Abstract
Quercetin is one of the most abundant flavonoids in terrestrial plants and pollen. In living plants, quercetin can function as a secondary metabolite to discourage insect herbivory. Literature on insect-quercetin interactions was searched and data synthesized to test the hypothesis that quercetin can become an effective biocide to reduce herbivory without disrupting natural enemies and pollinators. The USDA, National Agricultural Library, DigiTop Navigator platform was used to search the literature for harmful versus nonharmful effects of quercetin on insect behavior, physiology, and life history parameters. Quercetin effects were evaluated on herbivores in five insect orders, natural enemies in two orders, and pollinators in one order. Quercetin was significantly more harmful to Hemiptera, Diptera, and Lepidoptera but significantly more nonharmful to Coleoptera. Harmful and nonharmful effects to Orthoptera were indistinguishable. Quercetin had significantly more harmful (than nonharmful) effects on herbivores when data from the five insect orders were combined. Quercetin concentration (mg/mL) did not significantly affect these results. Quercetin was significantly more nonharmful to natural enemies (Coleoptera and Hymenoptera, combined) and pollinators (Hymenoptera). This study suggests that quercetin could prevent herbivory without disrupting natural enemies and pollinators, but field experiments are necessary to substantiate these results.
1. Introduction
Flavonoids represent a class of polyphenolic compounds known as secondary metabolites that can function as a host plant defense against attacks from plant pathogens and insect pests [1,2,3,4,5]. Some plant-feeding, i.e., herbivorous, insects can circumvent these defensive compounds and use them as cues to identify host plants and evaluate host plant quality [3,5,6,7,8,9,10]. Nevertheless, flavonoids are least toxic organic compounds that could suppress non-adapted herbivorous insects [3]. Quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one) could have such potential [11,12]. Quercetin is an organic compound classified as a flavonol, a flavonoid subclass (Figure 1). It is one of the most abundant flavonols found in plants and pollen [13,14,15]. Note that a sugar molecule (i.e., sugar moiety) is commonly bound to quercetin to form a quercetin glycoside in living plants.
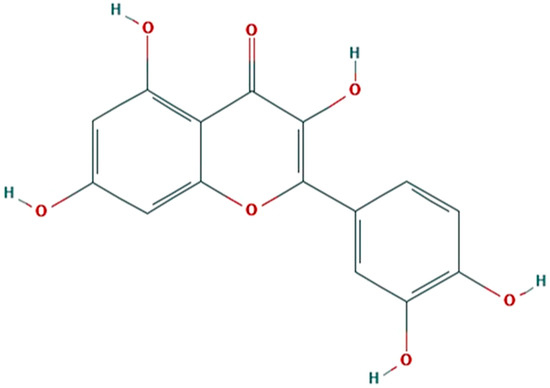
Figure 1.
Molecular structure of quercetin (C15H10O7). (Source: https://pubchem.ncbi.nlm.nih.gov).
Quercetin is known to facilitate interactions between insects and plants [7,9,10]. It is responsible for coloration in flower petals of some plants and, consequently, attracts insects (pollinators) to flowers and pollen [6]. Quercetin can function as a feeding stimulant [16], oviposition stimulant [7,9,17,18,19], or feeding deterrent [8,10,20]. Moreover, quercetin demonstrates repellency or insecticidal activity against pest herbivores, e.g., aphids [11], and maybe attract or not harm natural enemies. A cucumber cultivar (Storm) with high flavonoid (including quercetin) content and high trichome density, reduced development, and reproduction of Aphis gossypii Glover (Hemiptera: Aphididae) but increased the abundance of its predator Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae) [21]. The beneficial effects of quercetin on pollinators, e.g., western honeybee Apis mellifera L. (Hymenoptera: Apidae) has also been documented [22,23,24].
Ingestion of quercetin can induce the production of detoxification enzymes in non-adapted herbivores [9,10]. Ingestion of quercetin may increase the sensitivity of non-adapted herbivores to pesticides, which may or may not increase their ability to develop resistance to pesticides in the field. Quercetin ingestion may upregulate detoxification enzymes in pollinators (A. mellifera) with little or no harmful effects [23,24]. Quercetin ingestion can lessen the harmful effects of pesticide exposure on A. mellifera adult workers [23].
