Influence of Fertilization and Mycorrhizae on the Nutritional Status of Rhododendron (Rhododendron hybridum) in a Nursery
Abstract
1. Introduction
2. Materials and Methods
- Different fertilization modes.
- Mycorrhizal inoculation.
- Traditional fertilization. Single fertilizers: ammonium nitrate (NH4NO3, 34% N), monopotassium phosphate (KH2PO4, 22.9% P and 28% K), and potassium sulfate (MgSO4, 17.4% Mg) were applied in three doses at the beginning of the initial growing season. Microelements were used once (mg·L−1): Fe, 8.0 (iron citrate), Cu, 26.0 (CuSO4·5H2O), Zn, 1.7 (ZnSO4·7H2O), Mn, 8.0 (MnSO4·H2O), B, 3.4 (H3BO3), and Mo, 7.0 ((NH4)6Mo7O24·4H2O). Before the plants were transferred to containers, a 1/3 dose of N, P, K and Mg, as well as total microelements, were applied in each study year. Other amounts of nitrogen, phosphorus, potassium and magnesium were divided into two doses. A subsequent dose was supplied in the second ten days of May and in the last ten days of June. This fertilization method was regarded as the control.
- Slow-Release Fertilizer (SRF) Hortiform pH with the following composition: N, 17%, P, 3.5%, K, 12.5%, Mg, 1.8%, S, 10%, B, 0.01%, Co, 0.002%, Cu, 0.01%, Fe, 0.5%, Mn, 0.10%, Mo, 0.001% and Zn, 0.01% was used. The nutrients contained in this fertilizer have an extended 6-month period of availability for plants. A total 3 g·L−1 dose of Hortiform pH was applied before the start of vegetation in each study year.
- Fertigation is fertilization combined with irrigation. A nutrient medium with the composition 14 mg·L−1 N-NH4, 56 mg·L−1 N-NO3, 15 mg·L−1 P, 58 mg·L−1 K, 60 mg·L−1 Ca, 12 mg·L−1 Mg, 16 mg·L−1 S-SO4, 0.4 mg·L−1 Fe, 0.3 mg·L−1 Mn, 0.2 mg·L−1 Zn, 0.06 mg·L−1 Cu, 0.1 mg·L−1 B and 0.01 mg·L−1 Mo was applied every other day from the start of vegetation (end of April/beginning of May) to mid-September in each study year.
- mycelium-inoculated plants (M+),
- mycelium-uninoculated plants as control (M-).
