Conventional versus Nano Calcium Forms on Peanut Production under Sandy Soil Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Calcium Nitrate Ca(NO3)2 Nanoparticles Preparation
2.3. Cultural Practices
2.4. Experimental Design and Treatments
2.5. Traits and Measurements
2.6. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Variance, Growth and Yield Components
3.2. Quality Traits
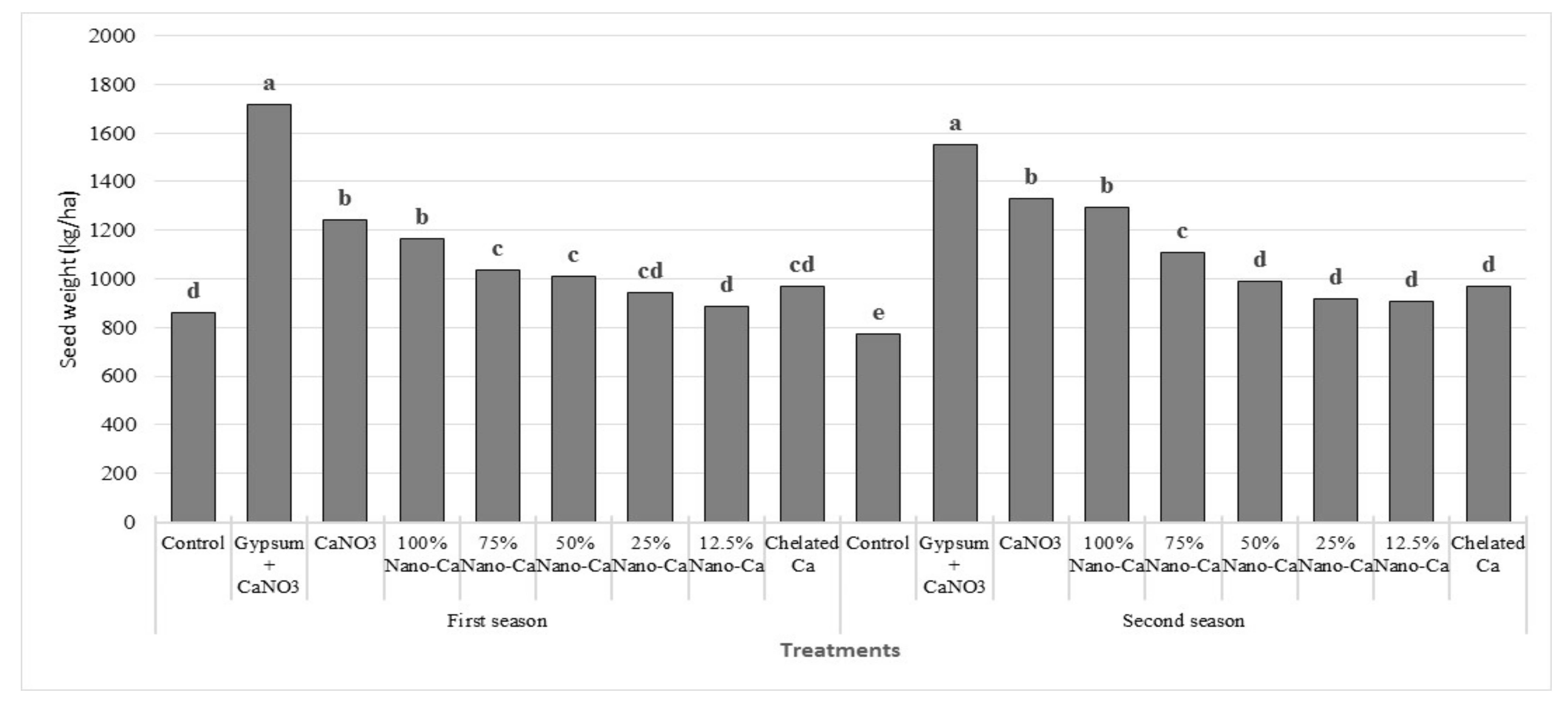
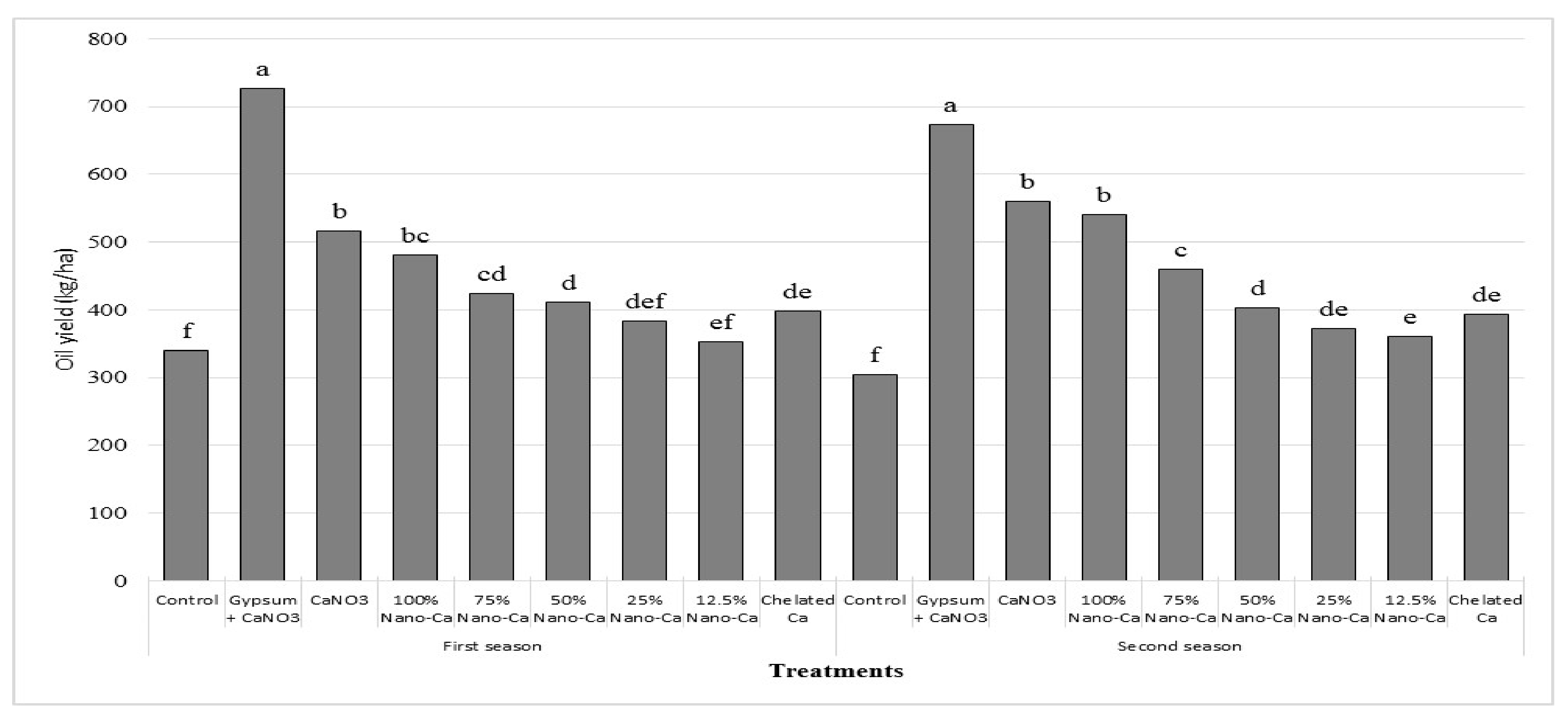
3.3. Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jambunathan, R. Groundnut quality characteristics. In Uses of Tropical Grain Legumes: Proceedings of the a Consultants Meeting, Patancheru, India, 27–30 March 1989; ICRISAT: Patancheru, India, 1991; pp. 267–275. [Google Scholar]
- Janila, P.; Nigam, S.N.; Pandey, M.K.; Nagesh, P.; Varshney, R.K. Groundnut improvement: Use of genetic and genomic tools. Front. Plant Sci. 2013, 4, 23. [Google Scholar] [CrossRef]
- Rameshaiah, G.N.; Pallavi, J.; Shabnam, S. Nano fertilizers and nano sensors—An attempt for developing smart agriculture. Int. J. Eng. Res. Gen. Sci. 2015, 3, 314–320. [Google Scholar]
- Das, R.; Kiley, P.J.; Segal, M.; Norville, J.; Yu, A.A.; Wang, L.; Trammel, S.A.; Reddick, L.E.; Kummar, R.; Stellacci, F.; et al. Integration of photosynthetic protein molecular complexes in solid-state electronic devices. Nano Lett. 2004, 4, 1079–1083. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Agrawal, A.; Raliya, R.; Kumar, P.; Burman, U.; Kaul, R.K. ZnO nanoparticles induced synthesis of polysaccharides and phosphatases by Aspergillus fungi. Adv. Sci. Eng. Med. 2012, 4, 324–328. [Google Scholar] [CrossRef]
- Prasad, R.; Kumar, V.; Prasad, K.S. Nanotechnology in sustainable agriculture: Present concerns and future aspects. Afr. J. Biotechnol. 2014, 13, 705–713. [Google Scholar] [CrossRef]
- Manjunatha, J.S.B.; Biradar, D.P.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm. Sci. 2016, 29, 1–13.2212. [Google Scholar]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; SergiMunné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Rizvi, T.F. Application of Nanofertilizer and Nanopesticides for Improvements in Crop Production and Protection. In Nanoscience and Plant–Soil Systems; Soil Biology; Ghorbanpour, M., Manika, K., Varma, A., Eds.; Springer: Cham, Switzerland, 2017; Volume 48, pp. 405–427. [Google Scholar] [CrossRef]
- Naderi, M.R.; Danesh-Shahraki, A. Nanofertilizers and their roles in sustainable agriculture. Int. J. Agric. Crop Sci. 2013, 5, 2229–2232. [Google Scholar]
