Hydrogels Are Reinforced with Colombian Fique Nanofibers to Improve Techno-Functional Properties for Agricultural Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Raw Material
2.3. Nanofibers Production
2.3.1. Chemical Treatment of the Raw Material
2.3.2. Obtaining Nanofibers
2.4. Reinforcing Hydrogels
2.5. Choosing the Best Hydrogel Formulation
2.5.1. Swelling Study
2.5.2. Mechanical Properties
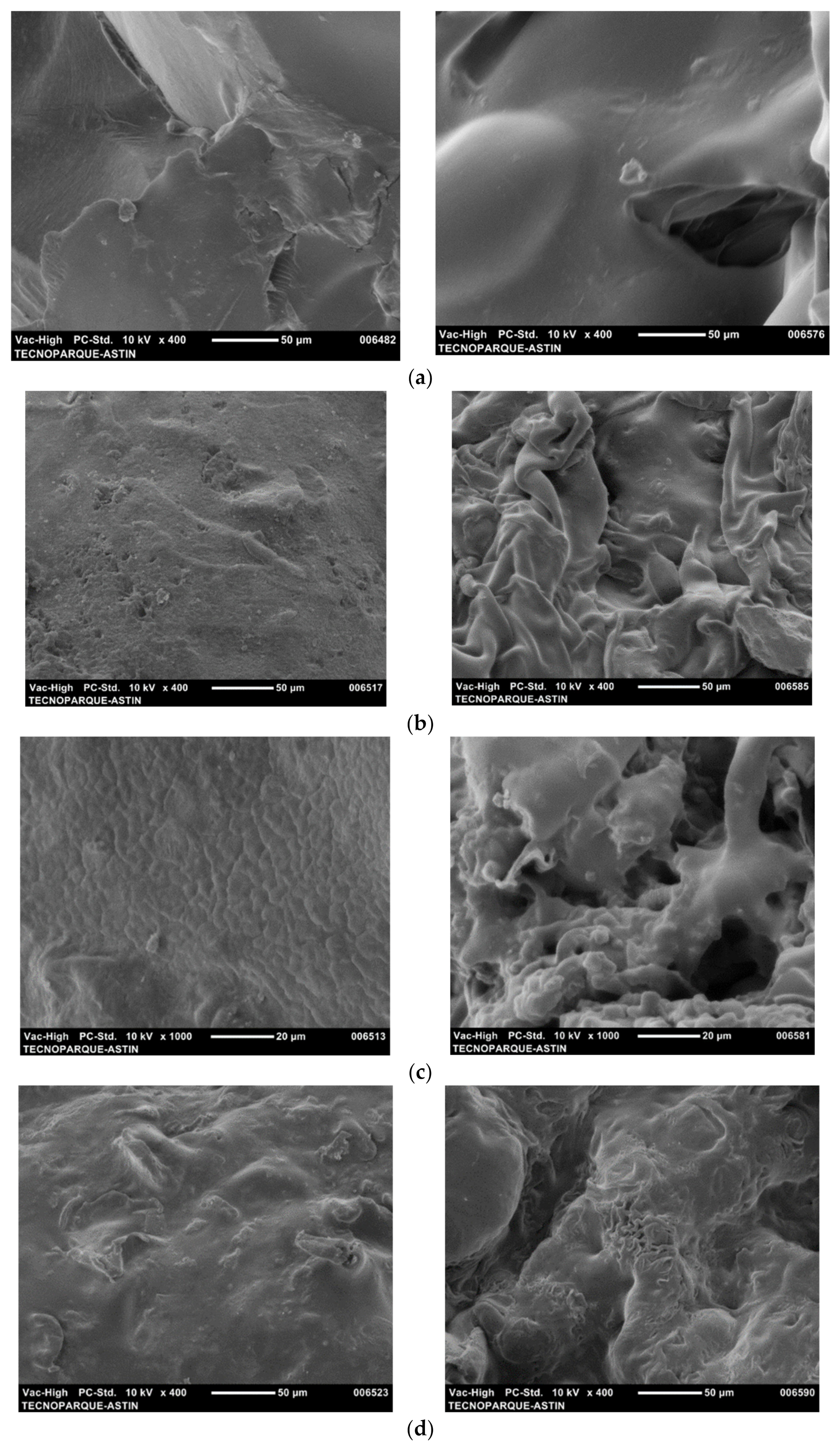
2.5.3. Scanning Electron Microscopy (SEM)
2.6. Experimental Design and Statistical Analysis
3. Results and Discussions
3.1. Swelling Behavior
3.2. Mechanical Properties
3.3. Morphologies of Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects the 2017 Revision: Key Findings and Advance Tables; United Nations: New York, NY, USA, 2017; p. 53. [Google Scholar]
- United Nations. Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/hunger/. (accessed on 10 January 2021).
