Aphid–Plant–Phytovirus Pathosystems: Influencing Factors from Vector Behaviour to Virus Spread
Abstract
1. Introduction
2. Vector Activity in Aphids
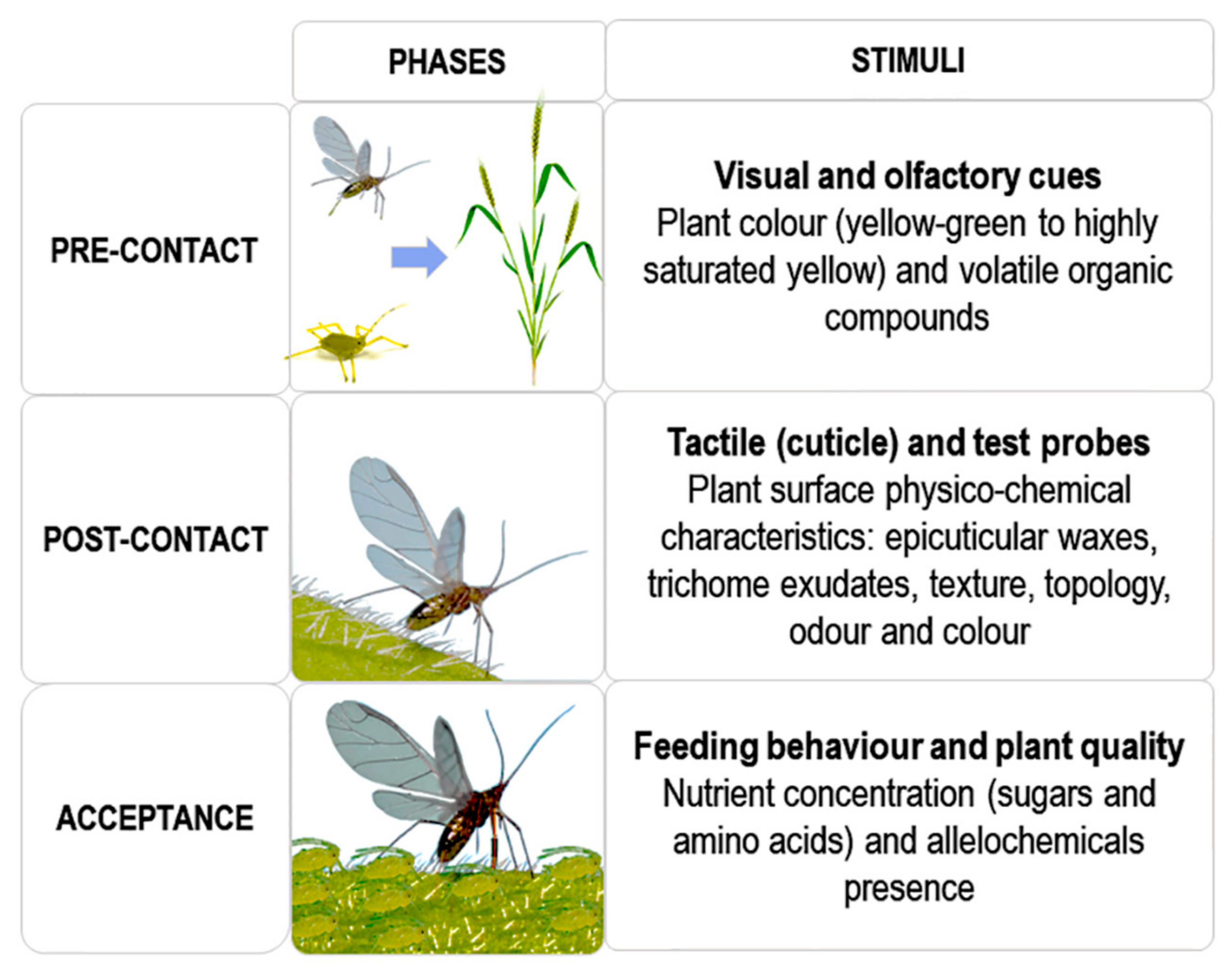
2.1. Host-Seeking Behaviour (HSB)
2.1.1. Pre-Contact
2.1.2. Post-Contact
2.1.3. Host Acceptance or Selection
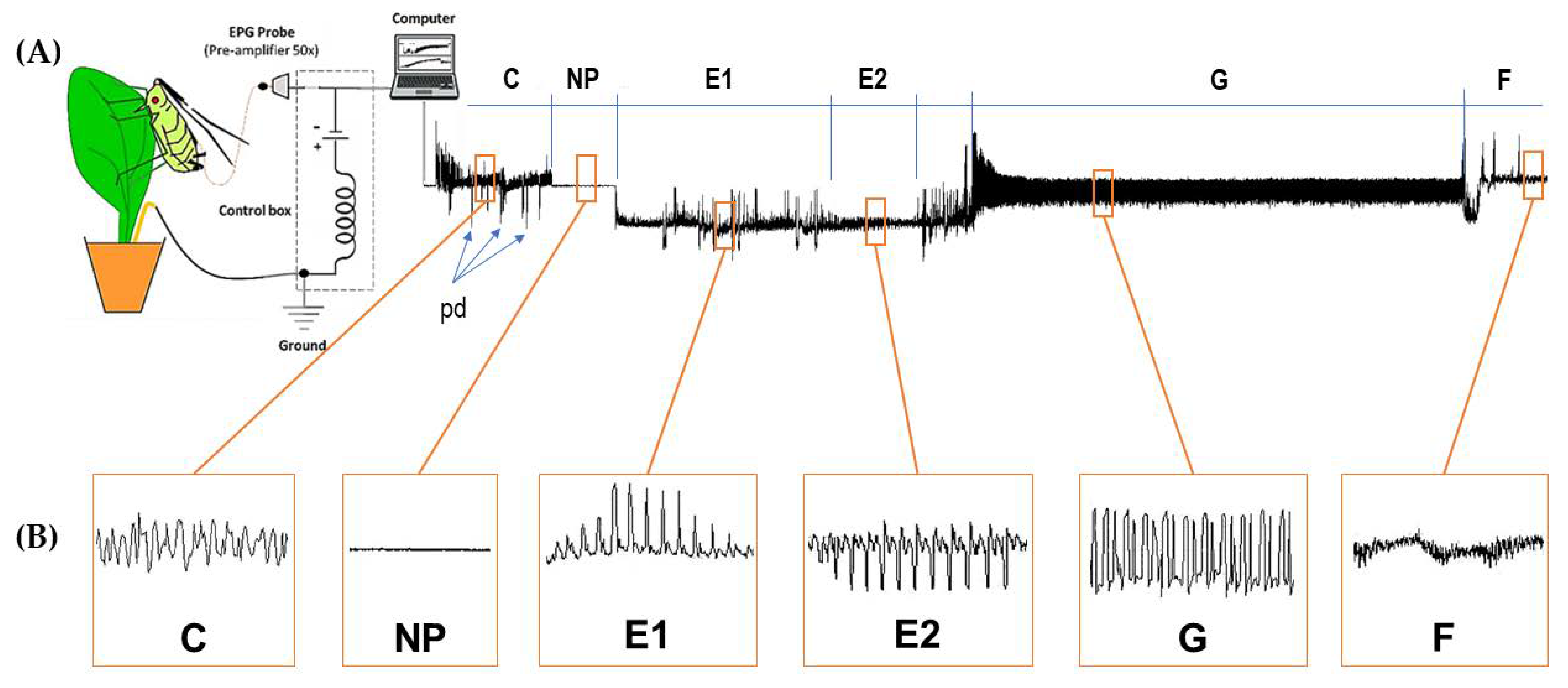
2.2. Probing and Feeding Behaviour (FB)
2.3. Shifting or Dispersal Behaviour (DB)
3. Vectorial Transmission Efficiency
3.1. Vector Efficiency
3.2. Interference Factors of Biological Materials and Experimental Methods
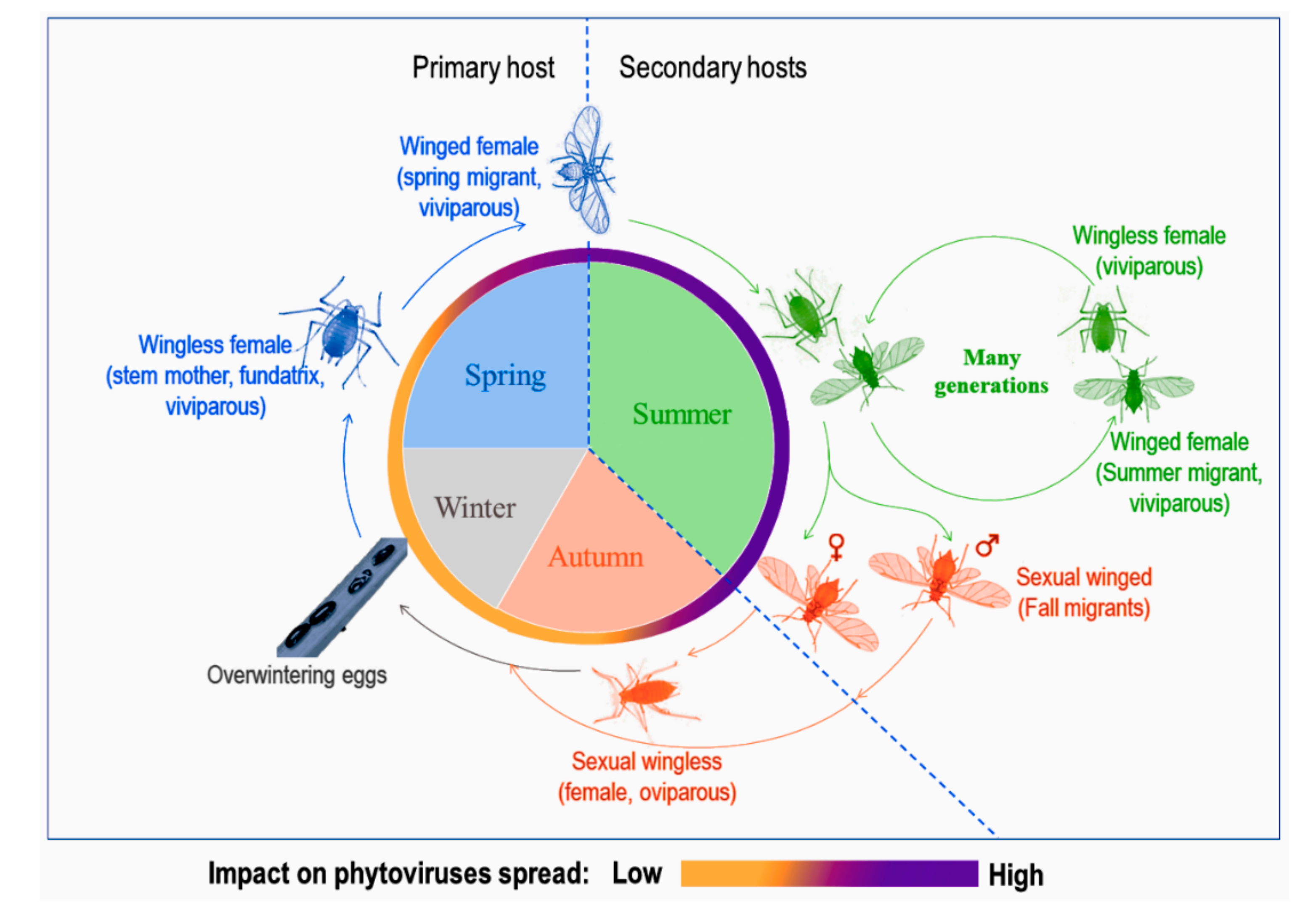
4. Life History Traits
5. Factors Due to External Components from Plant–Insect–Phytovirus Interaction
5.1. Biological Components
5.1.1. Macroorganisms
5.1.2. Microorganisms
5.2. Biochemical Components
5.3. Chemical and Physical Components
6. Concluding Remarks
- There are two main levels in phytovirus transmission process related to vector mobility and efficiency. The first includes the activities that allow the vector to reach its host plant and the site of phytovirus inoculation. Second concerns the vector capacity to transmit phytovirus to this host plant.
- Host-seeking is the first vector behaviour associated with phytovirus spread. It occurs in successive phases until final acceptance in relation to plant quality leading to sustainable sap ingestion from the host plant.
- Aphid life history performances with subsequent virus transmission efficiency are associated to variable nutrient content quality and the presence/absence of toxins and feeding deterrents.
- Vectorial transmission efficiency refers both to specific vector–phytovirus relationships and environmental conditions.
- All the aphid behaviours developed above could be manipulated by phytoviruses either directly by influencing their vectors or indirectly via their host plants in order to enhance their spread.
