Wild Plant Habitat Characterization in the Last Two Decades in the Nile Delta Coastal Region of Egypt
Abstract
:1. Introduction
2. Materials and Methods
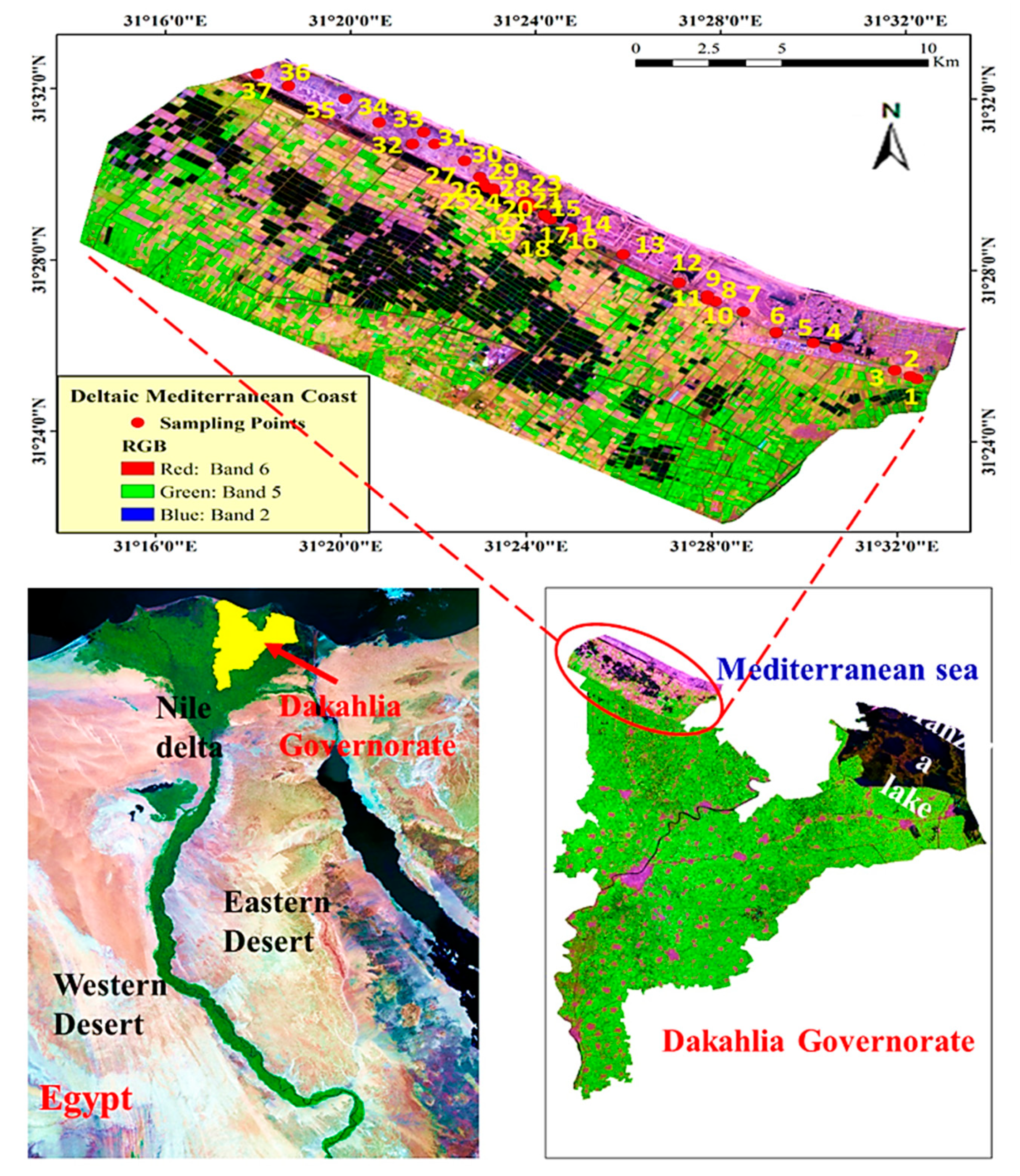
2.1. Study Area
2.2. Vegetation Analysis
2.3. Soil Analysis
2.4. Spectral Analysis
2.4.1. Satellite Image Acquisition and Preprocessing
2.4.2. Image Processing
2.4.3. Post-Classification Techniques
2.5. Data Analysis
3. Results and Discussion
3.1. Floristic Composition
3.2. Chorological Affinities
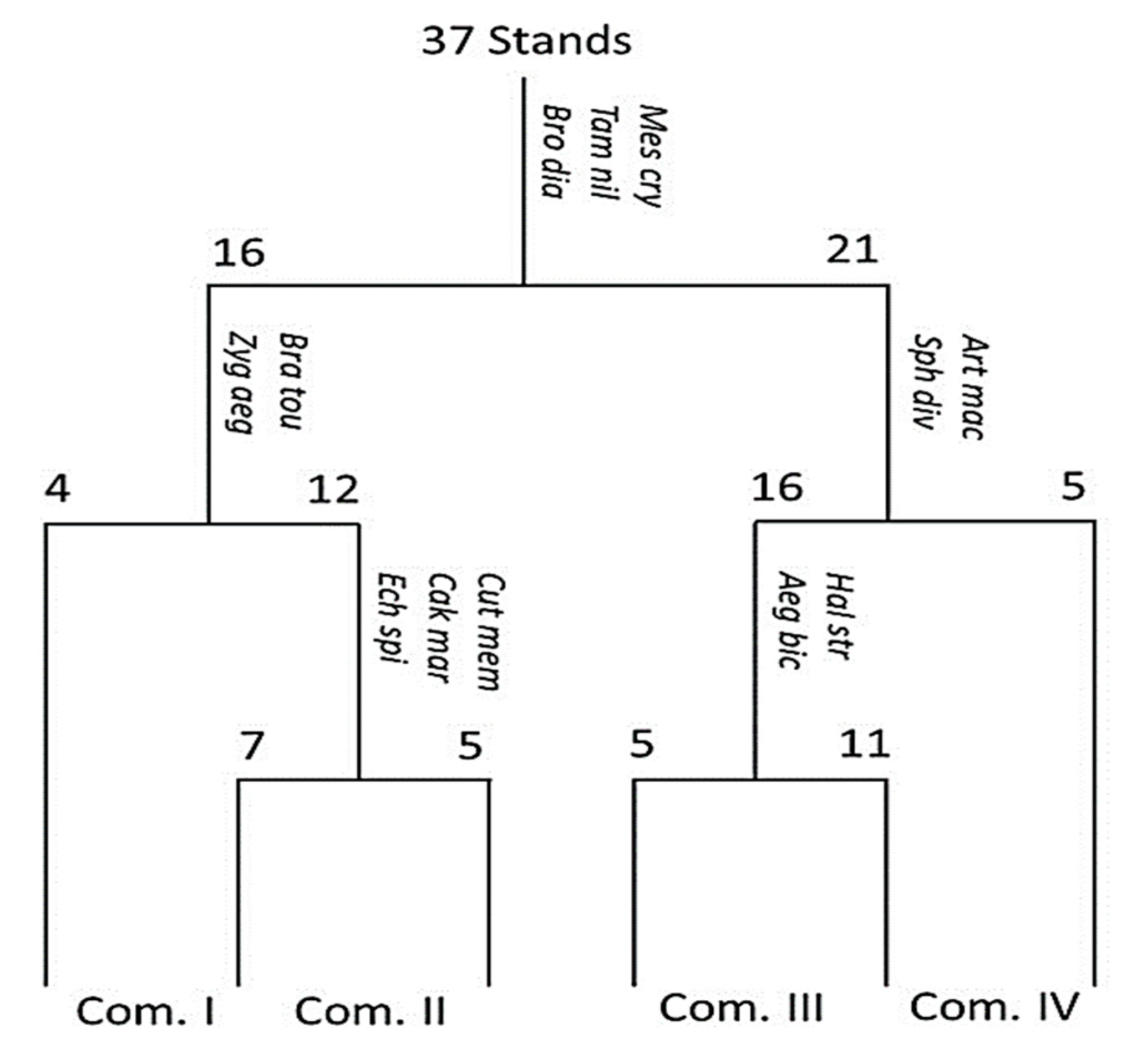
3.3. Classification of Vegetation
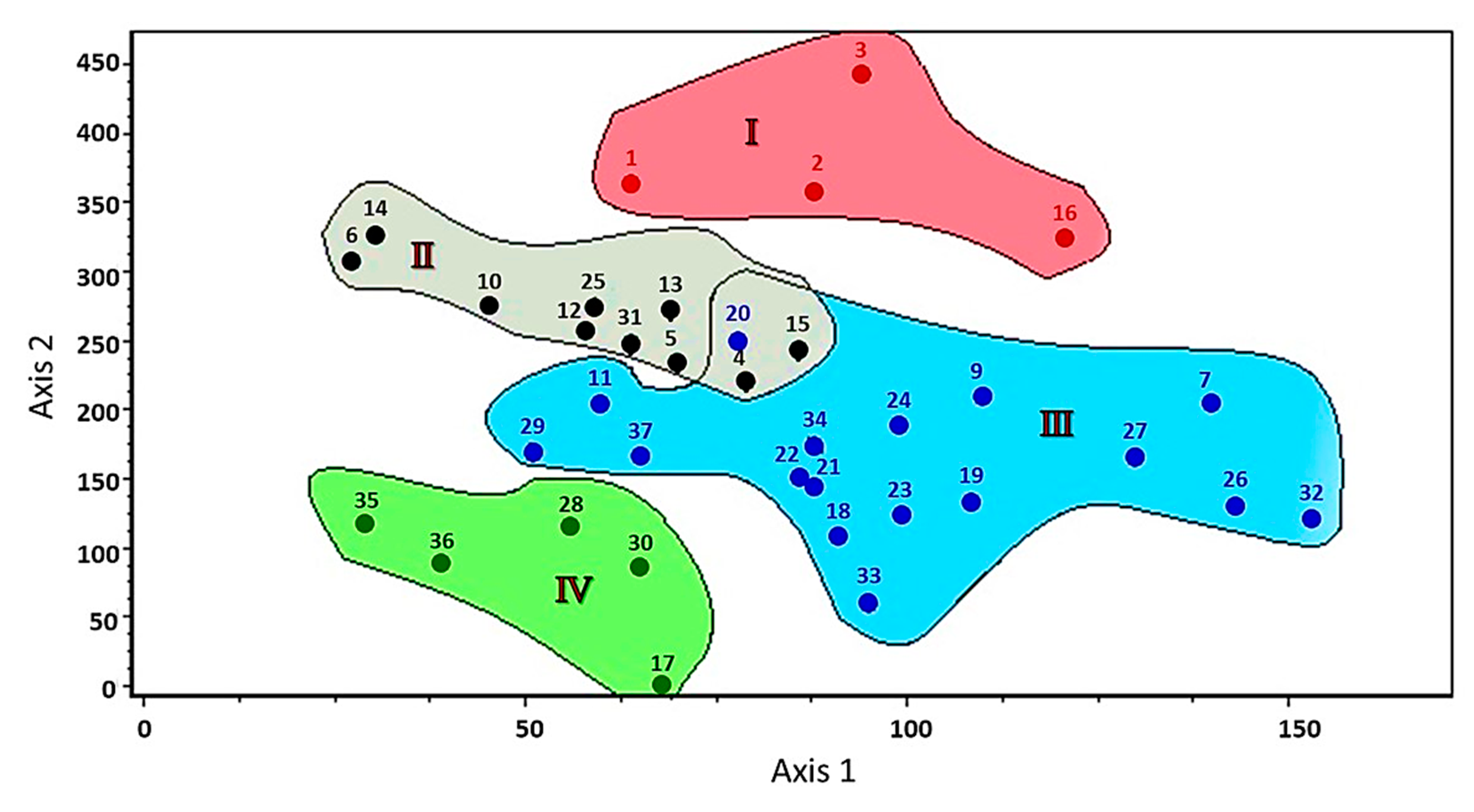
3.4. Ordination of Sampling Sites
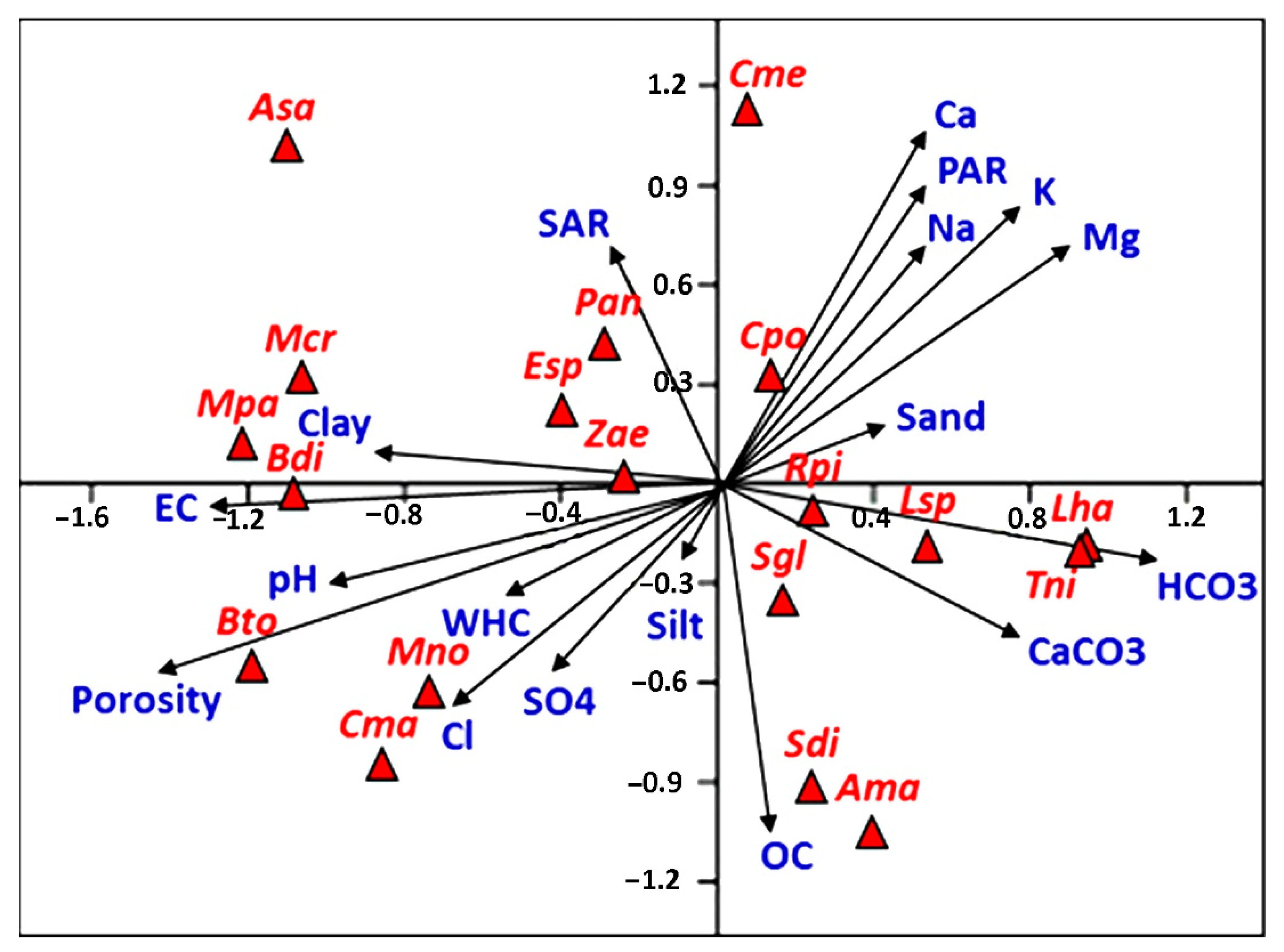
3.5. Soil–Vegetation Relationships
3.6. Spectral Analysis
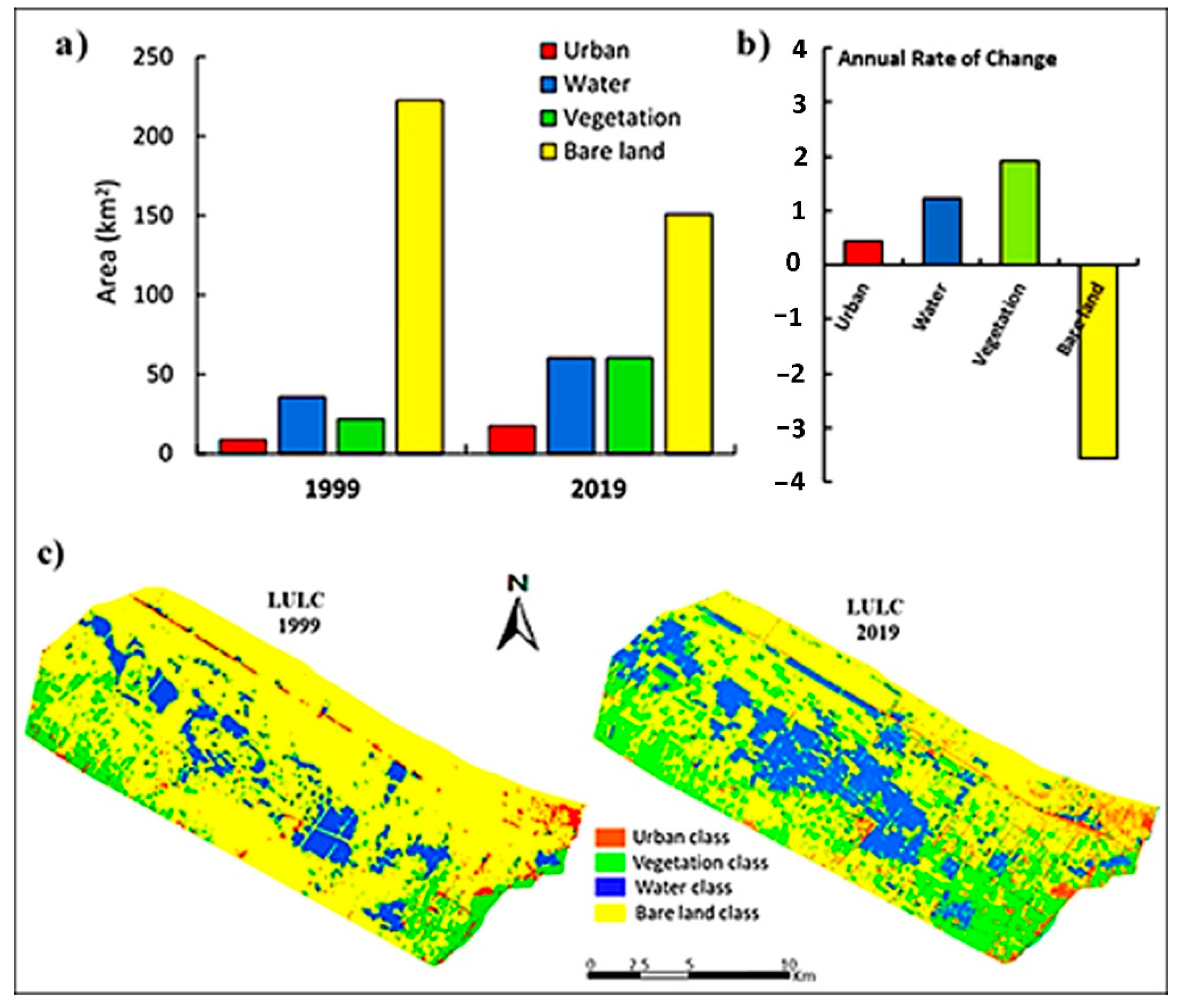
3.6.1. LULC analysis
3.6.2. Spectral Indices Assessment
Change Detection in NDVI
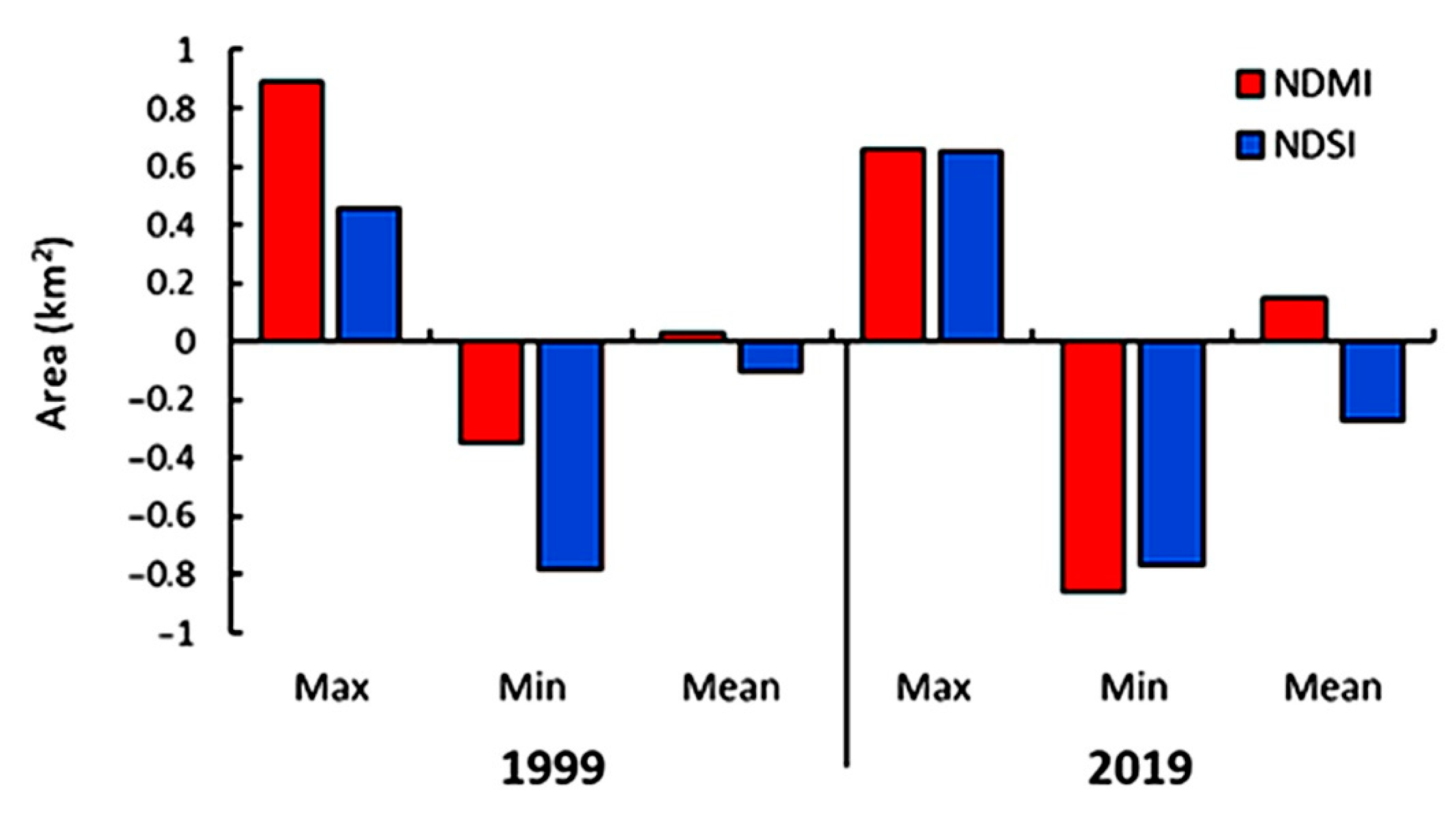
Change Detection in NDMI and NDSI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venâncio, C.; Ribeiro, R.; Lopes, I. Seawater intrusion: An appraisal of taxa at most risk and safe salinity levels. Biol. Rev. 2021, 97, 361–382. [Google Scholar] [CrossRef]