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Hyánková, E.; Kriška Dunajský, M.; Zedník, O.; Chaloupka, O.; Pumprlová Němcová, M. Irrigation with treated wastewater as an alternative nutrient source (for crop): Numerical simulation. Agriculture 2021, 11, 946. [Google Scholar] [CrossRef]
- Wilson, M.G.; Maggi, A.E.; Castiglioni, M.G.; Gabioud, E.A.; Sasal, M.C. Conservation of ecosystem services in argiudolls of Argentina. Agriculture 2020, 10, 649. [Google Scholar] [CrossRef]
- Kostrzewska, M.K.; Jastrzębska, M.; Treder, K.; Wanic, M. Phosphorus in spring barley and Italian rye-grass biomass as an effect of inter-species interactions under water deficit. Agriculture 2020, 10, 329. [Google Scholar] [CrossRef]
- Pérez-Blanco, C.D.; Hrast-Essenfelder, A.; Perry, C. Irrigation technology and water conservation: A review of the theory and evidence. Rev. Environ. Econ. Policy 2020, 14, 216–239. [Google Scholar] [CrossRef]
- Mehana, M.; Abdelrahman, M.; Emadeldin, Y.; Rohila, J.S.; Karthikeyan, R. Impact of genetic improvements of rice on its water use and effects of climate variability in Egypt. Agriculture 2021, 11, 865. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, C.; Li, J.; Yi, X.; He, Y.; Zhang, J.; Sun, Z. A separation and desalination process for farmland saline-alkaline water. Agriculture 2021, 11, 1001. [Google Scholar] [CrossRef]
- Haque, A.N.A.; Uddin, M.K.; Sulaiman, M.F.; Amin, A.M.; Hossain, M.; Solaiman, Z.M.; Mosharrof, M. Biochar with alternate wetting and drying irrigation: A potential technique for paddy soil management. Agriculture 2021, 11, 367. [Google Scholar] [CrossRef]