- Mousavi, S.R.; Rezaei, M. Nanotechnology in agriculture and food production. J. Appl. Environ. Biol. Sci. 2011, 1, 414–419. [Google Scholar]
- Srilatha, B. Nanotechnology in agriculture. J. Nanomedicnie Nanotechnol. 2011, 2, 1–5. [Google Scholar] [CrossRef]
- Ditta, A. How helpful is nanotechnology in agriculture? Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 033002. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Amanzadeh, T.; Sabaghnia, N.; Dashti, S. Impact of foliar application of nano micronutrient fertilizers and titanium dioxide nanoparticles on the growth and yield components of barley under supplemental irrigation. Acta Agric. Slov. 2016, 107, 265–276. [Google Scholar] [CrossRef]
- Tavan, T.; Niakan, M.; Norinia, A.A. Effect of nano-potassium fertilizer on growth factors, photosynthetic system and protein content in wheat (Triticum aestivum L. Cv. N8019). J. Plant Environ. Physiol. 2014, 9, 61–71. [Google Scholar]
- Mikkelsen, R. Nanofertilizer and nanotechnology: A quick look. Better Crops. Better Crop Plant Food 2018, 102, 18–19. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nano-level. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Pullagurala, V.L.R.; Adisa, I.O.; Rawat, S.; Kim, B.; Barrios, A.C.; Medina, I.A.; Gardea-Torresdey, J.L. Finding the conditions for the beneficial use of ZnO nanoparticles towards plants. Environ. Pollut. 2018, 241, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: New York, NY, USA, 1995. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Moller, I.S.; White, P. Functions of Macronutrients. In Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 171–178. [Google Scholar]
- Cox, F.R.; Adams, F.; Tucker, B.B. Liming, Fertilization, and Mineral Nutrition. In Peanut Science and Technology; Pattee, H.E., Young, C.T., Eds.; American Peanut Research and Education Soc.: Yoakum, TX, USA, 1982; pp. 139–163. [Google Scholar]
- Gascho, G.J.; Davis, J.G. Mineral Nutrition. In The Groundnut Crop; Smartt, J., Ed.; World Crop Series; Springer: Dordrecht, The Netherlands, 1994. [Google Scholar] [CrossRef]
- Zharare, G.E.; Blamey, F.P.C.; Asher, C.J. Calcium nutrition of peanut (Arachis hypogaea L.) grown in solution culture. II. Pod-zone and tissue calcium requirements for fruiting of a Virginia and a Spanish peanut. J. Plant Nutr. 2009, 32, 1843–1860. [Google Scholar] [CrossRef]
- Wiatrak, P.J.; Wright, D.L.; Marois, J.J.; Wilson, D. Influence of gypsum application on peanut yield and quality. Crop Manag. 2006, 5, 1–5. [Google Scholar] [CrossRef]