- Jiang, L.; Bao, A.; Guo, H.; Ndayisaba, F. Vegetation dynamics and responses to climate change and human activities in Central Asia. Sci. Total Environ. 2017, 599, 967–980. [Google Scholar] [CrossRef]
- Postel, S.; Richter, B. Rivers for Life: Managing Water for People and Nature; Island Press: Washington, DC, USA, 2012. [Google Scholar]
- Zahran, M.A.; Willis, A.J. The Vegetation of Egypt; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar]
- Balmford, A.; Bruner, A.; Cooper, P.; Costanza, R.; Farber, S.; Green, R.E.; Jenkins, M.; Jefferiss, P.; Jessamy, V.; Madden, J.; et al. Economic reasons for conserving wild nature. Science 2002, 297, 950–953. [Google Scholar] [CrossRef] [Green Version]
- Costanza, R.; Daly, H.; Goodland, R.; Norgaard, R.B. An Introduction to Ecological Economics; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Zlinszky, A.; Heilmeier, H.; Balzter, H.; Czúcz, B.; Pfeifer, N. Remote Sensing and GIS for Habitat Quality Monitoring: New Approaches and Future Research. Remote Sens. Environ. 2015, 7, 7987–7994. [Google Scholar] [CrossRef] [Green Version]
- Seress, G.; Lipovits, Á.; Bókony, V.; Czúni, L. Quantifying the urban gradient: A practical method for broad measurements. Landsc. Urban Plan. 2014, 131, 42–50. [Google Scholar] [CrossRef]
- Xu, L.; Ivanov, P.C.; Hu, K.; Chen, Z.; Carbone, A.; Stanley, H.E. Quantifying signals with power-law correlations: A comparative study of detrended fluctuation analysis and detrended moving average techniques. Phys. Rev. 2005, 71, 051101. [Google Scholar] [CrossRef] [Green Version]
- Elagami, S.A.; El-Zeiny, A.; El-Halawany, E.S.F.; El-Amier, Y.A. Monitoring Spatiotemporal Environmental Changes in Dakahlia Governorate, Egypt Using Landsat Imagery. Let. Appl. NanoBioSci. 2022, 11, 3843–3853. [Google Scholar]
- El-Amier, Y.A.; El-Zeiny, A.; El-Halawany, E.S.F.; Elsayed, A.; El-Esawi, M.A.; Noureldeen, A.; Elagami, S.A. Environmental and Stress Analysis of Wild Plant Habitat in River Nile Region of Dakahlia Governorate on Basis of Geospatial Techniques. Sustainability 2021, 13, 6377. [Google Scholar] [CrossRef]
