Biological Control May Fail on Pests Applied with High Doses of Insecticides: Effects of Sub-Lethal Concentrations of a Pyrethroid on the Host-Searching Behavior of the Aphid Parasitoid Aphidius colemani (Hymenoptera, Braconidae) on Aphid Pests
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphids
2.2. Parasitoids
2.3. Effect of Insecticides and Aphid Genotype on Patch Time Allocation in Parasitoids
2.4. Effect of Insecticide on Parasitoid Orientation
2.5. Statistical Analyses
3. Results
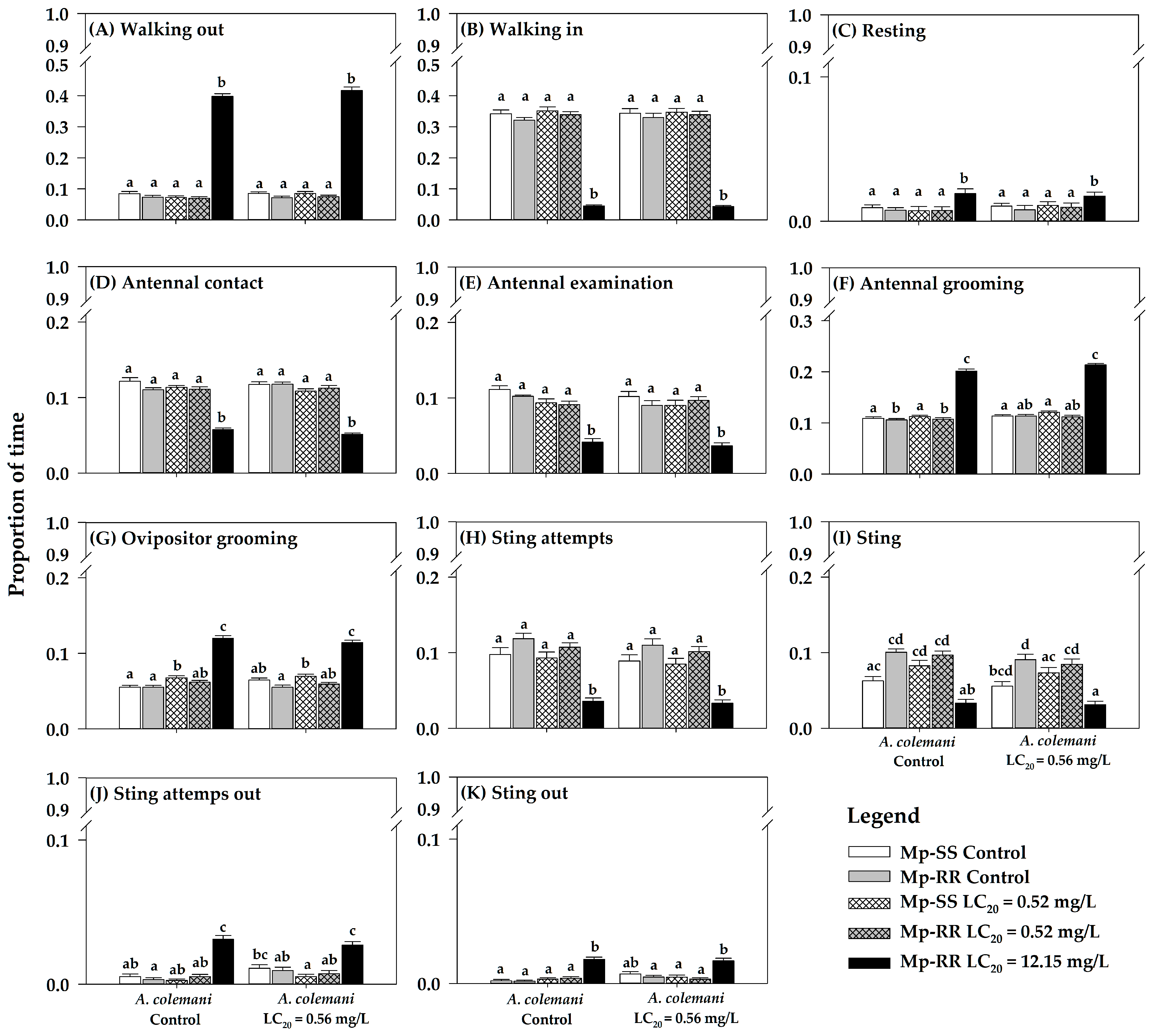
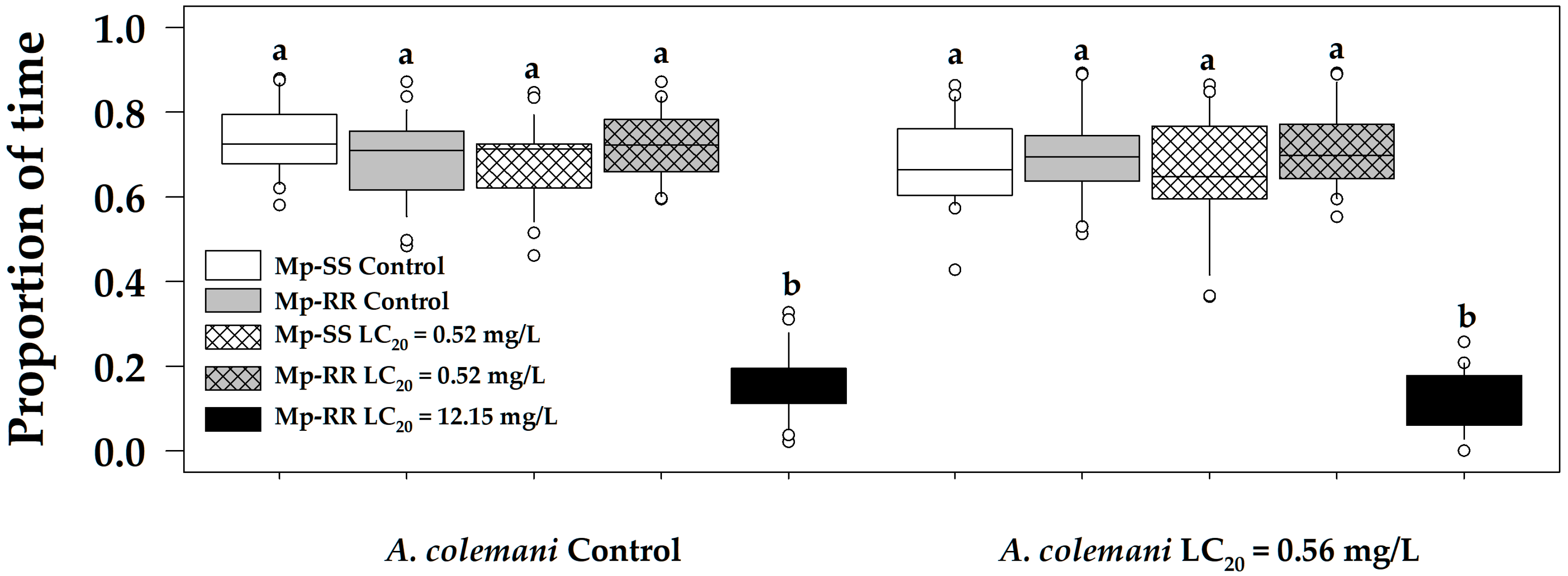
3.1. Effect of Insecticides and Aphid Genotype on the Time Allocation of Parasitoids
3.2. Influence of Insecticide Exposure on the Orientation Behavior of Parasitoids
4. Discussion
4.1. Parasitoids Avoid Parasitizing Insecticide-Resistant Aphids at High Doses
4.2. Non-Detrimental Effects of Low Insecticide Concentrations
4.3. Implications for Integrated Pest Management (IPM)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewar, A.M.; Denholm, I.; van Emden, H.F.; Harrington, R. Chemical control. In Aphids as Crop Pests, 2nd ed.; van Emden, H.F., Harrington, R., Eds.; CABI: Oxford, UK, 2017; pp. 398–425. [Google Scholar]
- Blackman, R.; Eastop, V.F. Taxonomic Issues. In Aphids as Crop Pests, 2nd ed.; van Emden, H.F., Harrington, R., Eds.; CABI: Oxford, UK, 2017; pp. 1–36. [Google Scholar]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef]
- Foster, S.P.; Devine, G.J.; Devonshire, A.L. Insecticide resistance. In Aphids as Crop Pests, 2nd ed.; van Emden, H.F., Harrington, R., Eds.; CABI: Oxford, UK, 2017; pp. 426–447. [Google Scholar]
- Silva, A.X.; Jander, G.; Samaniego, H.; Ramsey, J.S.; Figueroa, C.C. Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) I: A transcriptomic survey. PLoS ONE 2012, 7, e36366. [Google Scholar] [CrossRef]
- Martinez-Torres, D.; Foster, S.P.; Field, L.M.; Devonshire, A.L.; Williamson, M.S. A sodium channel point mutation is associated with resistance to DDT and pyrethroid insecticides in the peach-potato aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). Insect Mol. Biol. 1999, 8, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.P.; Tomiczek, M.; Thompson, R.; Denholm, I.; Poppy, G.; Kraaijeveld, A.; Powell, W. Behavioural side-effects of insecticide resistance in aphids increase their vulnerability to parasitoid attack. Anim. Behav. 2007, 74, 621–632. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.P.; Denholm, I.; Poppy, G.M.; Thompson, R.; Powell, W. Fitness trade-off in peach-potato aphids (Myzus persicae) between insecticide resistance and vulnerability to parasitoid attack at several spatial scales. Bull. Entomol. Res. 2011, 101, 659–666. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; van Lenteren, J.C. The status of biological control and recommendations for improving uptake for the future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef]