- N-NH4 and N-NO3 (N-min.) with the Bremner microdistillation method modified by Starck,
- P-PO4 with the vanadomolybdate method,
- S-SO4 with the nephelometric method with BaCl2
- K, Ca, and Mg with the atomic absorption spectrophotometry ASA method (Abalyst 300 Perkin Elmer)
- pH (H2O) with the potentiometric method
- salt concentration (EC) with the conductometric method, expressed as the level of electrical conductivity.
3. Results and Discussion
4. Conclusions
- As indicated in the first three years of azalea cultivation in containers, single mineral fertilizers and Hortiform pH (SRF) ensured an appropriate plant nutritional status in terms of the content of nitrogen, phosphorus, potassium, calcium and magnesium, while fertigation required further improvement of the nutrient solution.
- Plant mycorrhization exerted a beneficial effect on plant nutrition, as higher contents of macro elements in plants were demonstrated in the second and third years of azalea cultivation.
- The nutrients applied were largely utilized by the plants by the middle of the growing season, and almost complete utilization of the elements was noted at the end of the growing season. Therefore, the nutrients need to be supplemented in each subsequent year of vegetation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Aendekerk, T. Fertilization Guide for Nursery Crops; Boomteelt, Praktijkonderzoek: Boskoop, The Netherlands, 1997. [Google Scholar]
- Hamim, A.; Miché, L.; Douaik, A.; Mrabet, R.; Ouhammou, A.; Duponnois, R.; Hafidi, M. Diversity of fungal assemblages in roots of Ericaceae in two Mediterranean contrasting ecosystems. C. R. Biol. 2017, 340, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Vohník, M.; Sadowsky, J.J.; Kohout, P.; Lhotáková, Z.; Nestby, R.; Kolařík, M. Novel Root-Fungus Symbiosis in Ericaceae: Sheathed Ericoid Mycorrhiza Formed by a Hitherto Undescribed Basidiomycete with Affinities to Trechisporales. PLoS ONE 2012, 7, e39524. [Google Scholar] [CrossRef] [PubMed]