- Biological agents including microbial entomopathogens to control aphids are rarely integrated into phytovirus pathosystems even if significant impacts start to be observed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yvon, M.; Vile, D.; Brault, V.; Blanc, S.; van Munster, M. Drought reduces transmission of turnip yellows virus, an insect-vectored circulative virus. Virus Res. 2017, 241, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Mulot, M.; Monsion, B.; Boissinot, S.; Rastegar, M.; Meyer, S.; Bochet, N.; Brault, V. Transmission of turnip yellows virus by Myzus persicae is reduced by feeding aphids on double-stranded RNA targeting the ephrin receptor protein. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Carmo-Sousa, M.; Moreno, A.; Garzo, E.; Fereres, A. A non-persistently transmitted-virus induces a pull-push strategy in its aphid vector to optimize transmission and spread. Virus Res. 2014, 186, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Ferrari, M.J.; Roossinck, M.J. Manipulation of aphid behavior by a persistent plant virus. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Fereres, A.; Moreno, A. Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res. 2009, 141, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Carmo-Sousa, M.; Moreno, A.; Plaza, M.; Garzo, E.; Fereres, A. Cucurbit aphid-borne yellows virus (CABYV) modifies the alighting, settling and probing behaviour of its vector Aphis gossypii favouring its own spread. Ann. Appl. Biol. 2016, 169, 284–297. [Google Scholar] [CrossRef]
- Bragard, C.; Caciagli, P.; Lemaire, O.; Lopez-Moya, J.J.; MacFarlane, S.; Peters, D.; Susi, P.; Torrance, L. Status and prospects of plant virus control through interference with vector transmission. Annu. Rev. Phytopathol. 2013, 51, 177–201. [Google Scholar] [CrossRef] [PubMed]
- Dáder, B.; Moreno, A.; Viñuela, E.; Fereres, A. Spatio-temporal dynamics of viruses are differentially affected by parasitoids depending on the mode of transmission. Viruses 2012, 4, 3069–3089. [Google Scholar] [CrossRef]
- Boquel, S.; Ameline, A.; Giordanengo, P. Assessing aphids potato virus Y-transmission efficiency: A new approach. J. Virol. Methods 2011, 178, 63–67. [Google Scholar] [CrossRef]
- Powell, G.; Hardie, J. The chemical ecology of aphid host alternation: How do return migrants find the primary host plant? Appl. Entomol. Zool. 2001, 36, 259–267. [Google Scholar] [CrossRef]
- Watson, M.A. Epidemiology of aphid-transmitted plant-virus diseases. Outlook Agric. 1967, 5, 155–166. [Google Scholar] [CrossRef]
- Lin, F.-J.J.; Bosquée, E.; Liu, Y.-J.J.; Chen, J.-L.L.; Yong, L.; Francis, F. Impact of aphid alarm pheromone release on virus transmission efficiency: When pest control strategy could induce higher virus dispersion. J. Virol. Methods 2016, 235, 34–40. [Google Scholar] [CrossRef]
- Capinera, J.L. Green Peach Aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1727–1730. [Google Scholar] [CrossRef]
- Prado, E.C. Aphid-Plant Interactions at Phloem Level, a Behavioural Study; Landbouwuniversiteit: Wageningen, The Netherlands, 1997. [Google Scholar]
- Ishikawa, A.; Miura, T. Morphological differences between wing morphs of two Macrosiphini aphid species, Acyrthosiphon pisum and Megoura crassicauda (Hemiptera, Aphididae). Sociobiology 2007, 50, 881–893. [Google Scholar]
- Chen, Y.; Martin, C.; Fingu-Mabola, J.C.; Verheggen, F.; Wang, Z.; He, K.; Francis, F. Effects of host plants reared under elevated CO2 concentrations on the foraging behavior of different stages of corn leaf aphids Rhopalosiphum maidis. Insects 2019, 10, 182. [Google Scholar] [CrossRef]
- Quiroz, A.; Niemeyer, H.M. Olfactometer-assessed responses of aphid Rhopalosiphum padi to wheat and oat volatiles. J. Chem. Ecol. 1998, 24, 113–124. [Google Scholar] [CrossRef]
- Congdon, B.S.; Coutts, B.A.; Renton, M.; Flematti, G.R.; Jones, R.A.C. Establishing alighting preferences and species transmission differences for pea seed-borne mosaic virus aphid vectors. Virus Res. 2017, 241, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Döring, T.F.; Chittka, L. Visual ecology of aphids—a critical review on the role of colours in host finding. Arthropod. Plant. Interact. 2007, 1, 3–16. [Google Scholar] [CrossRef]