- Simon, J.C.; Peccoud, J. Rapid evolution of aphid pests in agricultural environments. Curr. Opin. Insect Sci. 2018, 26, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Prado, S.G.; Jandricic, S.E.; Frank, S.D. Ecological interactions affecting the efficacy of Aphidius colemani in greenhouse crops. Insects 2015, 6, 538–575. [Google Scholar] [CrossRef] [PubMed]
- Starý, P. The Aphidiidae of Chile (Hymenoptera, Ichneumonoidea, Aphidiidae). Dtsch. Entomol. Fachz. 1995, 42, 113–138. [Google Scholar] [CrossRef]
- Khatri, D.; He, X.Z.; Wang, Q. Effective biological control depends on life history strategies of both parasitoid and its host: Evidence from Aphidius colemani–Myzus persicae system. J. Econ. Entomol. 2017, 110, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Pham-Delègue, M.-H.; Kaiser, L. Effects of sub-lethal and lethal doses of lambda-cyhalothrin on oviposition experience and host-searching behaviour of a parasitic wasp, Aphidius ervi. Pest Manag. Sci. 2004, 60, 381–389. [Google Scholar] [CrossRef]
- Desneux, N.; Wajnberg, E.; Fauvergue, X.; Privet, S.; Kaiser, L. Oviposition behaviour and patch-time allocation in two aphid parasitoids exposed to deltamethrin residues. Entomol. Exp. Appl. 2004, 112, 227–235. [Google Scholar] [CrossRef]
- Desneux, N.; Denoyelle, R.; Kaiser, L. A multi-step bioassay to assess the effect of the deltamethrin on the parasitic wasp Aphidius ervi. Chemosphere 2006, 65, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Teder, T.; Knapp, M. Sublethal effects enhance detrimental impact of insecticides on non-target organisms: A quantitative synthesis in parasitoids. Chemosphere 2019, 214, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Zappalà, L.; Stark, J.D.; Desneux, N. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 2013, 8, e76548. [Google Scholar] [CrossRef] [PubMed]
- Salerno, G.; Colazza, S.; Conti, E. Sub-lethal effects of deltamethrin on walking behaviour and response to host kairomone of the egg parasitoid Trissolcus basalis. Pest Manag. Sci. 2002, 58, 663–668. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Kawazu, K.; Shimoda, T.; Suzuki, Y. Effects of insecticides on the foraging behaviour and survival of Cotesia vestalis, a larval parasitoid of the diamondback moth, Plutella xylostella. J. Appl. Entomol. 2011, 135, 647–657. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D.; Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef]