- Douds, D.D., Jr.; Johnson, C.R.; Koch, K.E. Carbon cost of the fungal symbiont relative to net leaf P accumulation in a split-root VA mycorrhizal symbiosis. Plant Physiol. 1988, 86, 491–496. [Google Scholar] [CrossRef]
- Bi, G.; Scagel, C.F.; Fuchigami, L.H.; Regan, R.P. Differences in growth, and nitrogen uptake and storage between two container-grown cultivars of Rhododendron. J. Environ. Hortic. 2007, 25, 13–20. [Google Scholar] [CrossRef]
- Marczyński, A. Kierunki Rozwoju Szkółkarstwa Ozdobnego w Polsce. Monografia: Ogrodnictwo Ozdobne Sektorem Gospodarki Narodowej Red; Rabiza-Świder, J., Skutnik, E., Eds.; Wyd. SGGW: Warszawa, Poland, 2013; pp. 109–114. (In Polish) [Google Scholar]
- Bosiacki, M.; Golcz-Polaszewska, M.; Kozik, E. Slow-release fertilizers in the production of horticultural plants. Part I. Effect of Osmocote exact standard fertilizer on the growth and condition of nourishing of selected taxons of ornamental trees and shrubs. J. Res. Appl. Agric. Eng. 2009, 54, 29–35. [Google Scholar]
- Michałojć, Z.; Koter, M. Effect of fertilization and mycorrhizal on growth and nutritional status of cranberry (Vacinnium macrocarpon Ait.) in the nursery. J. Hortic. Res. 2015, 23, 49–56. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Zhang, C.; Pan, D. A new Oidiodendron maius strain isolated from Rhododendron fortunei and its effects on nitrogen uptake and plant growth. Front. Microbiol. 2016, 7, 1327. [Google Scholar] [CrossRef]
- Douglas, G.C.; Heslin, M.C.; Reid, C. Isolation of Oidiodendron maius from Rhododendron and ultrastructural characterization of synthesized mycorrhizas. Can. J. Bot. 1989, 67, 2206–2212. [Google Scholar] [CrossRef]
- Perotto, S.; Perotto, R.; Faccio, A.; Schubert, A.; Varma, A.; Bonfante, P. Ericoid mycorrhizal fungi: Cellular and molecular bases of their interaction whit the host plant. Can. J. Bot. 1995, 73, 557–568. [Google Scholar] [CrossRef]