The literature was searched and data synthesized to test the hypothesis that quercetin can be utilized as an effective biocide to reduce herbivory without disrupting natural enemies and pollinators. The objectives of this review are to (1) compile the available evidence of harmful versus nonharmful effects of quercetin on herbivores, (2) estimate the influence of quercetin concentration on the observed effects on herbivores, and (3) determine the effects of quercetin on natural enemies and pollinators.
The United States Department of Agriculture (USDA), National Agricultural Library, online digital catalog system (DigiTop) Navigator platform, which includes research databases (such as AGRICOLA, BIOSIS Previews, CAB Abstracts, Scopus, Web of Science, Zoological Record, etc.) was used to retrieve abstracts and then the full text of manuscripts. The key words “quercetin and Hemiptera, Coleoptera, Diptera, Orthoptera, Lepidoptera, or Hymenoptera” were used to search for the effects of quercetin on insects. Only published research that tested pure quercetin was included in this study. Literature searches were restricted to these taxa (insect orders) to encompass herbivorous crop pests, natural enemies of crop pests, or pollinators of crop plants. To synthesize the available data, harmful and nonharmful effects of quercetin on insect species were tabulated. Harmful effects had negative consequences on behavior, physiology, and life history parameters, whereas non-harmful effects had positive or neutral consequences. Statistical analysis included a z-test for proportions to compare quercetin effects (harmful vs non-harmful) on herbivores (in five orders), natural enemies (in two orders), and pollinators (in one order). Second, a z-test compared quercetin effects on all herbivores, all taxa combined. A Mann–Whitney Rank Sum Test, with U statistic, compared the influence of chemical concentration (mg/mL), when available, on the observed effects (harmful vs nonharmful) on herbivores, five orders combined. A z-test was also used to compare quercetin effects on natural enemies (two orders combined) and pollinators (one order). Significant differences were indicated when p < 0.05. Statistical software, SigmaStat, interfaced within SigmaPlot 12.0, and JMP 14 were used for data analysis.
2. Effects of Quercetin on Herbivores
2.1. Hemiptera (True Bugs)
Four herbivorous hemipterans (three aphid species and one mirid species) were subjects in bioassays with quercetin based on the review of the literature (Table 1). Quercetin had harmful (negative) effects on survival rate of the aphid species Macrosiphum rosae (L.) and Acyrthosiphon pisum Harris, both nymphs and adults. Quercetin also had harmful (negative) effects on development, preoviposition time, and fecundity of A. pisum and fecundity of the aphid Sitobion miscanthi (Takahashi) via innate resistance in wheat ears in a field bioassay. In contrast, quercetin had nonharmful (positive) effects on the mirid Tupiocoris notatus (Distant); nymphs were attracted to quercetin treated leaves in the laboratory.

Table 1.
Exemplary research that tested the effects of quercetin on behavior and life history parameters of agriculturally important insect herbivores.
In a summary of this section, quercetin caused 0.857 and 0.143 proportional harmful and nonharmful effects on hemipteran species; the two effects were significantly different (z = 2.672, p = 0.008; n = 7). A concentration of 1 mg/mL or less was sufficient to cause harmful effects on M. rosae, A. pisum, and S. miscanthi, whereas an extremely low quercetin concentration (0.9 × 10−4 mg) caused nonharmful (positive) effects on T. notatus (Table 1). Quercetin concentrations were variable amongst these studies. Concentration data were not subjected to statistical analysis for this order, only for combined data for all five orders (see Section 2.6).