- dos Santos, R.C.; Peñaflor, M.F.G.V.; Sanches, P.A.; Nardi, C.; Bento, J.M.S. The effects of Gibberella zeae, barley yellow dwarf virus, and co-infection on Rhopalosiphum padi olfactory preference and performance. Phytoparasitica 2016, 44, 47–54. [Google Scholar] [CrossRef]
- Lacroix, C.; Jolles, A.; Seabloom, E.W.; Power, A.G.; Mitchell, C.E.; Borer, E.T. Non-random biodiversity loss underlies predictable increases in viral disease prevalence. J. R. Soc. Interface 2014, 11, 20130947. [Google Scholar] [CrossRef] [PubMed]
- Cilia, M.; Peter, K.A.; Bereman, M.S.; Howe, K.; Fish, T.; Smith, D.; Gildow, F.; MacCoss, M.J.; Thannhauser, T.W.; Gray, S.M. Discovery and targeted LC-MS/MS of purified polerovirus reveals differences in the virus-host interactome associated with altered aphid transmission. PLoS ONE 2012, 7, e48177. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef]
- Hodge, S.; Powell, G. Complex interactions between a plant pathogen and insect parasitoid via the shared vector-host: Consequences for host plant infection. Oecologia 2008, 157, 387–397. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Dutta, B.; Coolong, T.; Srinivasan, R. A non-persistent aphid-transmitted Potyvirus differentially alters the vector and non-vector biology through host plant quality manipulation. Sci. Rep. 2019, 9, 2503. [Google Scholar] [CrossRef]
- Jahan, S.M.H.; Lee, G.-S.; Lee, S.; Lee, K.-Y. Acquisition of tomato yellow leaf curl virus enhances attraction of Bemisia tabaci to green light emitting diodes. J. Asia Pac. Entomol. 2014, 17, 79–82. [Google Scholar] [CrossRef]
- Döring, T.F.; Archetti, M.; Hardie, J. Autumn leaves seen through herbivore eyes. Proc. R. Soc. B Biol. Sci. 2009, 276, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Rajabaskar, D.; Bosque-Pérez, N.A.; Eigenbrode, S.D. Preference by a virus vector for infected plants is reversed after virus acquisition. Virus Res. 2014, 186, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Ding, H.; Shiel, P.; Berger, P.H. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. London. Ser. B Biol. Sci. 2002, 269, 455–460. [Google Scholar] [CrossRef]
- Rajabaskar, D.; Ding, H.; Wu, Y.; Eigenbrode, S.D. Different reactions of potato varieties to infection by potato leafroll virus, and associated responses by its vector, Myzus persicae (Sulzer). J. Chem. Ecol. 2013, 39, 1027–1035. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Evidence of local adaptation in plant virus effects on host-vector interactions. Integr. Comp. Biol. 2014, 54, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Mwando, N.L.; Tamiru, A.; Nyasani, J.O.; Obonyo, M.A.; Caulfield, J.C.; Bruce, T.J.A.A.; Subramanian, S. Maize chlorotic mottle virus induces changes in host plant volatiles that attract vector thrips species. J. Chem. Ecol. 2018, 44, 681–689. [Google Scholar] [CrossRef]
- Ngumbi, E.; Eigenbrode, S.D.; Bosque-Pérez, N.A.; Ding, H.; Rodriguez, A. Myzus persicae is arrested more by blends than by individual compounds elevated in headspace of plrv-infected potato. J. Chem. Ecol. 2007, 33, 1733–1747. [Google Scholar] [CrossRef]
- Medina-Ortega, K.J.; Bosque-Pérez, N.A.; Ngumbi, E.; Jiménez-Martínez, E.S.; Eigenbrode, S.D.; Medina-ortega, A.K.J.; Bosque-Pérez, N.A.; Ngumbi, E.; Eigenbrode, S.D.; Ngumbi, E.; et al. Rhopalosiphum padi (Hemiptera: Aphididae) responses to volatile cues from barley yellow dwarf virus-infected wheat. Environ. Entomol. 2009, 38, 836–845. [Google Scholar] [CrossRef]
- Webster, B. The role of olfaction in aphid host location. Physiol. Entomol. 2012, 37, 10–18. [Google Scholar] [CrossRef]
- Powell, G.; Tosh, C.R.; Hardie, J. Host plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef]
- Westwood, J.H.; Stevens, M. Resistance to aphid vectors of virus disease. In Natural and Engineered Resistance to Plant Viruses, Part B; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 76, pp. 179–210. [Google Scholar] [CrossRef]