- Kerley, S.J.; Read, D.J. The biology of mycorrhiza in the Ericaceae XX. Plant and mycorrhizal necromass as nitrogenous substrates for the ericoid mycorrhizal fungus Hymenoscyphus ericae and its host. New Phytol. 1998, 139, 353–360. [Google Scholar] [CrossRef]
- Casarrubia, S.; Daghino, S.; Kohler, A.; Morin, E.; Khouja, H.R.; Daguerre, Y.; Vigneault-Fourrey, C.; Martin, F.M.; Perotto, S.; Martino, E. The hydrophobin-like mSSP1 may be an effector in the ericoid mycorrhizal symbiosis. Front. Plant Sci. 2018, 9, 546. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Kubiak, J. Mikoryzacja roślin i aplikacja szczepionek mikoryzowych. Probl. Inż. Rol. 2005, 2, 25–32. (In Polish) [Google Scholar]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, L.; Lanver, D.; Schweizer, G.; Tanaka, S.; Liang, L.; Tollot, M.; Zuccaro, A.; Reissmann, S.; Kahmann, R. Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 2015, 66, 513–545. [Google Scholar] [CrossRef]
- Konieczny, A.; Kowalska, I. Arbuscular Mycorrhiza—Partner in communication. Acta Sci. Pol. Hortorum Cultus 2017, 16, 73–78. [Google Scholar] [CrossRef][Green Version]
- Casarrubia, S.; Martino, E.; Daghino, S.; Kohler, A.; Morin, E.; Khouja, H.R.; Murat, C.; Barry, K.W.; Lindquist, E.A.; Martin, F.M.; et al. Modulation of Plant and Fungal Gene Expression upon Cd Exposure and Symbiosis in Ericoid Mycorrhizal Vaccinium myrtillus. Front. Microbiol. 2020, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Księżniak, A.; Orlikowski, L.B.; Szałański, W.; Wróblewska, B. Effect of ericoid mycorrhizal inoculants on the growth, development and healthiness of rhododendronvariety Nova Zembla and Northern highbush blueberry variety Bluecrop. Prog. Plant Prot. 2013, 53, 190–195. (In Polish) [Google Scholar]
- Krupa, A. Mikoryzy i ich wielofunkcyjna rola w środowisku. Chem. Environ. Biotechnol. 2010, 14, 175–182. [Google Scholar]
- Koide, R.T.; Mosse, B. A history of research on arbuscular mycorrhiza. Mycorrhiza 2004, 14, 145–163. [Google Scholar] [CrossRef]