- Desneux, N.; Ramirez-Romero, R.; Kaiser, L. Multistep bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ. Toxicol. Chem. 2006, 25, 2675. [Google Scholar] [CrossRef] [PubMed]
- D’Ávila, V.A.; Barbosa, W.F.; Guedes, R.N.C.; Cutler, G.C. Effects of spinosad, imidacloprid, and lambda-cyhalothrin on survival, parasitism, and reproduction of the aphid aarasitoid Aphidius colemani. J. Econ. Entomol. 2018, 111, 1096–1103. [Google Scholar] [CrossRef]
- Mills, N.J.; Heimpel, G.E. Could increased understanding of foraging behavior help to predict the success of biological control? Curr. Opin. Insect Sci. 2018, 27, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Eilenberg, J. Natural Enemies: An Introduction to Biological Control; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Contreras, E.; Figueroa, C.C.; Reyes, M.; Briones, L.M.; Niemeyer, H.M. Genetic diversity and insecticide resistance of Myzus persicae (Hemiptera: Aphididae) populations from tobacco in Chile: Evidence for the existence of a single predominant clone. Bull. Entomol. Res. 2004, 94, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Rubiano-Rodríguez, J.A.; Fuentes Conterras, E.; Figueroa, C.C.; Margaritopoulos, J.T.; Briones, L.M.; Ramírez, C.C. Genetic diversity and insecticide resistance during the growing season in the green peach aphid (Hemiptera: Aphididae) on primary and secondary hosts: A farm-scale study in Central Chile. Bull. Entomol. Res. 2014, 104, 182–194. [Google Scholar] [CrossRef]
- Anstead, J.A.; Williamson, M.S.; Eleftherianos, I.; Denholm, I. High-throughput detection of knockdown resistance in Myzus persicae using allelic discriminating quantitative PCR. Insect Biochem. Mol. Biol. 2004, 34, 871–877. [Google Scholar] [CrossRef]
- Anstead, J.A.; Williamson, M.S.; Denholm, I. New methods for the detection of insecticide resistant Myzus persicae in the U.K. suction trap network. Agric. For. Entomol. 2008, 10, 291–295. [Google Scholar] [CrossRef]
- Devonshire, A. Comparison of microplate esterase assays and immunoassay for identifying insecticide resistant variants of Myzus persicae (Homoptera: Aphididae). Bull. Entomol. Res. 1992, 82, 459–463. [Google Scholar] [CrossRef]
- Sepúlveda, D.A.; Zepeda-Paulo, F.; Ramírez, C.C.; Lavandero, B.; Figueroa, C.C. Diversity, frequency and geographic distribution of facultative bacterial endosymbionts in introduced aphid pests. Insect Sci. 2017, 24, 511–521. [Google Scholar] [CrossRef]
- Vorburger, C. Symbiont-conferred resistance to parasitoids in aphids—Challenges for biological control. Biol. Control 2018, 116, 17–26. [Google Scholar] [CrossRef]