- Feng, W.; Gao, J.; Cen, R.; Yang, F.; He, Z.; Wu, J.; Miao, Q.; Liao, H. Effects of polyacrylamide-based super absorbent polymer and corn straw biochar on the arid and semi-arid salinized soil. Agriculture 2020, 10, 519. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.A.; Neto, A.G.V.C.; Pereira, A.G.B.; Fajardo, A.R.; Radovanovic, E.; Rubira, A.F.; Muniz, E.C. Nanocomposites based on poly(acrylamide-co-acrylate) and cellulose nanowhiskers. Eur. Polym. J. 2012, 48, 454–463. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.; Pereira, A.; Fajardo, A.; Rubira, A.; Muniz, E. Superabsorbent hydrogel composite made of cellulose nanofibrils and chitosan-graft-poly(acrylic acid). Carbohydr. Polym. 2012, 87, 2038–2045. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Jia, X.; Yin, L. Hydrogel: Diversity of structures and applications in food science. Food Rev. Int. 2021, 37, 313–372. [Google Scholar] [CrossRef]
- Song, B.; Liang, H.; Sun, R.; Peng, P.; Jiang, Y.; She, D. Hydrogel synthesis based on lignin/sodium alginate and application in agriculture. Int. J. Biol. Macromol. 2020, 144, 219–230. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors – A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef]
- Mohite, S.; Prasad, E. Hydrogel Market Outlook - 2027; Allied Market Research: Pune, India, 2020; p. 363. [Google Scholar]
- Serna Cock, L.; Guancha-Chalapud, M.A. Natural fibers for hydrogels production and their applications in agriculture. Acta Agron. 2017, 66, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Prakash, P.; Rout, P.K.; Bhaladhare, S. Synthesis and characterization of superabsorbent cellulose-based hydrogel for agriculture application. Starch - Stärke 2021, 73, 1900284. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Lu, Z.; Zhang, H.; Huang, J.; Yan, K.; Wang, D. Nanofiber-reinforced bulk hydrogel: Preparation and structural, mechanical, and biological properties. J. Mater. Chem. B 2020, 8, 9794–9803. [Google Scholar] [CrossRef]