2.2. Coleoptera (Beetles)
Fourteen herbivorous coleopteran species were exposed to quercetin in bioassays (Table 1). The species included one bruchid Callosobruchus chinensis L., one tenebrionid Tribolium castaneum Herbst, two scarabaeids Melolontha melolontha (L.) and Popillia japonica Newman, one nitidulid Carpophilus hemipterus (L.), six chrysomelids Leptinotarsa decemlineata Say, Phaedon brassicae Baly, Oulema oryzae Kuwayama, Plagiodera versicolora Laicharting, Altica oleracea (L.), Altica nipponica (Ohno), one curculionid Anthonomus grandis Boheman, and one herbivorous coccinellid Epilachna paenulata (Germar). Quercetin had harmful (negative) effects and nonharmful (neutral and positive) effects on these species. For example, quercetin decreased the survival of C. chinensis eggs, L. decemlineata larvae, and E. paenulata larvae, and reduced feeding by T. castaneum adults, P. brassicae adults, and O. oryzae adults. In contrast, quercetin did not affect the survival of M. melolontha and E. paenulata larvae or feeding behavior by A. grandis and E. paenulata larvae. In other coleopteran species, quercetin increased feeding behavior (Table 1).
In summary, quercetin caused 0.304 and 0.696 proportional harmful and nonharmful effects, respectively, on herbivorous coleopterans (z = 2.652, p = 0.008, n = 23); nonharmful effects were predominant. There was variability in quercetin concentration and positive feeding responses between and within coleopteran species. For example, a concentration of 0.30 mg/mL stimulated feeding by P. japonica adults in one study; but a higher concentration of 30.22 mg/mL stimulated feeding of the same species in another study (Table 1). Quercetin had harmful effects on oviposition by C. chinensis in one study but not in another; quercetin concentration was at least five times greater in the bioassay indicating reduced oviposition than in the one indicating neutral effects. At 1–10 mg/mL, quercetin had nonharmful (neutral) effects on oviposition and feeding behavior by A. grandis in one study, but nonharmful (positive) effects on feeding behavior at a lower concentration, 0.5 mg/mL, in another study.
2.3. Lepidoptera (Moths/Butterflies)
Ten lepidopteran species, representing five families, were challenged with quercetin in bioassays (Table 1). The noctuids included Helicoverpa armigera (Hübner), Helicoverpa zea (Boddie), Heliothis virescens (F.), Spodoptera litura (F.), Spodoptera frugiperda (J. E. Smith), and Pectinophora gossypiella (Saunders). One arctiid Chilesia rudis (Butler), one lymantriid Lymantria dispar (L.), one bombycid Bombyx mori (L.), and one pyralid Ostrinia nubilalis (Hübner) were also challenged with quercetin.
Quercetin had harmful effects on development or body weight, i.e., growth, of noctuid larvae in most studies (Table 1). Effects on feeding behavior were variable, with nonharmful (positive) effects on S. frugiperda at low concentration (0.01 μg/cm2) on treated foliage as well as nonharmful (neutral) effects on H. virescens and H. zea at low concentration (0.10%, w/w) in an artificial diet. Quercetin also had nonharmful (positive) effects on feeding behavior of the arctiid C. rudis at 0.005 mg/mL on treated foliage. Quercetin had harmful effects on development, body weight, or survival of L. dispar, B. mori, and O. nubilalis at a concentration ranging from 0.1–2% (w/w) in an artificial diet (Table 1).
In summary, quercetin caused 0.792 and 0.208 proportional harmful and nonharmful effects on lepidopterans, respectively. A statistical analysis indicated a significant difference between the two effects (z = 4.046, p < 0.001, n = 24); harmful effects were predominant.
2.4. Diptera (True Flies)
Dipteran species subjected to quercetin in bioassays included two tephritids Rhagoletis pomonella (Walsh) and Bactrocera cucurbitae (Coquillett), one drosophilid Drosophila melanogaster Meigen and one sciarid Lycoriella pleuroti Yang & Zhang. Records indicated harmful (negative) effects of quercetin on B. cucurbitae, R. pomonella, and L. pleuroti after direct physical bodily contact with the compound in test arenas, in an artificial diet or artificial culture media at variable quercetin concentrations. For example, quercetin at 0.05–3.1 mg/mL, in bioassays involving B. cucurbitae, reduced egg hatch rate, pupation, adult emergence, oviposition, and survival rate (Table 1). In two studies, quercetin had nonharmful (positive) effects on development time and fecundity of D. melanogaster larvae and adult females, respectively. Quercetin concentration ranged from 1.7% to 5.0% across these two studies. In summary of this section, quercetin caused 0.75 and 0.25 proportional harmful and nonharmful effects on dipterans, respectively. The statistical analysis indicated a significant difference between the two effects (z = 2.00, p = 0.046, n = 8); harmful effects were more prevalent.