- Peccoud, J.; Bonhomme, J.; Mahéo, F.; de la Huerta, M.; Cosson, O.; Simon, J.-C. Inheritance patterns of secondary symbionts during sexual reproduction of pea aphid biotypes. Insect Sci. 2013, 21, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Abarca, P.; Felmer, S.; Allende, M.; Lemus, G.; Antunez, A.; Quiroz, C.; Hirzel, J.; Riquelme, S.; Carrasco, J.; Sepulveda, P. Manual de manejo del cultivo de Duraznero. In Boletin INIA-Instituto de Investigaciones Agropecuarias; Ministerio de Agricultura: Santiago, Chile, 2017. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Taxonomic Issues. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2007. [Google Scholar]
- Alfaro-Tapia, A.; Alvarez-Baca, J.K.; Figueroa, C.C.; Fuentes-Contreras, E. Sub-lethal effects of λ-cyhalothrin on behavior and development of the parasitoid Aphidius colemani (Hymenoptera: Braconidae) on susceptible and kdr resistant green peach aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2021. (in revision). [Google Scholar]
- Zepeda-Paulo, F.; Lavandero, B.; Mahéo, F.; Dion, E.; Outreman, Y.; Simon, J.-C.; Figueroa, C.C. Does sex-biased dispersal account for the lack of geographic and host-associated differentiation in introduced populations of an aphid parasitoid? Ecol. Evol. 2015, 5, 2149–2161. [Google Scholar] [CrossRef]
- Ottoni, E.B. EthoLog 2.2: A tool for the transcription and timing of behavior observation sessions. Behav. Res. Methods Instrum. Comput. 2000, 32, 446–449. [Google Scholar] [CrossRef][Green Version]
- Desneux, N.; Barta, R.J.; Hoelmer, K.A.; Hopper, K.R.; Heimpel, G.E. Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 2009, 160, 387–398. [Google Scholar] [CrossRef]
- Vet, L.E.; Lenteren, J.V.; Heymans, M.; Meelis, E. An airflow olfactometer for measuring olfactory responses of hymenopterous parasitoids and other small insects. Physiol. Entomol. 1983, 8, 97–106. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Browne, W.J.; Subramanian, S.V.; Jones, K.; Goldstein, H. Variance partitioning in multilevel logistic models that exhibit overdispersion. J. R. Stat. Soc. Ser. A 2005, 168, 599–613. [Google Scholar] [CrossRef]
- van de Pol, M.; Wright, J. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim. Behav. 2009, 77, 753–758. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: New York, NY, USA, 2011; ISBN 9781412975148. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M.; Schuetzenmeister, A.; Scheibe, S.; Hothorn, M.T. Multcomp: Simultaneous Inference in General Parametric Models; Project for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Desneux, N.; Rafalimanana, H.; Kaiser, L. Dose-response relationship in lethal and behavioural effects of different insecticides on the parasitic wasp Aphidius ervi. Chemosphere 2004, 54, 619–627. [Google Scholar] [CrossRef]