- Roland, B.S.; Christopher, B.L. Pod yield and mineral concentration of four peanut cultivars following gypsum application with subsurface drip irrigation. Peanut Sci. 2008, 35, 86–91. [Google Scholar] [CrossRef][Green Version]
- Bairagi, M.D.; David, A.A.; Thomas, T.; Gurjar, P.C. Effect of Different Level of N P K and Gypsum on Soil Properties and Yield of Groundnut (Arachis hypogaea L.) var. Jyoti. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 984–991. [Google Scholar] [CrossRef][Green Version]
- Thilakarathna, S.M.; Kirthisinghe, C.R.; Gunathilaka, J.P.; Dissanayaka, B.L.D.M.P.V. Influence of gypsum application on yield and visual quality of groundnut (Arachis hypogaea L.) grown in Maspotha in Kurunegala District of Sri Lanka. Trop. Agric. Res. 2014, 25, 432–436. [Google Scholar] [CrossRef]
- Yadav, R.; Jat, L.K.; Yadav, S.N.; Singh, R.P.; Yadav, P.K. Effect of gypsum on growth and yield of groundnut (Arachis hypogaea L.). Environ. Ecol. 2015, 33, 676–679. [Google Scholar]
- Arnold, J.A.; Beasley, J.P.; Harris, G.H.; Grey, T.L.; Cabrera, M. Effect of gypsum application rate, soil type, and soil calcium on yield, grade and seed quality of runner type peanut cultivars. Peanut Sci. 2017, 44, 13–18. [Google Scholar] [CrossRef][Green Version]
- Xiumei, L.; Fudao, Z.; Shuqing, Z.; Xusheng, H.; Rufang, W.; Zhaobin, F.; Yujun, W. Responses of peanut to Nano-calcium carbonate. J. Plant Nutr. Soil Sci. 2005, 11, 385–389. [Google Scholar]
- Pattee, H.E.; Stalker, H.T. Advances in Peanut Science; American Peanut Research and Education Society, Inc.: Stillwater, OK, USA, 1995. [Google Scholar]
- Pathak, B.P. Effect of calcium on peanut (Arachis hypogaea L.) pod and seed development under field conditions. Master’s Thesis, Graduate School, University of Florida, Florida, FL, USA, 2010. [Google Scholar]
- Keisling, T.C.; Walker, M.E. Calcium absorption efficiency of peanut fruit of various cultivars. J. Plant Nutr. 1982, 5, 91–95. [Google Scholar] [CrossRef]
- Yang, R. Calcium availability to runner-type peanut (Arachis hypogaea L.) in the Southeastern United States. Master’s Thesis, Faculty of Graduate, Auburn University, Auburn, AL, USA, 2015. [Google Scholar]
- Hepler, P.K.; Wayne, R.O. Calcium and plant development. Ann. Rev. Plant Physiol. 1985, 36, 397–439. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Tang, Z.; Guo, F.; Zhang, Y.; Zhang, J.; Meng, J.; Zheng, L.; Wan, S.; Li, X. Transcriptome of peanut kernel and shell reveals the mechanism of calcium on peanut pod development. Sci. Rep. 2020, 10, 15723. [Google Scholar] [CrossRef] [PubMed]