- Almario, J.; Jeena, G.; Wunder, J.; Langen, G.; Zuccaro, A.; Coupland, G.; Bucher, M. Root-associated fungal microbiota of nonmycorrhizal Arabis alpinaand itscontribution to plant phosphorus nutrition. Proc. Natl. Acad. Sci. USA 2017, 114, E9403–E9412. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Labidi, S.; Ben Jeddi, F.; Tisserant, B.; Debiane, D.; Rezgui, S.; Grandmougin-Ferjani, A.; Lounès-Hadj Sahraoui, A. Role of arbuscular mycorrhizal symbiosis in root mineral uptake under CaCO3 stress. Mycorrhiza 2012, 22, 337–345. [Google Scholar] [CrossRef]
- Cairney, J.W.G.; Sawyer, N.A.; Sharples, J.M.; Meharg, A.A. Intraspecific variation in nitrogen source utilization by isolates of the ericoid mycorrhizal fungus Hymenoscyphus ericae (Read) Korf and Kernan. Soil Biol. Biochem. 2000, 32, 1319–1322. [Google Scholar] [CrossRef]
- Michałojć, Z.; Koter, M.; Dzida, K.; Jarosz, Z.; Pitura, K.; Jamiołkowska, A.; Księżniak, A. Influence of fertilization and mycorrhiza on growth and development of rhododendron (Rhododendron hybridum) in a nursery. J. Elem. 2019, 24, 1349–1361. [Google Scholar] [CrossRef]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Metody Analizy i Oceny Właściwości Gleb i Roślin; Instytut Ochrony Środowiska: Warszawa, Poland, 1991. [Google Scholar]
- Yin, L.J.; Zhang, C.Y.; Yang, B. Characteristics of nitrogen absorbed by ericoid mycorrhizal fungi and impact on growth of Rhododendron fortunei. Sci. Agric. Sin. 2010, 43, 868–872. [Google Scholar]
- De Kreij, C.; Sonneveld, C.; Warmenhoven, M.G.; Straver, N. Normen Voor Gehalten Aan Voedingselementen Van Groenten En Bloemen Onder Glas. Ser. Voedingsplossingen Glas.-Bouv 1990, 15, 23. [Google Scholar]
- Michałojć, Z.; Koter, M. Effect of different fertilization on growth and nutrition of azalea (Rhododendron L.). Acta Agrobot. 2012, 65, 123–132. [Google Scholar] [CrossRef]