- Desneux, N.; Noel, B.; Kaiser, L. Sublethal effect of a pyrethroid on orientation behaviour of the parasitic wasp Aphidius ervi (Hymenoptera: Aphidiidae) in response to odour from oilseed rape infested by the aphid Myzus persicae. Bull. IOBC/WPRS 2000, 23, 55–64. [Google Scholar]
- Foster, S.P.; Young, S.; Williamson, M.S.; Duce, I.; Denholm, I.; Devine, G.J. Analogous pleiotropic effects of insecticide resistance genotypes in peach—Potato aphids and houseflies. Heredity 2003, 91, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.P.; Denholm, I.; Thompson, R.; Poppy, G.M.; Powell, W. Reduced response of insecticide-resistant aphids and attraction of parasitoids to aphid alarm pheromone; a potential fitness trade-off. Bull. Entomol. Res. 2005, 95, 37–46. [Google Scholar] [CrossRef]
- Foster, S.P.; Kift, N.B.; Baverstock, J.; Sime, S.; Reynolds, K.; Jones, J.E.; Thompson, R.; Tatchell, G.M. Association of MACE-based insecticide resistance in Myzus persicae with reproductive rate, response to alarm pheromone and vulnerability to attack by Aphidius colemani. Pest Manag. Sci. 2003, 59, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Ensley, S.M. Pyrethrins and Pyrethroids. In Veterinary Toxicology: Basic and Clinical Principles, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 515–520. ISBN 9780128114100. [Google Scholar]
- He, L.-M.; Troiano, J.; Wang, A.; Goh, K. Environmental chemistry, ecotoxicity, and fate of lambda-cyhalothrin. Rev. Environ. Contam. Toxicol. 2008, 195, 71–91. [Google Scholar]
- Fatouros, N.E.; Dicke, M.; Mumm, R.; Meiners, T.; Hilker, M. Foraging behavior of egg parasitoids exploiting chemical information. Behav. Ecol. 2008, 19, 677–689. [Google Scholar] [CrossRef]
- Chesnais, Q.; Ameline, A.; Doury, G.; Le Roux, V.; Couty, A. Aphid parasitoid mothers don’t always know best through the whole host selection process. PLoS ONE 2015, 10, e0135661. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef]
- Suma, P.; Zappalà, L.; Mazzeo, G.; Siscaro, G. Lethal and sub-lethal effects of insecticides on natural enemies of citrus scale pests. BioControl 2009, 54, 651–661. [Google Scholar] [CrossRef]
- Zhukovskaya, M.; Yanagawa, A.; Forschler, B. Grooming behavior as a mechanism of insect disease defense. Insects 2013, 4, 609–630. [Google Scholar] [CrossRef]
- Kawada, H.; Ohashi, K.; Dida, G.O.; Sonye, G.; Njenga, S.M.; Mwandawiro, C.; Minakawa, N. Insecticidal and repellent activities of pyrethroids to the three major pyrethroid-resistant malaria vectors in western Kenya. Parasit. Vectors 2014, 7, 208. [Google Scholar] [CrossRef]
- Belzunces, L.P.; Tchamitchian, S.; Brunet, J.-L. Neural effects of insecticides in the honey bee. Apidologie 2012, 43, 348–370. [Google Scholar] [CrossRef]