- Prado, E.; Tjallingii, W.F. Effects of previous plant infestation on sieve element acceptance by two aphids. Entomol. Exp. Appl. 1997, 82, 189–200. [Google Scholar] [CrossRef]
- Wamonje, F.O.; Donnelly, R.; Tungadi, T.D.; Murphy, A.M.; Pate, A.E.; Woodcock, C.; Caulfield, J.; Mutuku, J.M.; Bruce, T.J.A.; Gilligan, C.A.; et al. Different plant viruses induce changes in feeding behavior of specialist and generalist aphids on common bean that are likely to enhance virus transmission. Front. Plant Sci. 2020, 10, 1811. [Google Scholar] [CrossRef] [PubMed]
- Giordanengo, P.; Brunissen, L.; Rusterucci, C.; Vincent, C.; van Bel, A.; Dinant, S.; Girousse, C.; Faucher, M.; Bonnemain, J.-L. Compatible plant-aphid interactions: How aphids manipulate plant responses. C. R. Biol. 2010, 333, 516–523. [Google Scholar] [CrossRef]
- Ziegler-Graff, V. Molecular insights into host and vector manipulation by plant viruses. Viruses 2020, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Bosque-Pérez, N.A.; Eigenbrode, S.D. The influence of virus-induced changes in plants on aphid vectors: Insights from luteovirus pathosystems. Virus Res. 2011, 159, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.E.; Broglia, V.G.; Alberti D’Amato, A.M.; Wouters, D.; van der Vossen, E.; Garzo, E.; Tjallingii, W.F.; Dicke, M.; Vosman, B. Comparative analysis of Solanum stoloniferum responses to probing by the green peach aphid Myzus persicae and the potato aphid Macrosiphum euphorbiae. Insect Sci. 2013, 20, 207–227. [Google Scholar] [CrossRef]
- Chesnais, Q.; Mauck, K.E.; Bogaert, F.; Bamière, A.; Catterou, M.; Spicher, F.; Brault, V.; Tepfer, M.; Ameline, A. Virus effects on plant quality and vector behavior are species specific and do not depend on host physiological phenotype. J. Pest Sci. 2019, 92, 791–804. [Google Scholar] [CrossRef]
- Döring, T.F. How aphids find their host plants, and how they don’t. Ann. Appl. Biol. 2014, 165, 3–26. [Google Scholar] [CrossRef]
- Boquel, S.; Giordanengo, P.; Ameline, A. Vector activity of three aphid species (Hemiptera: Aphididae) modulated by host plant selection behaviour on potato (Solanales: Solanaceae). Ann. Soc. Entomol. Fr. 2014, 50, 141–148. [Google Scholar] [CrossRef]
- Tjallingii, W.F.; Prado, E. Analysis of circulative transmission by electrical penetration graphs. In Virus-Insect-Plant Interactions; Academic Press: Cambridge, MA, USA, 2001; pp. 69–85. [Google Scholar]
- Garzo, E.; Moreno, A.; Plaza, M.; Fereres, A. Feeding behavior and virus-transmission ability of insect vectors exposed to systemic insecticides. Plants 2020, 9, 895. [Google Scholar] [CrossRef]
- Giordanengo, P. EPG-Calc: A PHP-based script to calculate electrical penetration graph (EPG) parameters. Arthropod. Plant. Interact. 2014, 8, 163–169. [Google Scholar] [CrossRef]
- Tjallingiil, W.E.; Mayoral, A. Criteria for Host Plant Acceptance by Aphids; Springer: Dordrecht, The Netherlands, 1992; pp. 280–281. [Google Scholar]
- Martín, B.; Collar, J.L.; Tjallingii, W.F.; Fereres, A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 1997, 78 Pt 10, 2701–2705. [Google Scholar] [CrossRef]
- Collar, J.L.; Avilla, C.; Fereres, A. New correlations between aphid stylet paths and nonpersistent virus transmission. Environ. Entomol. 1997, 26, 537–544. [Google Scholar] [CrossRef]
- Powell, G. Intracellular salivation is the aphid activity associated with inoculation of non-persistently transmitted viruses. J. Gen. Virol. 2005, 86, 469–472. [Google Scholar] [CrossRef]
- Angelella, G.; Nalam, V.; Nachappa, P.; White, J.; Kaplan, I. Endosymbionts differentially alter exploratory probing behavior of a nonpersistent plant virus vector. Microb. Ecol. 2018, 76, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Boquel, S.; Giordanengo, P.; Ameline, A. Divergent effects of PVY-infected potato plant on aphids. Eur. J. Plant Pathol. 2011, 129, 507–510. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Effects of pathogens on sensory-mediated interactions between plants and insect vectors. Curr. Opin. Plant Biol. 2016, 32, 53–61. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, A.; Vijayanandraj, S.; Mandal, B. Cardamom bushy dwarf virus infection in large cardamom alters plant selection preference, life stages, and fecundity of aphid vector, Micromyzus kalimpongensis (Hemiptera: Aphididae). Environ. Entomol. 