- Klute, A. Methods of Soil Analysis. Part-I: Physical and Mineralogical Methods, 2nd ed.; American Society of Agronomy Madison: Madison, WI, USA, 1986. [Google Scholar]
- Page, A.I.; Miller, R.H.; Keeny, D.R. Methods of Soil Analysis Part II. Chemical and Microbiological Methods, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar]
- A.O.A.C. Official Methods of Analysis of A.O.A.C. International, 17th ed.; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000; Volume 2, pp. 66–68. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. Analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- SPSS Statistics 17.0; SPSS for Windows; SPSS Inc.: Chicago, IL, USA, 2008.
- Steel, R.G.D.; Torri, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; Mc Graw-Hill: New York, NY, USA, 1997; p. 666. [Google Scholar]
- Freed, R.; Einensmith, S.P.; Gutez, S.; Reicosky, D.; Smail, V.W.; Wolberg, P. User’s Guide to MSTAT-C Analysis of Agronomic Research Experiments; Michigan State University: East Lansing, IM, USA, 1989. [Google Scholar]
- Clark, R.B.; Ritchey, K.D.; Baligar, V.C. Benefits and constraints for use of FGD products on agricultural land. Fuel 2001, 80, 821–828. [Google Scholar] [CrossRef]
- Elrashicli, M.A.; West, L.T.; Seybold, C.A.; Benharn, E.C. Effects of gypsum addition on solubility of nutrients in soil amended with peat. Soil Sci. 2010, 175, 162–172. [Google Scholar] [CrossRef]
- Florence, R.J. Fertilization of peanut (Arachis hypogaea L.) with calcium: Influence of source, rate, and leaching on yield and seed quality. Master’s Thesis, Graduate Faculty of Auburn University, Alabama, AL, USA, 2011. [Google Scholar]
- Kahlon, U.Z.; Murtaza, G.; Ghafoor, A. Amelioration of saline-sodic soil with amendments using brackish water, canal water and their combination. Int. J. Agric. Biol. 2012, 14, 38–46. [Google Scholar]
- Murtaza, G.; Murtaza, B.; Usman, H.M.; Ghafoor, A. Amelioration of saline-sodic soil using gypsum and low-quality water in following sorghum-berseem crop rotation. Int. J. Agric. Biol. 2013, 5, 640–648. [Google Scholar]
- Arnold, J.A. Effect of soil calcium and gypsum application rate on yield, grade, and seed quality of two runner-type peanut cultivars. Master’s Thesis, Graduate Faculty of the University of Georgia, Georgia, IL, USA, 2011. [Google Scholar]
