.; Tan, Y.; Zhou, X.; Tan, X.; Wu, Q.; Jin, L. The Detection of Wolbachia in Tea Green Leafhopper (Empoasca onukii Matsuda) and Its Influence on the Host. Agriculture 2022, 12, 36. https://doi.org/10.3390/agriculture12010036
Zhang Q, Lan R, Ji D, Tan Y, Zhou X, Tan X, Wu Q, Jin L. The Detection of Wolbachia in Tea Green Leafhopper (Empoasca onukii Matsuda) and Its Influence on the Host. Agriculture. 2022; 12(1):36. https://doi.org/10.3390/agriculture12010036
Chicago/Turabian StyleZhang, Qiuqiu, Rongmeng Lan, Dezhong Ji, Yanni Tan, Xia Zhou, Xiaofeng Tan, Qiong Wu, and Linhong Jin. 2022. "The Detection of Wolbachia in Tea Green Leafhopper (Empoasca onukii Matsuda) and Its Influence on the Host" Agriculture 12, no. 1: 36. https://doi.org/10.3390/agriculture12010036
APA StyleZhang, Q., Lan, R., Ji, D., Tan, Y., Zhou, X., Tan, X., Wu, Q., & Jin, L. (2022). The Detection of Wolbachia in Tea Green Leafhopper (Empoasca onukii Matsuda) and Its Influence on the Host. Agriculture, 12(1), 36. https://doi.org/10.3390/agriculture12010036