- Li, T.; Bi, G.; Harkess, R.L.; Blythe, E.K. Mineral nutrient uptake of Encore Azalea ‘Chiffon’ affected by nitrogen, container, and irrigation frequency. HortScience 2019, 54, 2240–2248. [Google Scholar] [CrossRef]
- Ristvey, A.G.; Lea-Cox, J.D.; Ross, D.S. Nitrogen and phosphorus uptake efficiency and partitioning of container-grown azalea during spring growth. J. Am. Soc. Hortic. Sci. 2007, 132, 563–571. [Google Scholar] [CrossRef]
- Nowak, J. Effects of mycorrhization and phosphorus nutrition on nutrient uptake, growth and flowering of China aster (Callistephus chinensis L. Nees) cultivated on ebb-and-flow benches. Acta Agrobot. 2009, 62, 77–81. [Google Scholar] [CrossRef]
- Davidson, H.; Meclenburg, R.; Peterson, C. Nursery Management; Administration and Culture, 4th ed.; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 2000; p. 524. [Google Scholar]
- Jakobsen, I.; Abbott, L.K.; Robson, A.D. External Hyphae of Vesicular-Arbuscular Mycorrhizal Fungi Associated with Trifolium subterraneum L. I. Spread of Hyphae and Phosphorus Inflow into Roots. New Phytol. 1992, 120, 371–380. [Google Scholar] [CrossRef]
- Hawkins, H.J.; Johansen, A.; George, E. Uptake and Transport of Organic and Inorganic Nitrogen by Arbuscular Mycorrhizal Fungi. Plant Soil 2000, 226, 275–285. [Google Scholar] [CrossRef]
- Jin, H.; Pfeffer, P.E.; Douds, D.D.; Piotrowski, E.; Lammers, P.J.; Shachar-Hill, Y. The Uptake, Metabolism, Transport and Transfer of Nitrogen in an Arbuscular Mycorrhizal Symbiosis. New Phytol. 2005, 168, 687–696. [Google Scholar] [CrossRef]
- Püschel, D.; Janoušková, M.; Hujslová, M.; Slavíková, R.; Gryndlerová, H.; Jansa, J. Plant-fungus competition for nitrogen erases mycorrhizal growth benefits of Andropogon gerardii under limited nitrogen supply. Ecol. Evol. 2016, 6, 4332–4346. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizai symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Komosa, A. Żywienie roślin ozdobnych. In Rabiza-Świder. Biologia Roślin Ozdobnych. Wybrane Zagadnienia; Starc, Z., Ed.; Wyd. SGGW: Warszawa, Poland, 2015; pp. 59–123. [Google Scholar]