- Zeinali, M.; McConnell, L.L.; Hapeman, C.J.; Nguyen, A.; Schmidt, W.F.; Howard, C.J. Volatile organic compounds in pesticide formulations: Methods to estimate ozone formation potential. Atmos. Environ. 2011, 45, 2404–2412. [Google Scholar] [CrossRef]
- Degenhardt, D.C.; Greene, J.K. Influence of pyrethroid pesticide formulation on volatile emissions from cotton, Gossypium hirsutum L., leaves. J. Agric. Urban. Entomol. 2012, 28, 8–15. [Google Scholar] [CrossRef]
- Charreton, M.; Decourtye, A.; Henry, M.; Rodet, G.; Sandoz, J.-C.; Charnet, P.; Collet, C. A Locomotor deficit induced by sublethal doses of pyrethroid and neonicotinoid insecticides in the honeybee Apis mellifera. PLoS ONE 2015, 10, e0144879. [Google Scholar] [CrossRef]
- Dong, K.; Du, Y.; Rinkevich, F.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B.S. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef]
- Cabral, S.; Soares, A.O.; Garcia, P. Voracity of Coccinella undecimpunctata: Effects of insecticides when foraging in a prey/plant system. J. Pest Sci. 2011, 84, 373–379. [Google Scholar] [CrossRef]
- Moiroux, J.; Abram, P.K.; Louâpre, P.; Barrette, M.; Brodeur, J.; Boivin, G. Influence of temperature on patch residence time in parasitoids: Physiological and behavioural mechanisms. Sci. Nat. 2016, 103, 32. [Google Scholar] [CrossRef]
- Alix, A.; Cortesero, A.M.; Nénon, J.P.; Anger, J.P. Selectivity assessment of chlorfenvinphos reevaluated by including physiological and behavioral effects on an important beneficial insect. Environ. Toxicol. Chem. 2001, 20, 2530–2536. [Google Scholar] [CrossRef]
- Bayram, A.; Salerno, G.; Onofri, A.; Conti, E. Sub-lethal effects of two pyrethroids on biological parameters and behavioral responses to host cues in the egg parasitoid Telenomus busseolae. Biol. Control 2010, 53, 153–160. [Google Scholar] [CrossRef]
- Ranjbar, F.; Reitz, S.; Jalali, M.A.; Ziaaddini, M.; Izadi, H. Lethal and sublethal effects of two commercial insecticides on egg parasitoids (Hymenoptera: Scelionidae) of green stink bugs (Hem: Pentatomidae). J. Econ. Entomol. 2021, 114, 33–39. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Tang, Z. Host resistance to an insecticide favors selection of resistance in the parasitoid, Cotesia plutellae (Hymenoptera: Braconidae). Biol. Control 2003, 28, 137–143. [Google Scholar] [CrossRef]
- Meiners, T.; Peri, E. Chemical ecology of insect parasitoids: Essential elements for developing effective biological control programmes. In Chemical Ecology of Insect Parasitoids; Wajnberg, E., Colazza, S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 191–224. ISBN 9781118409589. [Google Scholar]
- Stanley, J.; Preetha, G. Pesticide Toxicity to Parasitoids: Exposure, Toxicity and Risk Assessment Methodologies. In Pesticide Toxicity to Non-Target Organisms; Springer: Dordrecht, The Netherlands, 2016; pp. 99–152. [Google Scholar]