2.5. Orthoptera (Grasshoppers)
Three acridid species were tested against quercetin in field cage and laboratory bioassays. These species included Calliptamus abbreviatus Ikonn, Oedaleus asiaticus Bey-Bienko, and Melanoplus sanguinipes (F.) (Table 1). Quercetin had harmful (negative) effects on development and survival of C. abbreviatus nymphs at a concentration of 0.10 mg/mL. Quercetin concentrations ranging from 0.10–10 mg/mL significantly reduced growth/development and survival of O. asiaticus nymphs. In contrast, body weight and survival rate of M. sanguinipes nymphs were unaffected by quercetin at a concentration of ranging from 0.125–4.0% (w/w). In summary, quercetin caused 0.67 and 0.33 proportional harmful and nonharmful effects on orthopterans, respectively. A statistical analysis did not indicate a significant difference between the two effects (z = 1.155, p = 0.248, n = 6).
2.6. Summary of Herbivores
The sections above indicated that quercetin caused more harmful effects to Hemiptera, Lepidoptera, and Diptera but more nonharmful effects to Coleoptera. In concluding the herbivore section, quercetin caused 0.618 and 0.382 proportional harmful and nonharmful effects on herbivores, respectively, across the five insect orders combined. The two effects were significantly different (z = 2.744, p = 0.006, n = 68); harmful effects were predominant. Quercetin concentration (mg/mL) did not significantly influence the observed harmful and nonharmful effects on herbivores, based on pooling of data, when available, across the five insect orders (U = 105.50; p = 0.583; n = 20 for harmful effects; n = 12 for nonharmful effects). Median values with 25% and 75% confidence intervals were 0.56 mg/mL (0.10, 2.76) for harmful effects and 1.00 mg/mL (0.09, 3.02) for nonharmful effects. Specific harmful effects of quercetin on herbivores, of five orders combined, are illustrated in Figure 2. Quercetin frequently affected survival rate and development/growth.
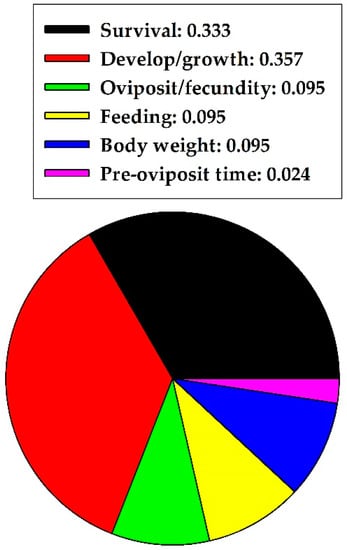
Figure 2.
Proportion of specific harmful effects of quercetin on herbivores in five insect orders combined.
3. Natural Enemies
Predators and Parasitoids
Limited research has been published on the effects of quercetin on natural enemies, i.e., predators and parasitoids. Quercetin had nonharmful (positive) effects on the coccinellid Coleomegilla maculata DeGeer, an important predator of aphids and other soft-bodied herbivores in agroecosystems (Table 2). In laboratory bioassays, females were attracted to quercetin (1 mg, 98% pure powder) and stimulated to lay more egg clutches in test cages than control cages. Quercetin had nonharmful (positive) effects on a trichogrammatid Trichogramma chilonis Ishii, a parasitoid of lepidopteran eggs. At low quercetin concentration (0.01–0.03 mg), T. chilonis adults were attracted to treated substrates and females were stimulated to oviposit into host eggs in laboratory and semi-field bioassays.

Table 2.
Research that tested the effects of quercetin on natural enemies.
In a summary of the natural enemy section, quercetin caused 0.00 and 1.0 proportional harmful and nonharmful effects, respectively, on natural enemies (Coleoptera and Hymenoptera taxa combined). The harmful and nonharmful effects were significantly different (z = 2.828, p = 0.005; n = 4); nonharmful effects were more prevalent. Specific nonharmful (all positive) effects of quercetin on two natural enemies are illustrated in Figure 3. Oviposition behavior was affected most frequently.