- Porras-Soriano, A.; Soriano-Martin, L.A.; Porras-Piedra, A.; Azcon, R. Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J. Plant Physiol. 2009, 166, 1350–1359. [Google Scholar] [CrossRef]
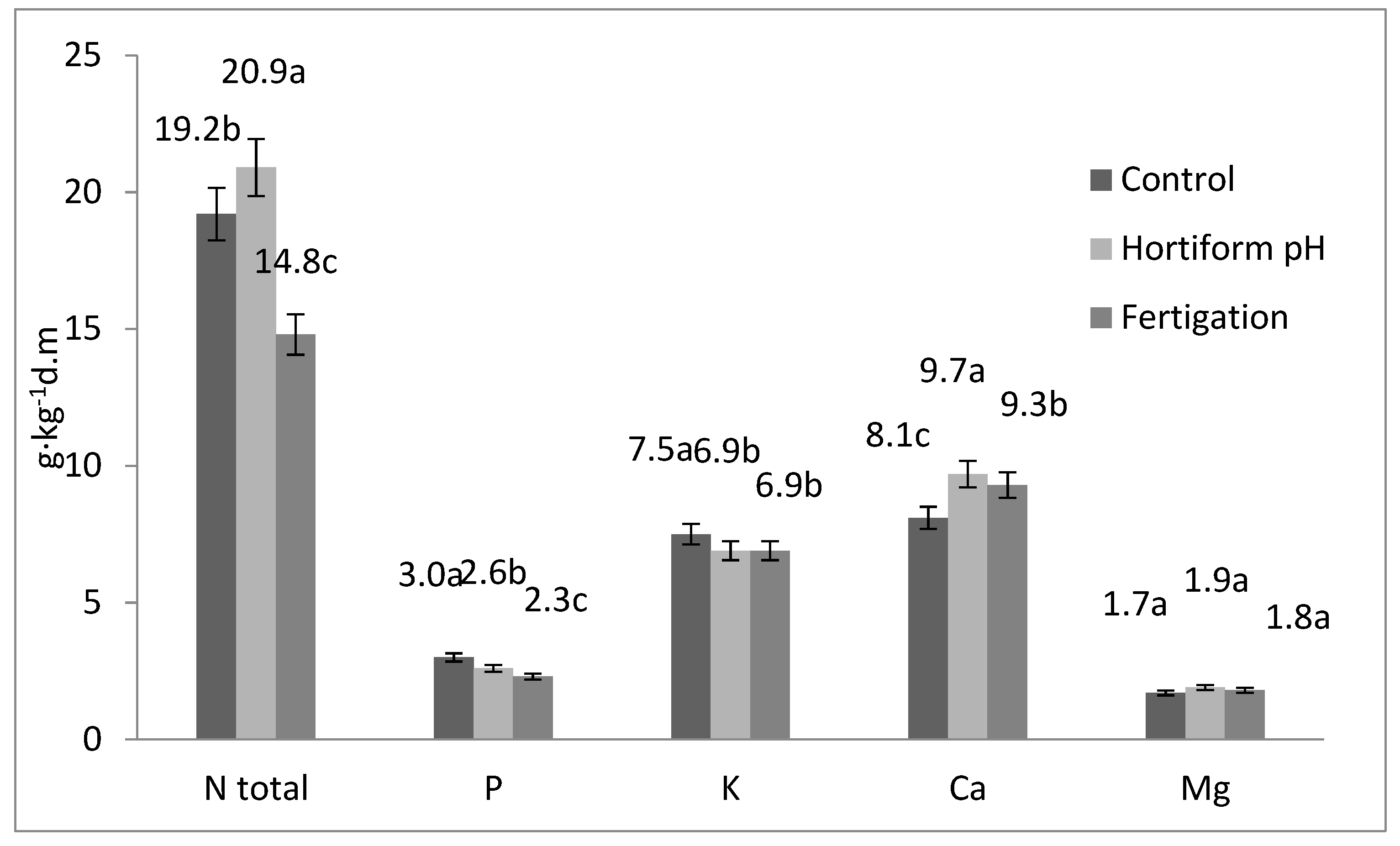
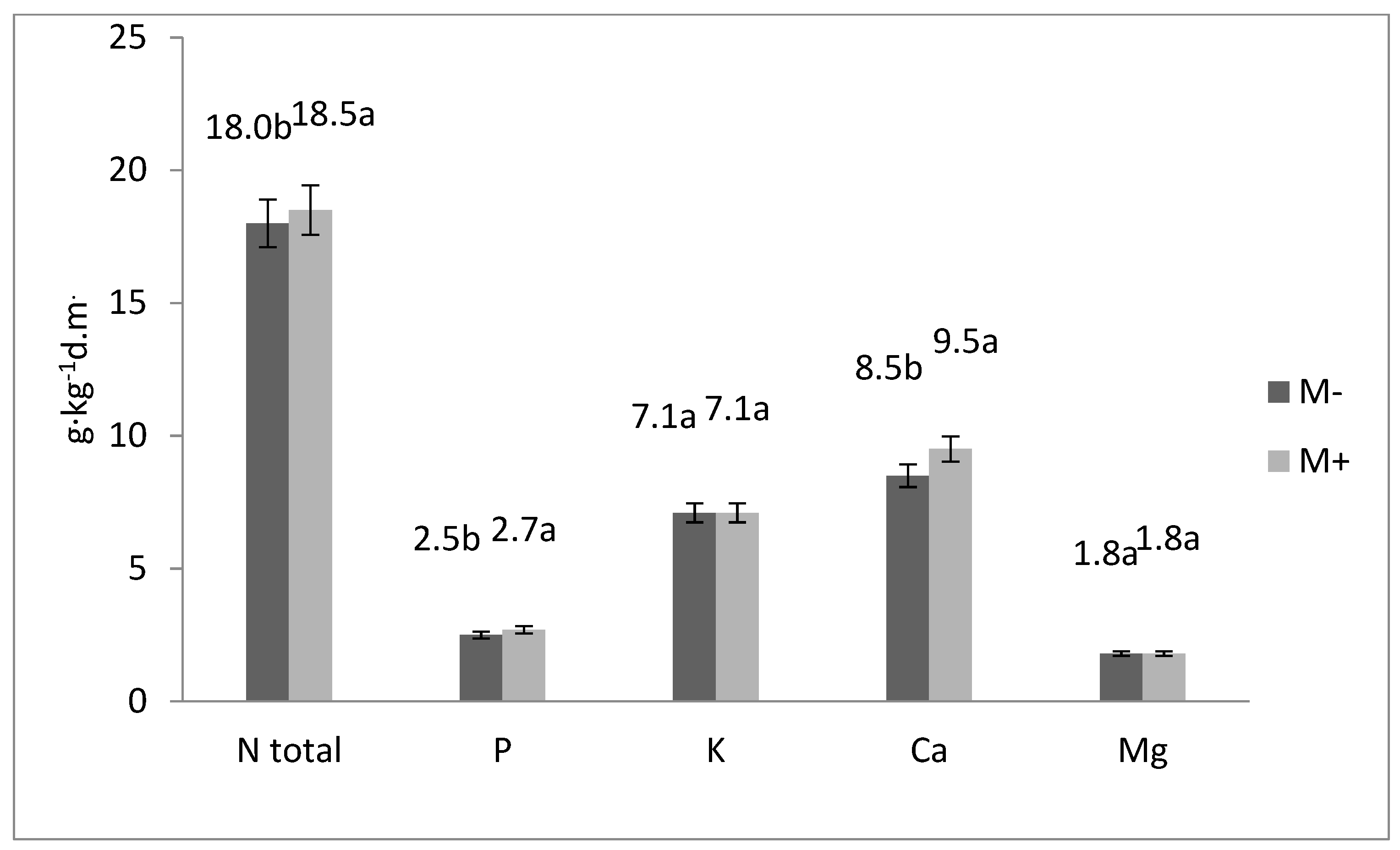
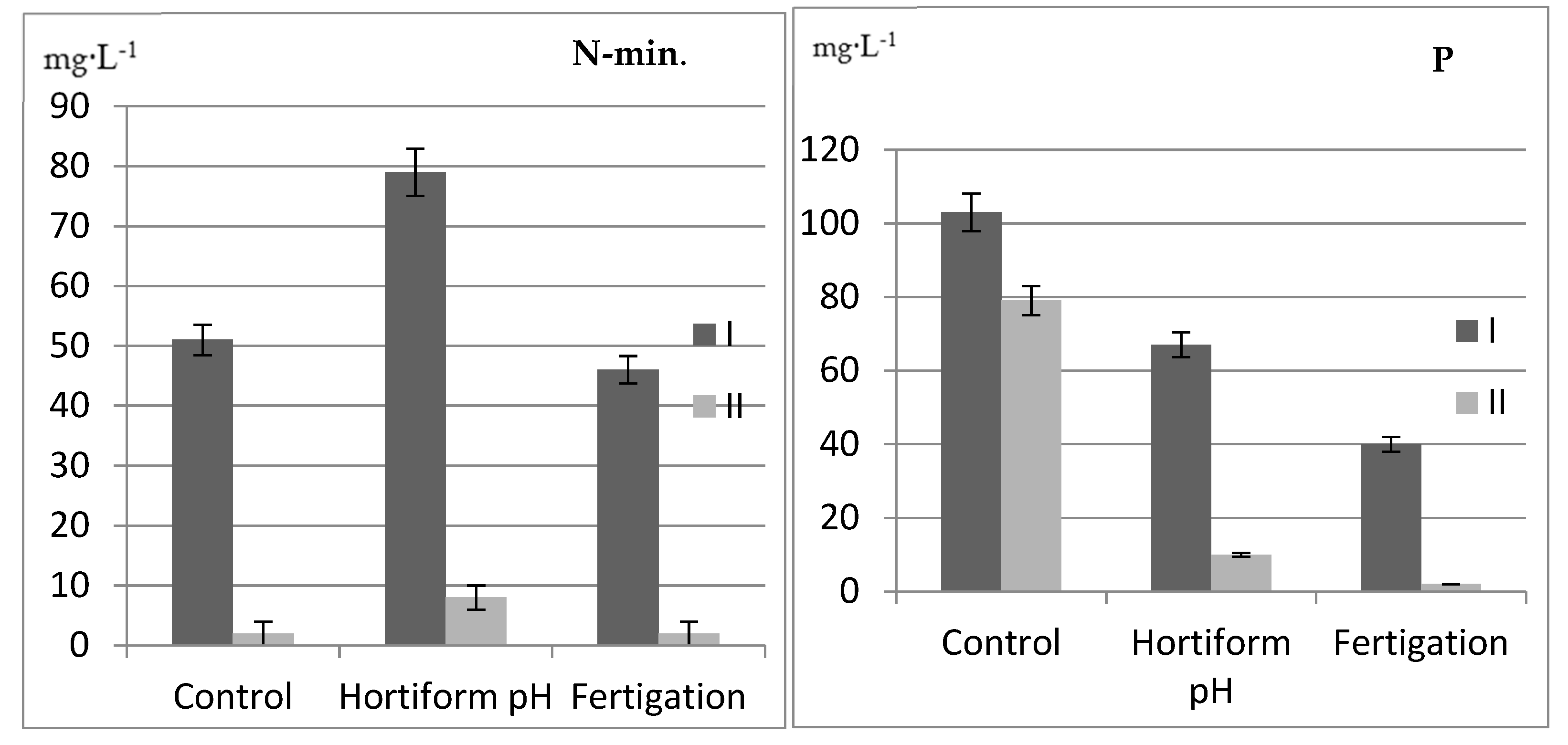
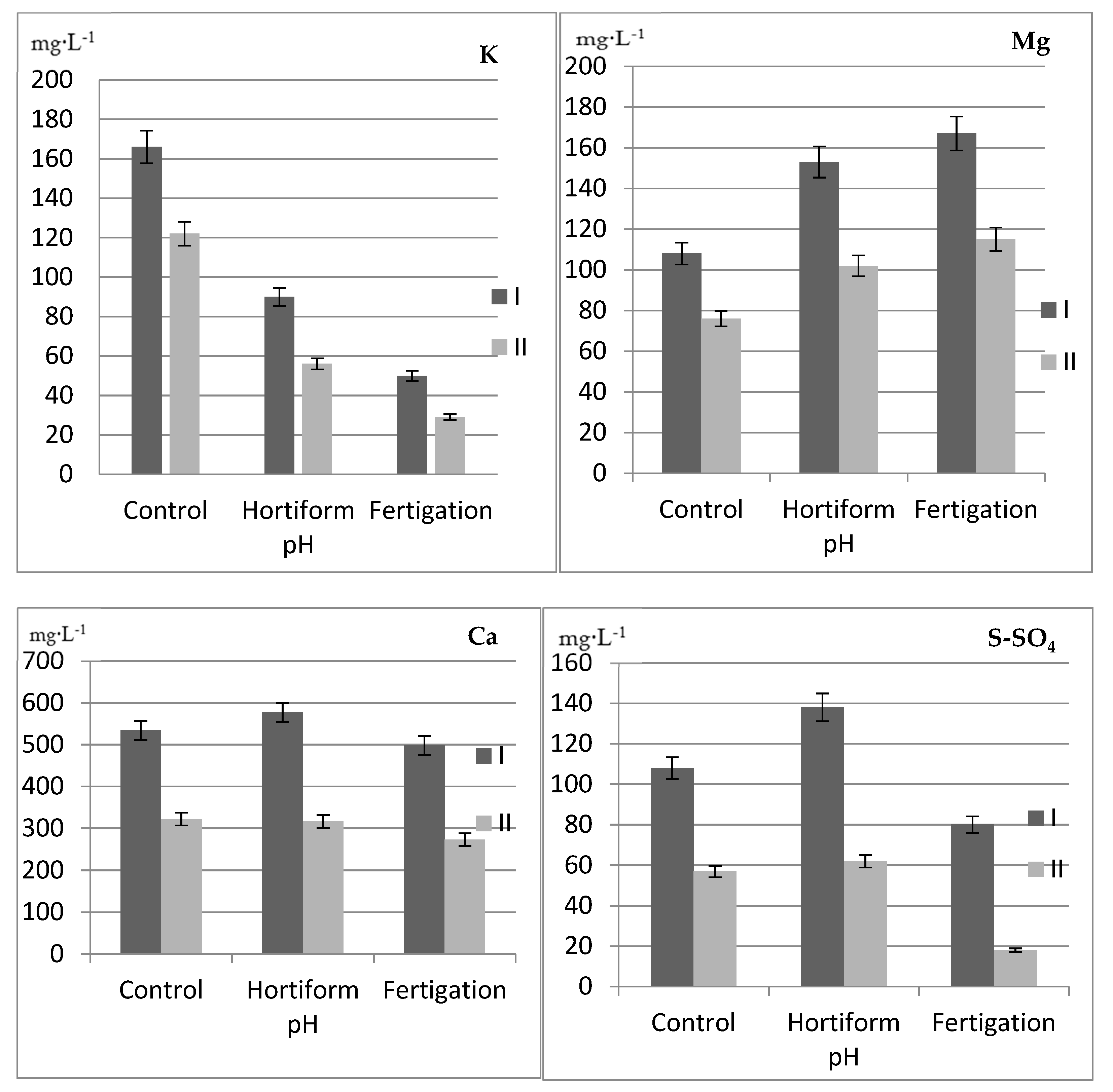
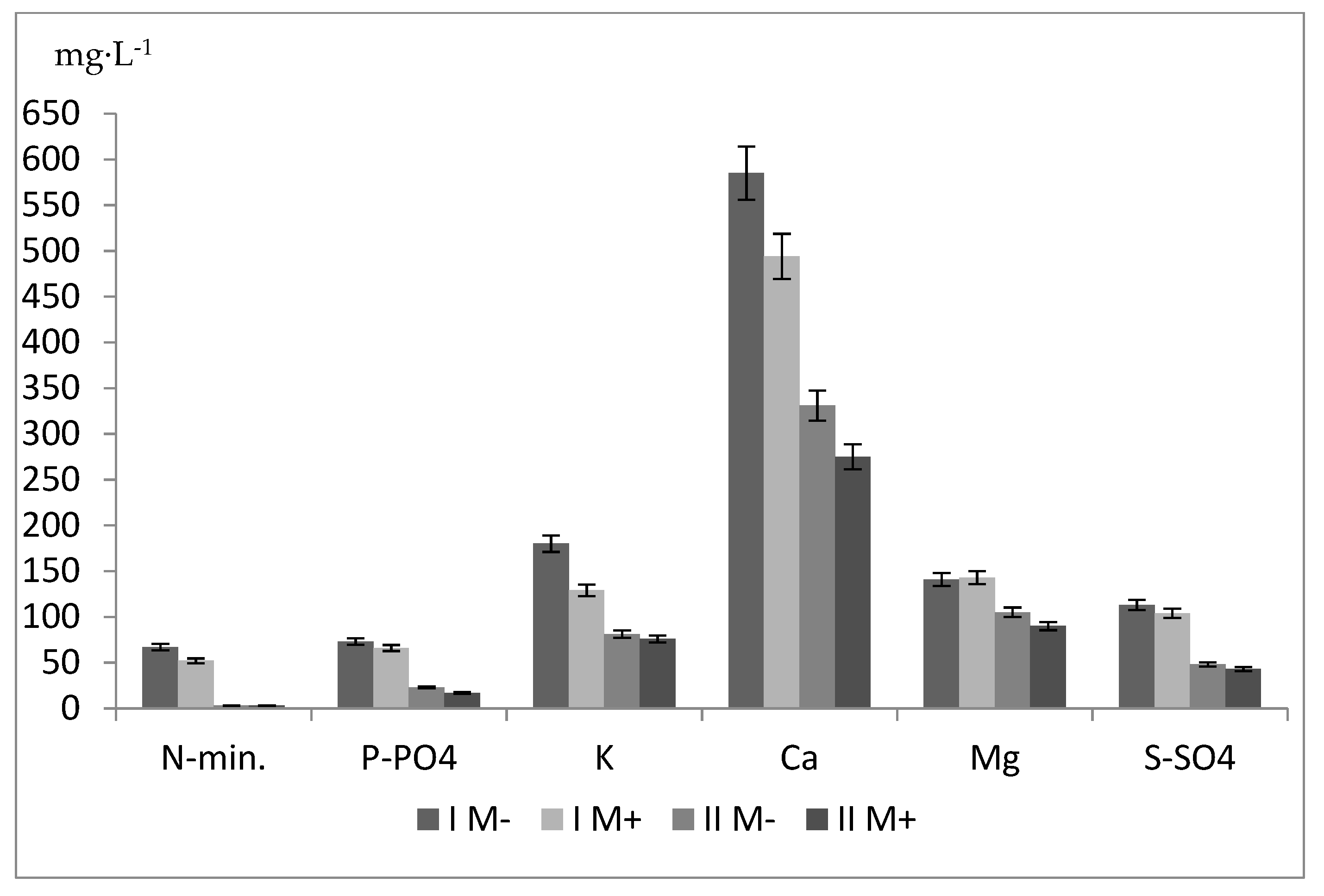
| Mycorrhizae (A) | Type of Fertilization (B) | Years | N-Total | P | K | Ca | Mg |
|---|---|---|---|---|---|---|---|
| Without ERM (M-) | Control | I | 17.8 | 2.50 | 7.40 | 7.50 | 1.60 |
| II | 19.4 | 3.00 | 7.40 | 7.80 | 1.50 | ||
| III | 20.3 | 3.60 | 8.80 | 7.80 | 1.80 | ||
| Mean for years | 19.2 b | 3.00 a | 7.90 a | 7.70 d | 1.60 | ||
| Hortiform pH (SRF) | I | 20.5 | 2.30 | 6.30 | 9.80 | 2.10 | |
| II | 20.3 | 2.40 | 6.80 | 9.20 | 1.90 | ||
| III | 20.8 | 2.60 | 6.90 | 8.80 | 1.50 | ||
| Mean for years | 20.5 a | 2.40 bc | 6.70 | 9.30 b | 1.80 | ||
| Fertigation | I | 13.9 | 2.00 | 5.70 | 8.80 | 2.60 | |
| II | 13.1 | 2.20 | 6.80 | 8.00 | 1.80 | ||
| III | 16.1 | 2.30 | 7.80 | 8.80 | 1.70 | ||
| Mean for years | 14.4 d | 2.22 c | 6.80 | 8.50 c | 2.00 | ||
| With ERM (M+) | Control | I | 17.5 | 2.50 | 7.30 | 8.10 | 1.80 |
| II | 19.6 | 3.00 | 6.90 | 8.00 | 1.70 | ||
| III | 20.5 | 3.30 | 7.20 | 9.10 | 1.60 | ||
| Mean for years | 19.2 b | 2.90 a | 7.10 b | 8.40 cd | 1.70 | ||
| Hortiform pH (SRF) | I | 19.1 | 2.60 | 6.90 | 10.9 | 2.30 | |
| II | 20.9 | 2.90 | 7.00 | 9.60 | 1.80 | ||
| III | 23.5 | 3.00 | 7.40 | 9.80 | 1.60 | ||
| Mean for years | 21.2 a | 2.80 ab | 7.10 b | 10.1 a | 1.90 | ||
| Fertigation | I | 12.5 | 2.10 | 6.70 | 10.6 | 2.10 | |
| II | 16.0 | 2.30 | 7.00 | 9.20 | 1.50 | ||
| III | 17.2 | 2.50 | 7.20 | 10.4 | 1.50 | ||
| Mean for years | 15.2 c | 2.30 c | 7.00 b | 10.0 ab | 1.70 | ||