- Irshaid, L.A.; Hasan, H.S. Bioresidual effect of two insecticides on melon aphid Aphis gossypii Glover (Homoptera: Aphididae) and its parasitoid Aphidius colemani Verick (Hymenoptera: Brachonidae). Environ. Sci. 2011, 11, 228–236. [Google Scholar]
- Bhardwaj, K.; Sharma, R.; Abraham, J.; Sharma, P. Pyrethroids: A Natural Product for Crop Protection. In Natural Bioactive Products in Sustainable Agriculture; Springer: Singapore, 2020; pp. 113–130. [Google Scholar]
- Gu, X.Z.; Zhang, G.Y.; Chen, L.; Dai, R.L.; Yu, Y.C. Persistence and dissipation of synthetic pyrethroid pesticides in red soils from the Yangtze River Delta area. Environ. Geochem. Health 2008, 30, 67–77. [Google Scholar] [CrossRef]
- Langhof, M.; Gathmann, A.; Poehling, H.-M.; Meyhöfer, R. Impact of insecticide drift on aphids and their parasitoids: Residual toxicity, persistence and recolonisation. Agric. Ecosyst. Environ. 2003, 94, 265–274. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro-Tapia, A.; Alvarez-Baca, J.K.; Fuentes-Contreras, E.; Figueroa, C.C. Biological Control May Fail on Pests Applied with High Doses of Insecticides: Effects of Sub-Lethal Concentrations of a Pyrethroid on the Host-Searching Behavior of the Aphid Parasitoid Aphidius colemani (Hymenoptera, Braconidae) on Aphid Pests. Agriculture 2021, 11, 539. https://doi.org/10.3390/agriculture11060539
Alfaro-Tapia A, Alvarez-Baca JK, Fuentes-Contreras E, Figueroa CC. Biological Control May Fail on Pests Applied with High Doses of Insecticides: Effects of Sub-Lethal Concentrations of a Pyrethroid on the Host-Searching Behavior of the Aphid Parasitoid Aphidius colemani (Hymenoptera, Braconidae) on Aphid Pests. Agriculture. 2021; 11(6):539. https://doi.org/10.3390/agriculture11060539
Chicago/Turabian StyleAlfaro-Tapia, Armando, Jeniffer K. Alvarez-Baca, Eduardo Fuentes-Contreras, and Christian C. Figueroa. 2021. "Biological Control May Fail on Pests Applied with High Doses of Insecticides: Effects of Sub-Lethal Concentrations of a Pyrethroid on the Host-Searching Behavior of the Aphid Parasitoid Aphidius colemani (Hymenoptera, Braconidae) on Aphid Pests" Agriculture 11, no. 6: 539. https://doi.org/10.3390/agriculture11060539
APA StyleAlfaro-Tapia, A., Alvarez-Baca, J. K., Fuentes-Contreras, E., & Figueroa, C. C. (2021). Biological Control May Fail on Pests Applied with High Doses of Insecticides: Effects of Sub-Lethal Concentrations of a Pyrethroid on the Host-Searching Behavior of the Aphid Parasitoid Aphidius colemani (Hymenoptera, Braconidae) on Aphid Pests. Agriculture, 11(6), 539. https://doi.org/10.3390/agriculture11060539