- Olorunfemi, I.E.; Fasinmirin, J.T.; Olufayo, A.A.; Komolafe, A.A. GIS and remote sensing-based analysis of the impacts of land use/land cover change (LULCC) on the environmental sustainability of Ekiti State, southwestern Nigeria. Environ. Dev. Sustain. 2020, 22, 661–692. [Google Scholar] [CrossRef]
- Benzer, N. Using the geographical information system and remote sensing techniques for soil erosion assessment. Pol. J. Environ. Stud. 2010, 19, 881–886. [Google Scholar]
- Bing, P.; Hui-min, X.; Tao, H.; Asundi, A. Measurement of coefficient of thermal expansion of films using digital image correlation method. Polym. Test. 2009, 28, 75–83. [Google Scholar] [CrossRef]
- Bakr, N.; Weindorf, D.C.; Bahnassy, M.H.; Marei, S.M.; El-Badawi, M.M. Monitoring land cover changes in a newly reclaimed area of Egypt using multi-temporal Landsat data. Appl. Geogr. 2010, 30, 592–605. [Google Scholar] [CrossRef]
- Ormsby, J.P.; Choudhury, B.J.; Owe, M. Vegetation spatial variability and its effect on vegetation indices. Int. J. Remote Sens. 1987, 8, 1301–1306. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, C.L.; Richardson, A.J.; Kanemasu, E.T. Leaf Area Index Estimates for Wheat from LANDSAT and Their Implications for Evapotranspiration and Crop Modeling 1. Agron. J. 