- Tillman, B.I.; Gomillion, M.W.; Person, G.; Mackowiak, C.I. Variation in response of calcium fertilization among four runner-type of peanut. Agron. J. 2010, 102, 469–474. [Google Scholar] [CrossRef]
- Webb, A.J.; Hansen, A.P. Histological changes of the peanut (Arachis hypogaea) gynophore and fruit surface during development, and their potential significance for nutrient uptake. Ann. Bot. 1989, 64, 351–357. [Google Scholar] [CrossRef]
- Skelton, B.J.; Shear, G.M. Calcium translocation in the peanut (Arachis hypogaea L.). Agron. J. 1971, 63, 409–412. [Google Scholar] [CrossRef]
- Ghosh, A.; Singh, V.J.; Srihima, G.; Sahoo, P.; Chakraborti, S.K. Effect of gypsum and lime on seed yield parameters of groundnut (Arachis hypogaea). J. Crop Weed 2015, 11, 5–9. [Google Scholar]
- Yang, S.; Li, L.; Zhang, J.; Geng, Y.; Guo, F.; Wang, J.; Meng, J.; Sui, N.; Wan, S.; Li, X. Transcriptome and differential expression profiling analysis of the mechanism of Ca2+ regulation in peanut (Arachis hypogaea) pod development. Front. Plant Sci. 2017, 8, 1609. [Google Scholar] [CrossRef]
- Gashti, A.H.; Vishekaei, M.N.S.; Hosseinzadeh, M.H. Effect of potassium and calcium application on yield, yield components and qualitative characteristics of peanut (Arachis hypogaea L.) In Guilan Province, Iran. World Appl. Sci. J. 2012, 16, 540–546. [Google Scholar]
- Chirwa, M.; Mrema, J.P.; Mtakwa, P.W.; Kaaya, A.; Lungu, O.I. Yield response of groundnut (Arachis hypogaea L.) to boron, calcium, nitrogen, phosphorus and potassium fertilizer application. Int. J. Soil Sci. 2017, 12, 18–24. [Google Scholar] [CrossRef]
- Kamara, E.G. Effect of calcium and phosphorus fertilization on the growth, yield and seed quality of two groundnut (Arachis hypogaea L.) varieties. Master’s Thesis, Faculty of Agriculture, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, 2010. [Google Scholar]
- Rahman, M.A. Effect of Calcium and Bradyrhizobium inoculation of the Growth, Yield and quality of groundnut (Arachis hypogaea L.). Bangladesh J. Sci. Ind. Res. 2006, 41, 181–188. [Google Scholar] [CrossRef]
- Zharare, G.E.; Blamey, F.C.; Asher, C.J. Effects of pod-zone calcium supply on dry matter distribution at maturity in two groundnut cultivars grown in solution culture. J. Plant Nutr. 2012, 35, 1542–1556. [Google Scholar] [CrossRef]
- Hussein, S.M.A.; El-Melegy, A.M.; Haikel, M.A. Effect of nitrogen frequency, gypsum application, plant density, and their interaction on growth and yield of peanut under drip irrigation system in North Sinai. J. Agric. Sci. Mansoura Univ. 2000, 25, 2427–2438. [Google Scholar]