2016, 45, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Van Munster, M.; Yvon, M.; Vile, D.; Dader, B.; Fereres, A.; Blanc, S. Water deficit enhances the transmission of plant viruses by insect vectors. PLoS ONE 2017, 12, e0174398. [Google Scholar] [CrossRef]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2012, 2, 578. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; Chesnais, Q.; Shapiro, L.R. Evolutionary determinants of host and vector manipulation by plant viruses. In Environmental Virology and Virus Ecology; Malmstrom, C.M., Ed.; Academic Press: Amsterdam, The Netherlands, 2018; pp. 189–250. [Google Scholar] [CrossRef]
- Hodge, S.; Powell, G. Conditional facilitation of an aphid vector, Acyrthosiphon pisum, by the plant pathogen, Pea enation mosaic virus. J. Insect Sci. 2010, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Westwood, J.H.; Groen, S.C.; Du, Z.; Murphy, A.M.; Anggoro, D.T.; Tungadi, T.; Luang-In, V.; Lewsey, M.G.; Rossiter, J.T.; Powell, G.; et al. A trio of viral proteins tunes aphid-plant interactions in Arabidopsis thaliana. PLoS ONE 2013, 8, e83066. [Google Scholar] [CrossRef]
- Carr, J.P.; Tungadi, T.; Donnelly, R.; Bravo-Cazar, A.; Rhee, S.-J.; Watt, L.G.; Mutuku, J.M.; Wamonje, F.O.; Murphy, A.M.; Arinaitwe, W.; et al. Modelling and manipulation of aphid-mediated spread of non-persistently transmitted viruses. Virus Res. 2020, 277, 197845. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.; Cunniffe, N.J.; Carr, J.P.; Gilligan, C.A. Pathogenic modification of plants enhances long-distance dispersal of nonpersistently transmitted viruses to new hosts. Ecology 2019, 100. [Google Scholar] [CrossRef]
- Belliure, B.; Amorós-Jiménez, R.; Fereres, A.; Marcos-García, M.Á. Antipredator behaviour of Myzus persicae affects transmission efficiency of broad bean wilt virus 1. Virus Res. 2011, 159, 206–214. [Google Scholar] [CrossRef]
- Boquel, S.; Delayen, C.; Couty, A.; Giordanengo, P.; Ameline, A. Modulation of aphid vector activity by potato virus Y on in vitro potato plants. Plant Dis. 2012, 96, 82–86. [Google Scholar] [CrossRef]
- Andret-Link, P.; Fuchs, M. Transmission specificity of plant viruses by vectors. J. Plant Pathol. 2005, 87, 153–165. [Google Scholar] [CrossRef]
- Ng, J.C.K.; Perry, K.L. Transmission of plant viruses by aphid vectors. Mol. Plant Pathol. 2004, 5, 505–511. [Google Scholar] [CrossRef]
- Blanc, S.; Drucker, M.; Uzest, M. Localizing viruses in their insect vectors. Annu. Rev. Phytopathol. 2014, 52, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479–480, 278–289. [Google Scholar] [CrossRef]
- Cervantes, F.A.; Alvarez, J.M. Within plant distribution of potato virus Y in hairy nightshade (Solanum sarrachoides): An inoculum source affecting PVY aphid transmission. Virus Res. 2011, 159, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, M.; Piron, P.G.M.; Dullemans, A.M.; Cuperus, C.; Van Der Vlugt, R.A.A. Determination of aphid transmission efficiencies for N, NTN and Wilga strains of potato virus Y. Ann. Appl. Biol. 2010, 156, 39–49. [Google Scholar] [CrossRef]
- Kersch-Becker, M.F.; Thaler, J.S. Virus strains differentially induce plant susceptibility to aphid vectors and chewing herbivores. Oecologia 2014, 174, 883–892. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, J.F.J.M.; Boerma, T.M.; Peters, D. Transmission of potato leafroll virus from plants and artificial diets by Myzus persicae. Phytopathology 1991, 81, 150–154. [Google Scholar] [CrossRef]
- Collar, J.L.; Fereres, A. Nonpersistent virus transmission efficiency determined by aphid probing behavior during intracellular punctures. Environ. Entomol. 1998, 27, 583–591. [Google Scholar] [CrossRef]
- Sylvester, E.S. Aphid transmission of nonpersistent plant viruses with special reference to the brassica nigra virus. Hilgardia 1954, 23, 53–98. [Google Scholar] [CrossRef]
- Robert, Y.; Bourdin, D. Aphid transmission of potato viruses. In Virus and Virus-like Diseases of Potatoes and Production of Seed-Potatoes; Loebenstein, G., Berger, P.H., Brunt, A.A., Lawson, R.H., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 195–225. [Google Scholar]