1979, 71, 336–342. [Google Scholar] [CrossRef]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E., III. Ratio analysis of reflectance spectra (RARS): An algorithm for the remote estimation of the concentrations of chlorophyll a, chlorophyll b, and carotenoids in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Wiegand, C.L.; Maas, S.J.; Aase, J.K.; Hatfield, J.L.; Pinter, P.J., Jr.; Jackson, R.D.; Lapitan, R.L. Multi-site analyses of spectral-biophysical data for wheat. Remote Sens. Environ. 1992, 42, 1–21. [Google Scholar] [CrossRef]
- Zahran, M.A.; Willis, A.J. Plant Life in the River Nile in Egypt; Mars Publishing House: Reyadh, Saudi Arabia, 2003. [Google Scholar]
- UNESCO United Nations Educational. Map of the World Distribution of Arid Regions, 7th ed.; MAB Technical Notes; UNESCO: Paris, France, 1979. [Google Scholar]
- Ayyad, M.G.; Floc’h, L. An Ecological Assessment of Renewable Resources for Rural Agricultural Development in the Western Mediterranean Coastal Region of Egypt Case Study: El Omayed Test-Area; Ministère des Affaires Étrangères and C.N.R.S./C.E.P.E.L. Emberger: Montpellier, France, 1983; 127p.