- Nobahara, A.; Zakerina, H.R.; Radb, M.M.; Sayfzadeha, S.; Valadabadya, A.R. Response of yield and some physiological traits of groundnut (Arachis hypogaea L.) to topping height and application methods of Zn and Ca nano-chelates. Commun. Soil Sci. Plant Anal. 2019, 50, 749–762. [Google Scholar] [CrossRef]
- Chalwe, H.M.; Lungu, O.I.; Mweetwa, A.M.; Phiri, E.; Yengwe, J.; Njoroge, S.C.; Brandenburg, R. The effects of gypsum on pod-yield and pre-harvest aflatoxin contamination in selected peanut cultivars of Zambia. Afr. J. Plant Sci. 2020, 14, 134–138. [Google Scholar] [CrossRef]
- Kamara, E.G.; Olympio, N.S.; Asibuo, J.Y.; Kabbia, M.K.; Yila, K.M.; Conteh, A.R. Effect of calcium and phosphorus fertilizer on seed yield and nutritional quality of groundnut (Arachis hypogaea L.). Int. J. Agric. For. 2017, 7, 129–133. [Google Scholar] [CrossRef]
- Howe, J.A.; Florence, R.J.; Haris, G.; Van Santen, E.; Beasley, J.P.; Bostick, J.P.; Balkcom, K.B. Effect of cultivar, irrigation, and soil calcium on runner peanut response to gypsum. Agron. J. 2012, 104, 1312–1320. [Google Scholar] [CrossRef]
- Vidya-Sagar, D.R.M.S.; Dawson, J.; Reddy, R.U.K. Effect of phosphorus and gypsum on growth, yield and economics of groundnut (Arachis hypogea L.). Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1635–1638. [Google Scholar] [CrossRef]
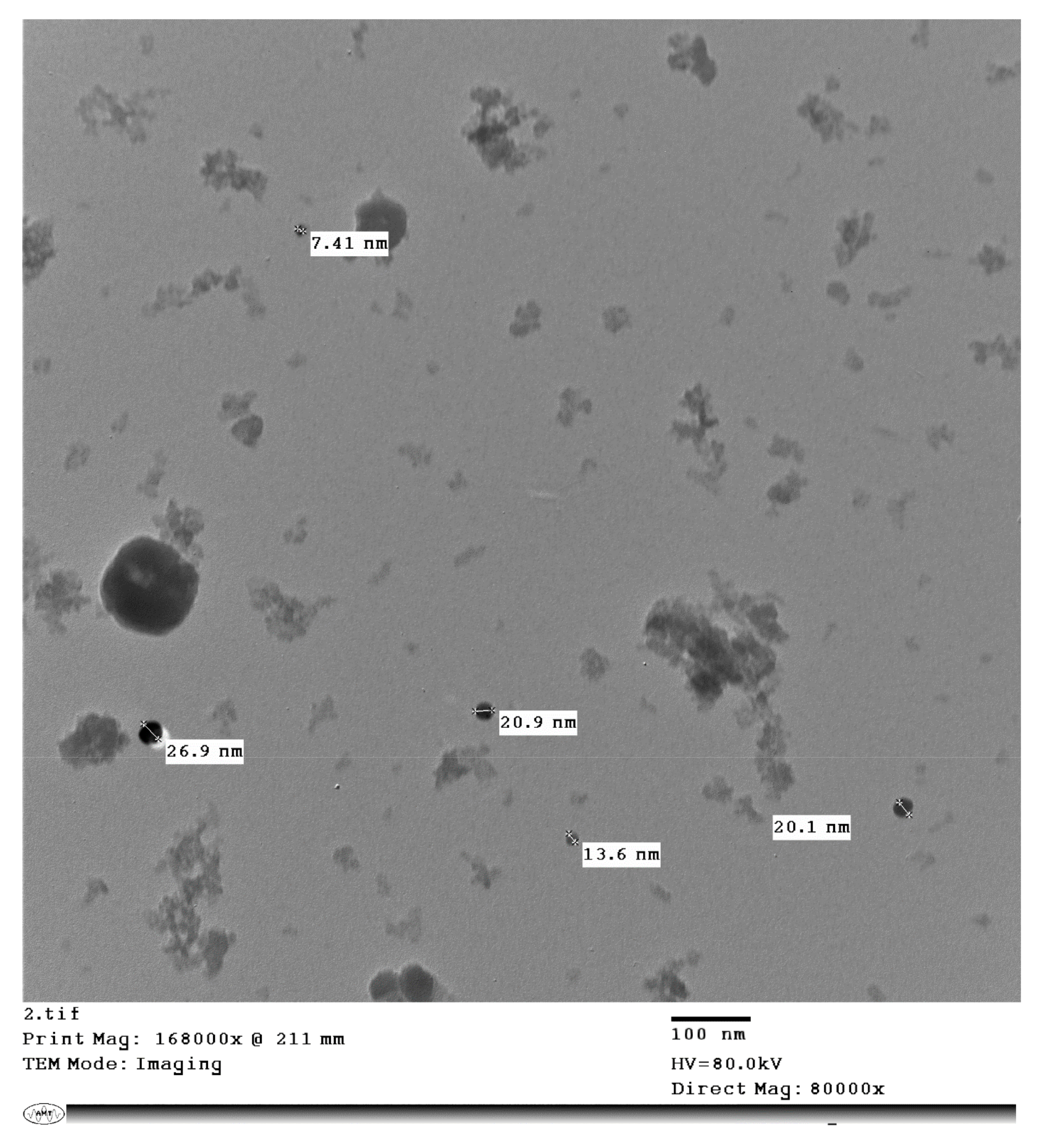
| Month | 2015 | 2016 | ||||
|---|---|---|---|---|---|---|
| Temperature (°C) | Relative Humidity (%) | Rainfall (mm) | Temperature (°C) | Relative Humidity (%) | Rainfall (mm) | |
| May | 22.3 | 63.7 | 0 | 23.7 | 63.0 | 0 |
| June | 24.6 | 64.7 | 0 | 26.9 | 59.0 | 0 |
| July | 26.7 | 67.0 | 0 | 27.6 | 61.3 | 0 |
| August | 19.4 | 43.0 | 0 | 18.8 | 43.7 | 0 |
| September | 28.2 | 56.0 | 2 | 26.0 | 58.0 | 0 |
| Properties | Season | |
|---|---|---|
| 2015 | 2016 | |
| Physical Properties | ||
| Sand % | 91.84 | 93.61 |
| Silt % | 3.64 | 2.84 |
| Clay % | 4.52 | 3.55 |
| Texture | Sandy | Sandy |
| Chemical Properties | ||
| Soil pH (1:1) | 7.31 | 7.53 |
| EC (1:1, dS m−1) | 4.73 | 4.46 |
| Soluble anions (meq L−1) | ||
| Cl‒ | 28.50 | 32.60 |
| SO42‒ | 10.28 | 11.02 |
| HCO3− | 1.80 | 1.93 |
| Soluble cations (meq L−1) | ||
| K+ | 2.26 | 2.62 |
| Ca2+ | 6.62 | 7.00 |
| Mg2+ | 8.44 | 8.62 |
| Na+ | 23.60 | 26.88 |
| Organic Matter (%) | 0.28 | 0.30 |
| Total carbonate content | 2.75 | 3.10 |
| Available N (mg kg−1) | 0.67 | 0.87 |
| Available P (mg kg−1) | 2.54 | 2.45 |