- Sadeghi, E.; Dedryver, C.; Gauthier, J. Role of acquisition and inoculation time in the expression of clonal variation for BYDV-PAV transmission in the aphid species Rhopalosiphum padi. Plant Pathol. 1997, 502–508. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X.; Chen, D.; Wang, X.; Fan, X.; Liu, X. Potato virus Y-infected tobacco affects the growth, reproduction, and feeding behavior of a vector aphid, Myzus persicae (Hemiptera: Aphididae). Appl. Entomol. Zool. 2015, 50, 239–243. [Google Scholar] [CrossRef]
- McMenemy, L.S.; Hartley, S.E.; MacFarlane, S.A.; Karley, A.J.; Shepherd, T.; Johnson, S.N. Raspberry viruses manipulate the behaviour of their insect vectors. Entomol. Exp. Appl. 2012, 144, 56–68. [Google Scholar] [CrossRef]
- Albittar, L.; Ismail, M.; Lohaus, G.; Ameline, A.; Visser, B.; Bragard, C.; Hance, T. Bottom-up regulation of a tritrophic system by beet yellows virus infection: Consequences for aphid-parasitoid foraging behaviour and development. Oecologia 2019, 191, 113–125. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.F.; Long, E.Y.; Finke, D.L. A negative effect of a pathogen on its vector? A plant pathogen increases the vulnerability of its vector to attack by natural enemies. Oecologia 2014, 174, 1169–1177. [Google Scholar] [CrossRef][Green Version]
- Long, E.Y.; Finke, D.L. Predators indirectly reduce the prevalence of an insect-vectored plant pathogen independent of predator diversity. Oecologia 2015, 177, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- González-Mas, N.; Quesada-Moraga, E.; Plaza, M.; Fereres, A.; Moreno, A. Changes in feeding behaviour are not related to the reduction in the transmission rate of plant viruses by Aphis gossypii (Homoptera: Aphididae) to melon plants colonized by Beauveria bassiana (Ascomycota: Hypocreales). Biol. Control 2019, 130, 95–103. [Google Scholar] [CrossRef]
- Bayendi Loudit, S.M.; Boullis, A.; Verheggen, F.; Francis, F. Identification of the alarm pheromone of cowpea aphid, and comparison with two other aphididae species. J. Insect Sci. 2018, 18. [Google Scholar] [CrossRef]
- Laney, A.G.; Chen, P.; Korth, K.L. Interactive effects of aphid feeding and virus infection on host gene expression and volatile compounds in salt-stressed soybean, Glycine max (L.) Merr. Arthropod. Plant. Interact. 2018, 12, 401–413. [Google Scholar] [CrossRef]
- Nachappa, P.; Culkin, C.T.; Saya, P.M.; Han, J.; Nalam, V.J. Water Stress Modulates Soybean Aphid Performance, Feeding Behavior, and Virus Transmission in Soybean. Front. Plant Sci. 2016, 7, 552. [Google Scholar] [CrossRef]
- del Toro, F.J.; Choi, K.S.; Rakhshandehroo, F.; Aguilar, E.; Tenllado, F.; Canto, T. Ambient conditions of elevated temperature and CO2 levels are detrimental to the probabilities of transmission by insects of a potato virus Y isolate and to its simulated prevalence in the environment. Virology 2019, 530, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khelifa, M. Possible induction of potato plant defences against potato virus Y by mineral oil application. Eur. J. Plant Pathol. 2017, 147, 339–348. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fingu-Mabola, J.C.; Francis, F. Aphid–Plant–Phytovirus Pathosystems: Influencing Factors from Vector Behaviour to Virus Spread. Agriculture 2021, 11, 502. https://doi.org/10.3390/agriculture11060502
Fingu-Mabola JC, Francis F. Aphid–Plant–Phytovirus Pathosystems: Influencing Factors from Vector Behaviour to Virus Spread. Agriculture. 2021; 11(6):502. https://doi.org/10.3390/agriculture11060502
Chicago/Turabian StyleFingu-Mabola, Junior Corneille, and Frédéric Francis. 2021. "Aphid–Plant–Phytovirus Pathosystems: Influencing Factors from Vector Behaviour to Virus Spread" Agriculture 11, no. 6: 502. https://doi.org/10.3390/agriculture11060502
APA StyleFingu-Mabola, J. C., & Francis, F. (2021). Aphid–Plant–Phytovirus Pathosystems: Influencing Factors from Vector Behaviour to Virus Spread. Agriculture, 11(6), 502. https://doi.org/10.3390/agriculture11060502