- Omran, E.-S.E.; Negm, A.M. Adaptive management zones of Egyptian coastal lakes. In Egyptian Coastal Lakes and Wetlands: Part I; Springer: Berlin/Heidelberg, Germany, 2018; pp. 37–60. [Google Scholar]
- Shukla, R.S.; Chandel, P.S. A Textbook of Plant Ecology including Ethobotany and Soil Science, 10th ed.; S. Chand & Company Ltd.: New Delhi, India, 1989. [Google Scholar]
- Canfield, R.H. Application of the line interception method in sampling range vegetation. J. For. 1941, 39, 388–394. [Google Scholar]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 1999; Volume 1. [Google Scholar]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 2002; Volume 3. [Google Scholar]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 2005; Volume 4. [Google Scholar]
- Täckholm, V. Student’s Flora of Egypt, 2nd ed.; Cairo University Press: Cairo, Egypt, 1974. [Google Scholar]
- Raunkięr, C. Plant Life Forms; Clarendon Press: Oxford, UK, 1937. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Interscience Publishers, Inc.: New York, NY, USA, 1947. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Constable and Co., Ltd.: London, UK, 1962. [Google Scholar]
- Pierce, W.C.; Haenisch, E.L.; Sawyer, D.T. Quantitative Analysis; Wiley Toppen: Tokyo, Japan, 1958. [Google Scholar]
- McKell, C.M.; Goodin, J.K. A brief overview of the saline lands of the United States. In Research and Development Seminar on Forage and Fuel Production from Salt-Affected Wasteland; CABI: Cunderdin, Australia, 1984. [Google Scholar]
- Jensen, J.R. Introductory Digital Image Processing: A Remote Sensing Perspective, 2nd ed.; Prentice-Hall Inc.: Hoboken, NJ, USA, 1996. [Google Scholar]
- Gad, A.A.; El-Zeiny, A. Spatial analysis for sustainable development of El Fayoum and Wadi El Natrun desert depressions, Egypt with the aid of remote sensing and GIS. J. Geog. Environ. Earth Sci. Int. 2016, 8, 1–18. [Google Scholar] [CrossRef]
- Rouse, J.W. Monitoring the Vernal Advancement and Retro Gradation of Natural Vegetation; NASA: Texas A&M University, Remote Sensing Center: College Station, TX, USA, 1973. [Google Scholar]
- Khan, N.M.; Rastoskuev, V.V.; Sato, Y.; Shiozawa, S. Assessment of hydrosaline land degradation by using a simple approach of remote sensing indicators. Agric. Water Manag. 2005, 77, 96–109. [Google Scholar] [CrossRef]
- Foody, G.; Boyd, D. Detection of partial land cover change associated with the migration of inter-class transitional zones. Int. J. Remote Sens. 1999, 20, 2723–2740. [Google Scholar] [CrossRef]
- Hill, M.O.; Šmilauer, P. WinTWINS. TWINSPAN for Windows Version, 2.3; Centre for Ecology and Hydrology, University of South Bohemia: Huntingdon, UK; Ceské Budějovice, Czech, 2005. [Google Scholar]
- Ter Braak, C.J. CANOCO-aFORTRAN Program for Canonical Community Ordination by Partial Detrended Correspondence Analysis, Principal. Component Analysis and Redunancy Analysis; Servier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Van Oudtshoorn, K.v.R.; van Rooyen, M.W. Restriction of dispersal due to reduction of dispersal structures. In Dispersal Biology of Desert Plants; Springer: Berlin/Heidelberg, Germany, 1999; pp. 93–119. [Google Scholar]
- Stanley, K.E. Evolutionary Trends in the Grasses (Poaceae): A Review; Michigan Publishing, University of Michigan Library: Ann Arbor, MI, USA, 1999. [Google Scholar]
- Funk, V.A.; Susanna, A.; Stuessy, T.; Bayer, R. Systematics, Evolution, and Biogeography of the Compositae; IAPT: Vienna, Austria, 2009. [Google Scholar]
- Walters, D.R.; Keil, D.J. Vascular Plant Taxonomy; Kendall Hunt: Dubuque, IO, USA, 1996. [Google Scholar]
- El-Amier, Y.A. Vegetation structure and soil characteristics of five common geophytes in desert of Egypt. Egypt. J. Basic Appl. Sci. 2016, 3, 172–186. [Google Scholar] [CrossRef] [Green Version]
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Assaeed, A.M.; Al-Rowaily, S.L. Interspecific variations in the habitats of Reichardia tingitana (L.) Roth leading to changes in its bioactive constituents and allelopathic activity. Saudi J. Biol. Sci. 2020, 27, 489–499. [Google Scholar] [CrossRef] [PubMed]
- El-Amier, Y.A.; El-Halawany, E.F.; Abdullah, T.J. Composition and diversity of plant communities in sand formations along the northern coast of the Nile Delta in Egypt. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 826–847. [Google Scholar]
- Abd El-Gawad, A.M. Ecology and allelopathic control of Brassica tournefortii in reclaimed areas of the Nile Delta, Egypt. Turk. J. Bot. 2014, 38, 347–357. [Google Scholar] [CrossRef]
- Asri, Y. Plant Diversity in Touran Biosphere Reservoir; Publishing Research Institute of Forests and Rangelands: Tehran, Iran, 2003; p. 306. [Google Scholar]