| Available K (mg kg−1) | 190 | 178 |
| Season | pH | EC | Soluble Ions (meq L−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (dS m−1) | HCO3− | Cl− | SO42‒ | Ca2+ | Mg2+ | Na+ | K+ | |||
| 2015 | 7.09 | 4.02 | 1.9 | 31.0 | 8.0 | 4.7 | 3.8 | 30.8 | 1.52 | |
| 2016 | 7.10 | 3.82 | 2.2 | 29.4 | 7.7 | 4.5 | 3.2 | 31.2 | 0.44 | |
| SOV | Growth and Yield Components | |||||
|---|---|---|---|---|---|---|
| Plant Height, cm | Branches Number Plant−1 | Pods Number Plant−1 | Immature Pods Number Plant−1 | Pods weight Plant−1, g | Seeds Weight Plant−1, g | |
| Year (Y) | ** | ** | ** | ** | NS | ** |
| Treatment (T) | ** | ** | ** | ** | ** | ** |
| T × Y | ** | NS | ** | ** | ** | ** |
| SOV | Quality Characteristics | |||||
| Pod Index, g | Seed Index, g | Shelling, % | Pops, % | Oil, % | Protein, % | |
| Year (Y) | * | * | * | NS | NS | NS |
| Treatment (T) | ** | ** | ** | ** | ** | ** |
| T × Y | NS | ** | NS | NS | NS | NS |
| SOV | Yields | |||||
| Biological Yield, ton ha−1 | Pods Yield, ton ha−1 | Seed Yield, ton ha−1 | Oil Yield, kg ha−1 | Protein Yield, kg ha−1 | ||
| Year (Y) | ** | * | NS | NS | NS | |
| Treatment (T) | ** | ** | ** | ** | ** | |
| T × Y | NS | ** | ** | * | * | |
| Plant Height, cm | Branches Number Plant−1 | Pods Number Plant−1 | Immature Pods Number Plant−1 | Pods Weight Plant−1, g | Seeds Weight Plant−1, g | |
|---|---|---|---|---|---|---|
| Control | 36.9 e † | 15.1 de | 19.2 h | 20.5 b | 22.5 f | 16.3 g |
| Gypsum + Ca(NO3)2 | 51.3 a | 25.7 a | 32.9 a | 8.7 h | 45.1 a | 31.5 a |
| Ca(NO3)2 | 49.8 b | 20.4 b | 26.4 b | 10.5 g | 39.9 b | 27.0 b |
| 100% nano Ca(NO3)2 | 49.8 b | 20.0 b | 24.7 cd | 10.9 fg | 38.6 b | 26.3 b |
| 75% nano Ca(NO3)2 | 49.1 b | 19.3 b | 23.9 de | 11.8 f | 33.4 d | 23.3 c |
| 50% nano Ca(NO3)2 | 48.9 b | 18.4 bc | 23.4 ef | 12.8 e | 29.5 e | 21.0 d |
| 25% nano Ca(NO3)2 | 47.3 c | 18.1 bc | 21.7 g | 16.7 d | 28.2 e | 18.7 ef |
| 12.5% nano Ca(NO3)2 | 43.8 d | 16.4 cd | 22.3 fg | 19.3 c | 24.3 f | 17.7 fg |
| Chelated Ca | 30.9 f | 12.7 e | 25.3 bc | 23.8 a | 36.6 c | 20.8 de |
| Mean | 45.3 | 18.5 | 24.4 | 15.0 | 33.1 | 22.5 |
| CV % | 6.8 | 4.7 | 5.7 | 4.1 | 12.3 | 2.4 |
| LSD 0.05 | 1.3 | 2.7 | 1.2 | 1.0 | 1.8 | 2.2 |
| Pod Index, g | Seed Index, g | Shelling, % | Pops, % | Oil, % | Protein, % | |
|---|---|---|---|---|---|---|
| Control | 115.0 f | 45.3 f | 54.2 e | 26.3 b | 39.5 e | 19.3 f |
| Gypsum + Ca(NO3)2 | 188.2 a | 64.3 a | 65.2 a | 12.2 e | 42.9 a | 23.2 a |
| Ca(NO3)2 | 171.9 b | 60.7 b | 63.1 ab | 15.2 d | 41.8 b | 22.1 b |