- Karadag, E.; Baris, O.; Saraydin, D. Water uptake in chemically crosslinked poly(acrylamide-co-crotonic acid) hydrogels. Mater. Des. 2005, 26, 265–270. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q. A novel polyacrylamide nanocomposite hydrogel reinforced with natural chitosan nanofibers. Colloids Surfaces B Biointerfaces 2011, 84, 155–162. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Yue, Y.; Zhang, Q. Application of rod-shaped cellulose nanocrystals in polyacrylamide hydrogels. J. Colloid Interface Sci. 2011, 353, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Guancha-Chalapud, M.A.; Gálvez, J.; Serna-Cock, L.; Aguilar, C.N. Valorization of Colombian fique (Furcraea bedinghausii) for production of cellulose nanofibers and its application in hydrogels. Sci. Rep. 2020, 10, 11637. [Google Scholar] [CrossRef]
- Ovalle-Serrano, S.A.; Gómez, F.N.; Blanco-Tirado, C.; Combariza, M.Y. Isolation and characterization of cellulose nanofibrils from Colombian Fique decortication by-products. Carbohydr. Polym. 2018, 189, 169–177. [Google Scholar] [CrossRef]
- Ovalle-Serrano, S.A.; Blanco-Tirado, C.; Combariza, M.Y. Exploring the composition of raw and delignified Colombian fique fibers, tow and pulp. Cellulose 2018, 25, 151–165. [Google Scholar] [CrossRef]
- Zhong, K.; Zheng, X.-L.; Mao, X.-Y.; Lin, Z.-T.; Jiang, G.-B. Sugarcane bagasse derivative-based superabsorbent containing phosphate rock with water–fertilizer integration. Carbohydr. Polym. 2012, 90, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Spagnol, C.; Rodrigues, F.; Pereira, A.; Fajardo, A.; Rubira, A.; Muniz, E. Superabsorbent hydrogel nanocomposites based on starch- g -poly ( sodium acrylate) matrix filled with cellulose nanowhiskers. Cellulose 2012, 19, 1225–1237. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.R.; Duan, J.F.; Xu, F.; Sun, R.C. Mechanical and viscoelastic properties of cellulose nanocrystals reinforced poly(ethylene glycol) nanocomposite hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