- El-Husseini, N.; El-Ghani, M.M.A.; El-Naggar, S.M. Biogeography and diversity of the tubiflorae in Egypt. Pol. Bot. J. 2008, 53, 105–124. [Google Scholar]
- Danin, A.; Orshan, G. The distribution of Raunkiaer life forms in Israel in relation to the environment. J. Veg. Sci. 1990, 1, 41–48. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Abdul-Kader, O.M. Vegetation and species diversity in the northern sector of Eastern Desert, Egypt. West Afr. J. Appl. Ecol. 2015, 23, 75–95. [Google Scholar]
- EI Hadidi, M.N. Natural vegetation. In The Agriculture in Egypt; Oxford University Press: Oxford, UK, 1993; Volume 3. [Google Scholar]
- El-Amier, Y.A.; El Hayyany, L.Y. Floristic composition and species diversity of plant communities associated with genus Atriplex in Nile Delta coast, Egypt. Asian J. Conserv. Biol. 2020, 9, 11–24. [Google Scholar]
- El-Demerdash, M.; Hegazy, A.; Zilay, A. Distribution of the plant communities in Tihamah coastal plains of Jazan region, Saudi Arabia. Vegetation 1994, 112, 141–151. [Google Scholar] [CrossRef]
- Zahran, M.A.; El-Amier, Y.A. Non-traditional fodders from the halophytic vegetation of the deltaic Mediterranean coastal desert Egypt. J. Biol. Sci. 2013, 13, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Alghanem, S.M.; Al-Taisan, W.a.A.; El-Amier, Y.A. Nutritional Value, Mineral Composition, Secondary Metabolites, and Antioxidant Activity of Some Wild Geophyte Sedges and Grasses. Plants 2019, 8, 569. [Google Scholar] [CrossRef] [Green Version]
- Andreasen, C.; Skovgaard, I.M. Crop and soil factors of importance for the distribution of plant species on arable fields in Denmark. Agric. Ecosyst. Environ. 2009, 133, 61–67. [Google Scholar] [CrossRef]
- Allam, M.; Bakr, N.; Elbably, W. Multi-temporal assessment of land use/land cover change in arid region based on landsat satellite imagery: Case study in Fayoum Region, Egypt. Remote Sens. Appl. Soc. Environ. 2019, 14, 8–19. [Google Scholar] [CrossRef]
- Bossard, C.; Randall, J.; Hoshovsky, M. Invasive Plants of California’s Wildlands; University of California Press: Berkeley, CA, USA, 2000; p. 360. [Google Scholar]
- Vivekananda, G.; Swathi, R.; Sujith, A. Multi-temporal image analysis for LULC classification and change detection. Eur. J. Remote Sens. 2021, 54, 189–199. [Google Scholar] [CrossRef]
- El-Zeiny, A.M.; Effat, H.A. Environmental analysis of soil characteristics in El-Fayoum Governorate using geomatics approach. Environ. Monit. Assess. 2019, 191, 463. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.J.; Gatlin, J.A.; Schneider, S.R. Monitoring vegetation in the Nile Delta with NOAA-6 and NOAA-7 AVHRR imagery. Photogramm. Eng. Remote Sens. 1984, 50, 53–61. [Google Scholar]
- International Panel on Climate Change. Summary for Policymakers. In Global Warming of 1.5 °C: An IPCC Special Report; World Meteorological Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Eid, A.N.M.; Olatubara, C.; Ewemoje, T.; Farouk, H.; El-Hennawy, M.T. Coastal wetland vegetation features and digital Change Detection Mapping based on remotely sensed imagery: El-Burullus Lake, Egypt. Int. Soil Water Conserv. Res. 2020, 8, 66–79. [Google Scholar]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Karan, S.K.; Samadder, S.R.; Maiti, S.K. Assessment of the capability of remote sensing and GIS techniques for monitoring reclamation success in coal mine degraded lands. J. Environ. Manag. 2016, 182, 272–283. [Google Scholar] [CrossRef]
- Metternicht, G.; Zinck, A. Remote Sensing of Soil Salinization: Impact on Land Management; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Wiegand, L.; Rhoades, J.D.; Escobar, D.E.; Everitt, J.H. Photographic and videographic observations for determining and mapping the response of cotton to soil salinity. Remote Sens. Environ. 1994, 49, 212–223. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Kamruzzaman, M. Monitoring of Wildlife Suitability Habitat Changes in Nature Reserves. Rev. Cient. Fac. Cienc. Vet. Univ. 2019, 29, 991–999. [Google Scholar]


| Index | Formula | Classes (Ranges) | References |
|---|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | −1–0.25 No vegetation | [37,38] | |
| 0.25–0.5 Sparse vegetation | |||
| 0.5–0.75 Moderate vegetation | |||
| 0.75–1 Dense vegetation | |||
| Normalized Difference Salinity Index (NDSI) | −1–0 Non saline | [39] | |
| 0–0.25 Slightly saline | |||
| 0.25–0.5 Moderately saline | |||
| 0.5–1 Highly saline | |||
| Normalized Difference Moisture Index (NDMI) | NDMI ˃ 0.1 High humidity | [37] | |
| NDMI ˂ 0.1 Low humidity |
| Com. | Stand No. | Total Species | Habitats | Dominant Species (IV ± SD) | Other Important Species |