| 100% nano Ca(NO3)2 | 168.2 bc | 60.6 b | 62.1 bc | 15.7 d | 41.4 bc | 21.7 bc |
| 75% nano Ca(NO3)2 | 165.2 c | 59.7 bc | 61.3 bc | 17.5 cd | 41.1 bc | 21.5 bcd |
| 50% nano Ca(NO3)2 | 155.1 d | 52.2 d | 60.7 c | 19.0 c | 40.8 bcd | 21.3 cd |
| 25% nano Ca(NO3)2 | 150.7 d | 49.7 e | 58.3 d | 24.5 b | 40.5 cde | 20.8 de |
| 12.5% nano Ca(NO3)2 | 117.0 f | 46.8 f | 57.2 d | 25.5 b | 39.7 de | 20.2 e |
| Chelated Ca | 124.1 e | 57.5 c | 58.0 d | 30.2 a | 40.8 bc | 21.2 cd |
| Mean | 150.6 | 55.2 | 60.0 | 20.7 | 40.9 | 21.3 |
| CV % | 2.9 | 2.2 | 9.8 | 3.0 | 3.8 | 3.5 |
| LSD 0.05 | 6.2 | 2.7 | 2.1 | 2.4 | 1.0 | 0.7 |
| Biological Yield, ton ha −1 | Pods Yield, ton ha −1 | Seed Yield, ton ha −1 | Oil Yield, kg ha−1 | Protein Yield, kg ha−1 | |
|---|---|---|---|---|---|
| Control | 8.9 c | 1.5 f | 0.8 f | 322.6 f | 158.0 g |
| Gypsum + Ca(NO3)2 | 15.4 a | 2.5 a | 1.6 a | 700.3 a | 380.1 a |
| Ca(NO3)2 | 14.4 a | 2.1 b | 1.3 b | 538.2 b | 284.8 b |
| 100% nano Ca(NO3)2 | 13.8 a | 2.0 b | 1.2 b | 510.3 b | 267.0 b |
| 75% nano Ca(NO3)2 | 11.8 b | 1.8 c | 1.1 c | 441.7 c | 230.5 c |
| 50% nano Ca(NO3)2 | 11.1 b | 1.7 de | 1.0 cd | 407.5 d | 212.9 cd |
| 25% nano Ca(NO3)2 | 10.9 b | 1.6 def | 0.9 de | 377.5 de | 194.0 ef |
| 12.5% nano Ca(NO3)2 | 10.6 b | 1.8 ef | 0.9 e | 356.8 e | 181.0 f |
| Chelated Ca | 11.3 b | 1.7 cd | 1.0 de | 395.5 d | 205.9 de |
| Mean | 12.0 | 1.9 | 1.1 | 450.0 | 234.9 |
| CV % | 6.8 | 6.2 | 5.7 | 4.5 | 11.5 |
| LSD 0.05 | 1.6 | 0.1 | 0.1 | 32.6 | 18.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, M.; Abbas, M.; Abd Elrahman, A.; Helal, M.; Shahba, M. Conventional versus Nano Calcium Forms on Peanut Production under Sandy Soil Conditions. Agriculture 2021, 11, 767. https://doi.org/10.3390/agriculture11080767
Hamza M, Abbas M, Abd Elrahman A, Helal M, Shahba M. Conventional versus Nano Calcium Forms on Peanut Production under Sandy Soil Conditions. Agriculture. 2021; 11(8):767. https://doi.org/10.3390/agriculture11080767
Chicago/Turabian StyleHamza, Mohamed, Mohamed Abbas, Asmaa Abd Elrahman, Mohamed Helal, and Mohamed Shahba. 2021. "Conventional versus Nano Calcium Forms on Peanut Production under Sandy Soil Conditions" Agriculture 11, no. 8: 767. https://doi.org/10.3390/agriculture11080767
APA StyleHamza, M., Abbas, M., Abd Elrahman, A., Helal, M., & Shahba, M. (2021). Conventional versus Nano Calcium Forms on Peanut Production under Sandy Soil Conditions. Agriculture, 11(8), 767. https://doi.org/10.3390/agriculture11080767