| General mean | 18.3 | 2.60 | 7.10 | 9.00 | 1.80 | ||
| LSD0.05 | |||||||
| for A × B | 1.00 | 0.40 | 0.50 | 0.70 | n.s. | ||
| Mycorrhizae (A) | Type of Fertilization (B) | pH | EC *** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | 3rd Year | mS·cm−1 | ||||||
| I * | II ** | I | II | I | II | I | II | ||
| Without ERM (M-) | Control | 4.51 | 4.94 | 4.29 | 5.77 | 5.00 | 5.15 | 0.47 | 0.31 |
| Hortiform pH | 4.28 | 5.11 | 4.19 | 5.57 | 4.28 | 4.52 | 0.65 | 0.48 | |
| Fertigation | 4.25 | 5.08 | 4.45 | 5.28 | 4.27 | 5.25 | 0.31 | 0.24 | |
| With ERM (M+) | Control | 4.23 | 5.03 | 4.30 | 5.69 | 4.46 | 4.63 | 0.45 | 0.42 |
| Hortiform pH | 4.31 | 4.68 | 4.25 | 5.79 | 4.04 | 4.31 | 0.67 | 0.57 | |
| Fertigation | 4.09 | 4.52 | 4.70 | 5.28 | 4.20 | 5.38 | 0.28 | 0.24 | |
| Total range | 4.09–5.11 | 4.19–5.79 | 4.04–5.38 | 0.47 | 0.38 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz, Z.; Michałojć, Z.; Pitura, K.; Dzida, K.; Koter, M. Influence of Fertilization and Mycorrhizae on the Nutritional Status of Rhododendron (Rhododendron hybridum) in a Nursery. Agriculture 2021, 11, 538. https://doi.org/10.3390/agriculture11060538
Jarosz Z, Michałojć Z, Pitura K, Dzida K, Koter M. Influence of Fertilization and Mycorrhizae on the Nutritional Status of Rhododendron (Rhododendron hybridum) in a Nursery. Agriculture. 2021; 11(6):538. https://doi.org/10.3390/agriculture11060538
Chicago/Turabian StyleJarosz, Zbigniew, Zenia Michałojć, Karolina Pitura, Katarzyna Dzida, and Michał Koter. 2021. "Influence of Fertilization and Mycorrhizae on the Nutritional Status of Rhododendron (Rhododendron hybridum) in a Nursery" Agriculture 11, no. 6: 538. https://doi.org/10.3390/agriculture11060538
APA StyleJarosz, Z., Michałojć, Z., Pitura, K., Dzida, K., & Koter, M. (2021). Influence of Fertilization and Mycorrhizae on the Nutritional Status of Rhododendron (Rhododendron hybridum) in a Nursery. Agriculture, 11(6), 538. https://doi.org/10.3390/agriculture11060538