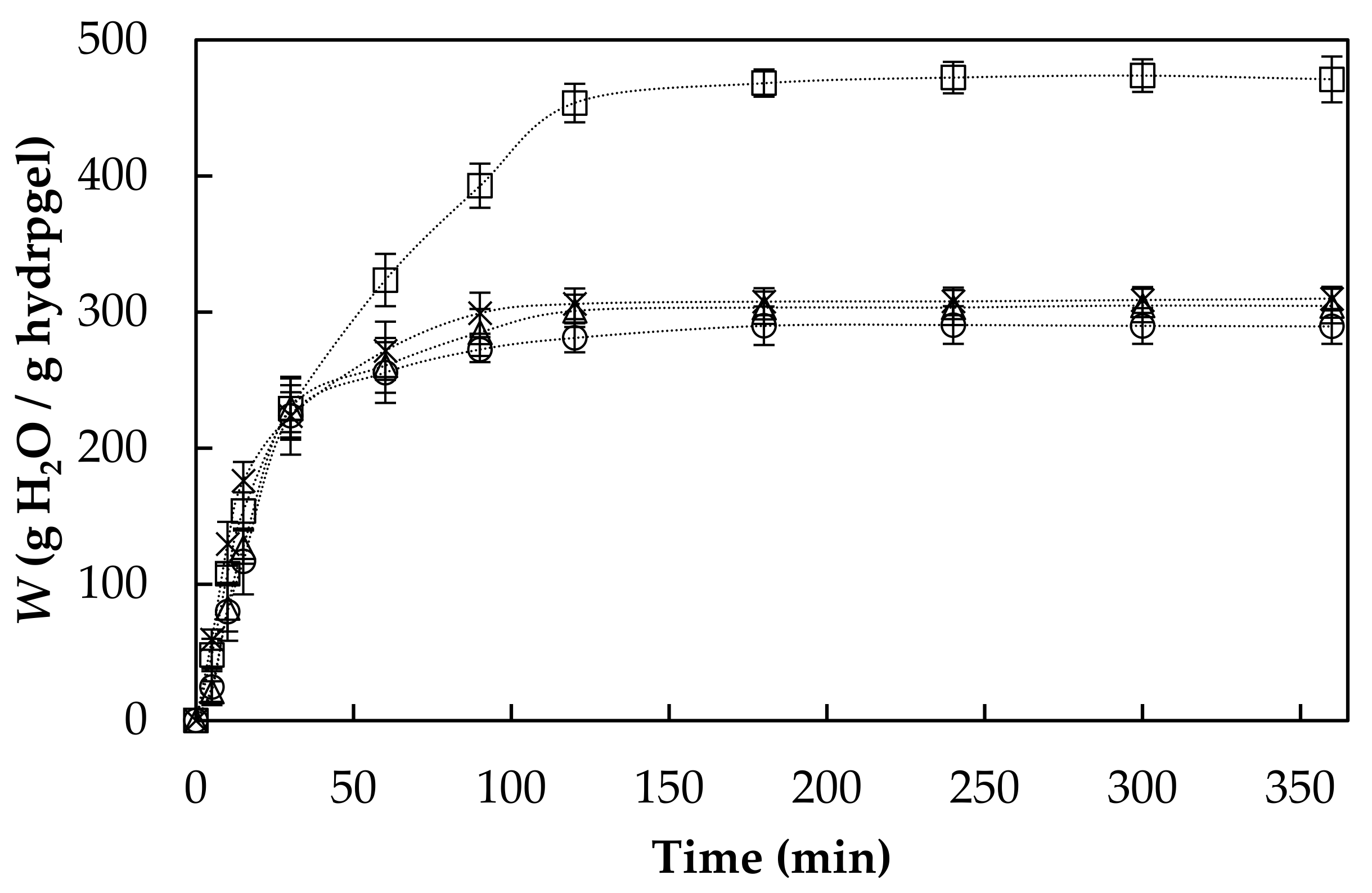
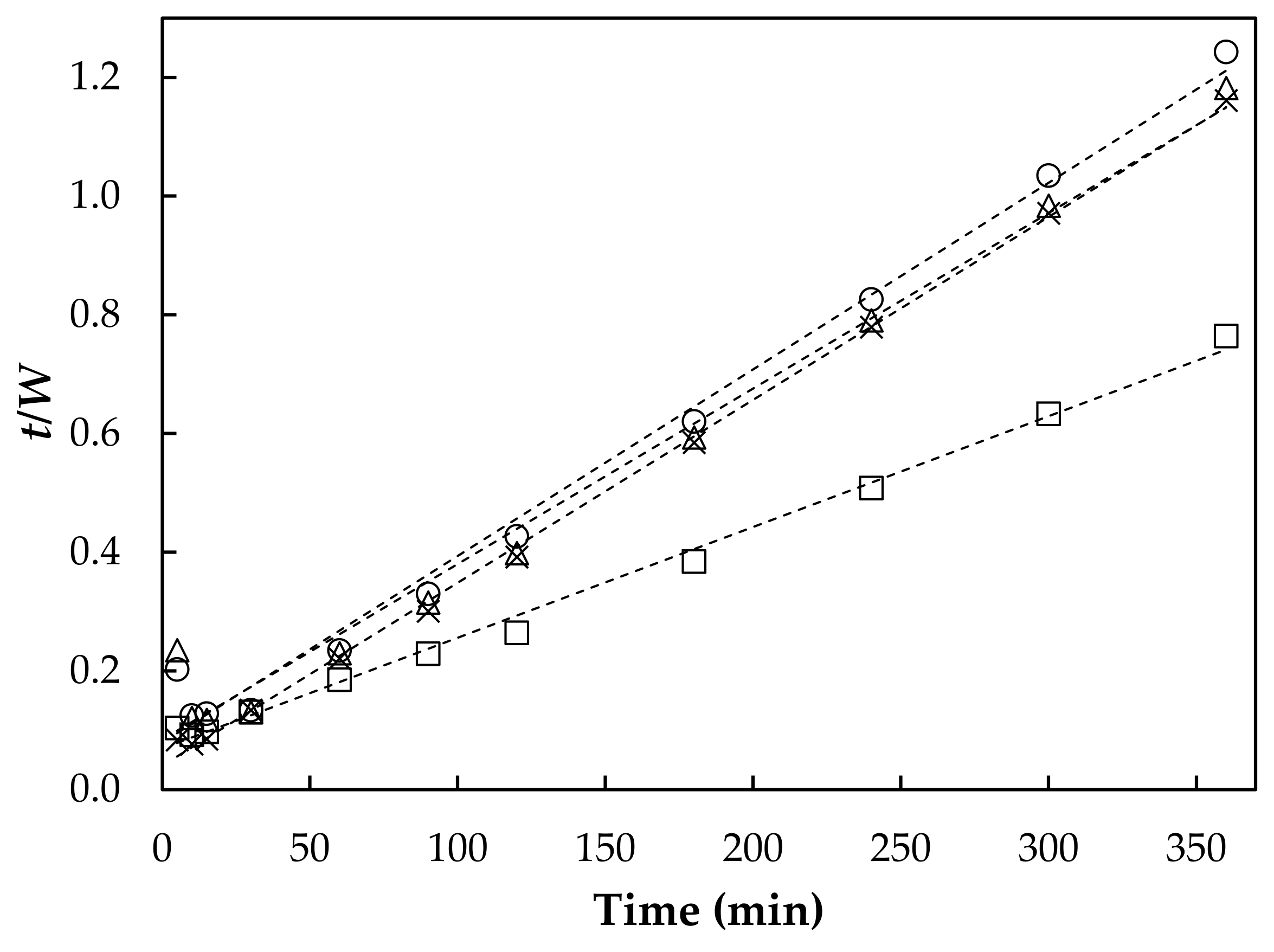
| Hydrogel | * Weq | ** Wt | *** ks × 10−4 | **** kis |
|---|---|---|---|---|
| AHR0 | 310 ± 9 b | 324 | 2.4 | 24.8 |
| AHR3 | 474 ± 12 c | 536 | 0.5 | 14.4 |
| AHR5 | 305 ± 12 a,b | 338 | 1.0 | 11.8 |
| AHR10 | 291 ± 14 a | 318 | 1.2 | 12.6 |
| Hydrogel | RC (kPa) | Strain (%) |
|---|---|---|
| AHR0 | 6 ± 2 a | 5 ± 1 |
| AHR3 | 8 ± 3 a,b | 6 ± 1 |
| AHR5 | 16 ± 4 c | 25 ± 2 |
| AHR10 | 10 ± 1 b | 17 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guancha-Chalapud, M.A.; Serna-Cock, L.; Tirado, D.F. Hydrogels Are Reinforced with Colombian Fique Nanofibers to Improve Techno-Functional Properties for Agricultural Purposes. Agriculture 2022, 12, 117. https://doi.org/10.3390/agriculture12010117
Guancha-Chalapud MA, Serna-Cock L, Tirado DF. Hydrogels Are Reinforced with Colombian Fique Nanofibers to Improve Techno-Functional Properties for Agricultural Purposes. Agriculture. 2022; 12(1):117. https://doi.org/10.3390/agriculture12010117
Chicago/Turabian StyleGuancha-Chalapud, Marcelo A., Liliana Serna-Cock, and Diego F. Tirado. 2022. "Hydrogels Are Reinforced with Colombian Fique Nanofibers to Improve Techno-Functional Properties for Agricultural Purposes" Agriculture 12, no. 1: 117. https://doi.org/10.3390/agriculture12010117
APA StyleGuancha-Chalapud, M. A., Serna-Cock, L., & Tirado, D. F. (2022). Hydrogels Are Reinforced with Colombian Fique Nanofibers to Improve Techno-Functional Properties for Agricultural Purposes. Agriculture, 12(1), 117. https://doi.org/10.3390/agriculture12010117