|---|---|---|---|---|---|
| I | 4 | 25 | SF | Echinopus spinosus (25.53 ± 0.79) * | Brassica tournefortii (20.29 ± 0.70) |
| Bromus diandrus (18.38 ± 0.79) | |||||
| Acacia saligna (17.19 ± 2.00) | |||||
| Mesembryanthemum crystallinum (17.23 ± 1.52) | |||||
| Rumex pictus (13.26 ± 1.27) | |||||
| Malva parviflora (10.62 ± 0.83) | |||||
| II | 12 | 43 | SF, SM | Zygophyllum aegyptium (25.42 ± 0.60) | Senecio glaucus (19.04 ± 0.44) |
| Calligonum polygnoides (23.14 ± 2.00) | Cakile maritima (13.93 ± 1.01) | ||||
| Bromus diandrus (13.63 ± 0.89) | |||||
| Echinopus spinosus (12.76 ± 0.93) | |||||
| Poa annua (11.37 ± 0.81) | |||||
| Rumex pictus (10.51 ± 0.78) | |||||
| III | 16 | 41 | SF | Calligonum polygnoides (38.20 ± 0.54) | Ifloga spicata (18.84 ± 0.63) |
| Senecio glaucus (16.94 ± 0.55) | |||||
| Lotus halophilus (11.96 ± 0.72) | |||||
| Rumex pictus (11.58 ± 0.59) | |||||
| Tamarix nilotica (11.00 ± 0.88) | |||||
| Cutandia memphitica (10.54 ± 1.53) | |||||
| IV | 5 | 24 | SM | Arthrocnemum macrostachyum (32.99 ± 0.49) | Ifloga spicata (17.08 ± 1.00) |
| Sphenopus divaricatus (30.61 ± 0.49) | Rumex pictus (14.15 ± 0.62) | ||||
| Senecio glaucus (13.59 ± 0.64) | |||||
| Lotus halophilus (11.12 ± 0.92) | |||||
| Tamarix nilotica (11.03 ± 1.40) |
| Sediment Variables | Mean (n = 24) | TWINSPAN Vegetation Groups | LSD0.05 | |||
|---|---|---|---|---|---|---|
| I (n = 4) | II (n = 12) | III (n = 16) | IV (n = 5) | |||
| Sand (%) | 89.76 ± 0.84 # | 88.07 a ± 1.87 | 88.37 a ± 0.65 | 90.16 a ± 0.30 | 92.44 a ± 0.55 | 4.99 ns |
| Silt (%) | 6.79 ± 0.52 | 7.12 a ± 0.91 | 7.75 a ± 0.43 | 6.98 a ± 0.27 | 5.29 b ± 0.45 | 1.22 ** |
| Clay (%) | 3.46 ± 0.36 | 4.81 a ± 0.97 | 3.88 ab ± 0.27 | 2.86 b ± 0.04 | 2.27 b ± 0.15 | 1.88 ns |
| Porosity (%) | 38.27 ± 1.16 | 39.59 a ± 2.31 | 41.95 a ± 0.53 | 32.14 b ± 0.43 | 39.40 a ± 1.35 | 5.84 * |
| WHC (%) | 35.98 ± 0.98 | 32.48 b ± 0.99 | 41.93 a ± 1.36 | 34.10 b ± 0.45 | 35.42 b ± 1.11 | 6.21 * |
| CaCO3 (%) | 5.65 ± 0.43 | 5.42 a ± 0.61 | 5.08 a ± 0.22 | 6.42 a ± 0.21 | 5.67 a ± 0.68 | 1.92 ns |
| OC (%) | 0.40 ± 0.04 | 0.35 a ± 0.07 | 0.34 a ± 0.02 | 0.34 a ± 0.02 | 0.55 a ± 0.06 | 0.41 ns |
| pH | 8.36 ± 0.13 | 9.36 a ± 0.13 | 8.07 a ± 0.07 | 7.55 a ± 0.12 | 8.46 a ± 0.18 | 2.26 ns |
| EC (mS.cm−1) | 0.95 ± 0.04 | 0.74 a ± 0.07 | 0.99 a ± 0.05 | 0.62 a ± 0.01 | 1.44 a ± 0.04 | 5.72 ns |
| Cl− (%) | 0.53 ± 0.15 | 0.83 a ± 0.29 | 0.59 b ± 0.07 | 0.14 c ± 0.01 | 0.57 b ± 0.23 | 0.19 *** |
| SO42− (%) | 0.52 ± 0.11 | 0.66 a ± 0.21 | 0.56 a ± 0.05 | 0.32 a ± 0.02 | 0.53 a ± 0.16 | 0.94 ns |
| HCO3− (%) | 0.67 ± 0.10 | 0.21 d ± 0.01 | 0.54 c ± 0.08 | 1.15 a ± 0.09 | 0.77 b ± 0.21 | 0.06 *** |
| Na+ (mg/100g dry soil) | 137.64 ± 12.52 | 91.75 c ± 3.62 | 150.32 b ± 12.74 | 237.75 a ± 18.75 | 70.75 d ± 14.97 | 10.17 *** |
| K+ (mg/100g dry soil) | 45.42 ± 4.63 | 15.05 c ± 0.52 | 54.06 b ± 6.75 | 100.32 a ± 8.80 | 12.24 c ± 2.45 | 3.58 *** |
| Ca++ (mg/100g dry soil) | 41.63 ± 3.74 | 23.21 c ± 1.00 | 47.83 b ± 4.75 | 81.08 a ± 6.55 | 14.40 d ± 2.64 | 7.15 *** |
| Mg++ (mg/100g dry soil) | 52.01 ± 5.40 | 11.63 c ± 0.28 | 57.27 b ± 7.61 | 126.66 a ± 11.35 | 12.46 c ± 2.37 | 4.52 *** |
| SAR | 19.27 ± 1.16 | 21.92 a ± 0.49 | 19.62 ab ± 0.71 | 19.69 ab ± 0.72 | 15.86 b ± 2.70 | 5.84 ns |
| PAR | 4.50 ± 0.32 | 3.60 bc ± 0.06 | 4.97 ab ± 0.36 | 6.64 a ± 0.37 | 2.77 c ± 0.50 | 2.17 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Zeiny, A.; Elagami, S.A.; Nour-Eldin, H.; El-Halawany, E.-S.F.; Bonanomi, G.; Abd-ElGawad, A.M.; Soufan, W.; El-Amier, Y.A. Wild Plant Habitat Characterization in the Last Two Decades in the Nile Delta Coastal Region of Egypt. Agriculture 2022, 12, 108. https://doi.org/10.3390/agriculture12010108
El-Zeiny A, Elagami SA, Nour-Eldin H, El-Halawany E-SF, Bonanomi G, Abd-ElGawad AM, Soufan W, El-Amier YA. Wild Plant Habitat Characterization in the Last Two Decades in the Nile Delta Coastal Region of Egypt. Agriculture. 2022; 12(1):108. https://doi.org/10.3390/agriculture12010108
Chicago/Turabian StyleEl-Zeiny, Ahmed, Shrouk A. Elagami, Hoda Nour-Eldin, El-Sayed F. El-Halawany, Giuliano Bonanomi, Ahmed M. Abd-ElGawad, Walid Soufan, and Yasser A. El-Amier. 2022. "Wild Plant Habitat Characterization in the Last Two Decades in the Nile Delta Coastal Region of Egypt" Agriculture 12, no. 1: 108. https://doi.org/10.3390/agriculture12010108
APA StyleEl-Zeiny, A., Elagami, S. A., Nour-Eldin, H., El-Halawany, E.-S. F., Bonanomi, G., Abd-ElGawad, A. M., Soufan, W., & El-Amier, Y. A. (2022). Wild Plant Habitat Characterization in the Last Two Decades in the Nile Delta Coastal Region of Egypt. Agriculture, 12(1), 108. https://doi.org/10.3390/agriculture12010108