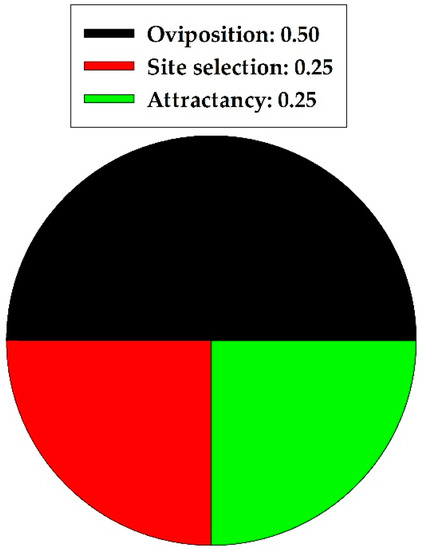
Figure 3.
Proportion of specific nonharmful effects of quercetin on natural enemies in two insect orders combined.
4. Pollinators
Domesticated Honeybee (Hymenoptera)
Honeybee, A. mellifera, workers can contact pesticide residues while collecting and consuming pollen. Since quercetin is one of the main constituents in pollen, research has addressed the interaction of quercetin and pesticides on the health of A. mellifera (Table 3). Ten studies were identified in the published literature; two studies indicated harmful effects of quercetin. For example, feeding A. mellifera adults a diet containing quercetin and an acaricide (for varroa mites) reduced survival rate. Feeding workers a diet containing quercetin and a fungicide reduced the ability of workers to produce energy, i.e., ATPs. All other studies indicated nonharmful effects of quercetin with or without incorporating pesticides in experimental designs (Table 3). Quercetin ameliorated harmful effects of pesticide exposure and increased A. mellifera survival in laboratory bioassays. Ingestion of fungicide residues with pollen or nectar reduced flight performance, i.e., wing beat frequency, of A. mellifera in an indoor flight mill experiment. Incorporating quercetin in the diet, restored wing beat frequency. Quercetin functioned as an attractant and feeding stimulant. Moreover, quercetin increased maturation of ovaries in A. mellifera workers.

Table 3.
Research that tested the effects of quercetin on behavior and life history parameters of agriculturally important pollinators.
The documented effects of quercetin on A. mellifera health do not appear to be influenced by quercetin concentration. Because of limited studies, quercetin concentration data were not analyzed for pollinators. Note that in the two studies indicating harmful effects, quercetin concentration ranged from 0.075 to 0.302 mg/mL; in the eight studies indicating nonharmful effects, quercetin concentration ranged from 0.003 mg/mL to 10 mg/g (Table 3).
In summarizing the pollinator section, quercetin caused 0.2 and 0.8 proportional harmful and nonharmful effects, respectively, on A. mellifera workers. A statistical analysis indicated significant differences between the two effects (z = 2.683, p = 0.007, n = 10); nonharmful effects were predominant. Specific nonharmful effects of quercetin on A. mellifera are displayed in Figure 4. An increased capacity to tolerate pesticide toxicity and an increase in survival rate were most frequent.
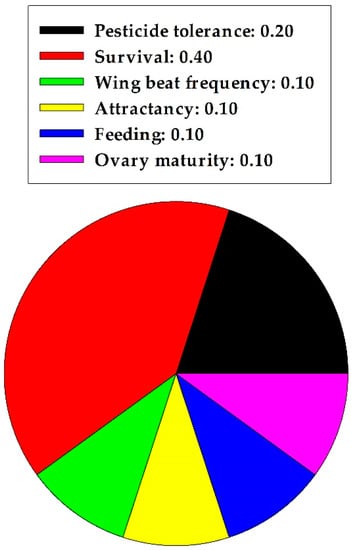
Figure 4.
Proportion of specific nonharmful effects of quercetin on pollinators in one insect order.
5. Synthesis and Conclusions
5.1. Synthesis
The potential of using quercetin as an effective biocide to discourage insect herbivory without disrupting natural enemies and pollinators has been evaluated in this study. Quercetin had significantly more harmful (negative) than nonharmful (positive or neutral) effects on behavior and life history parameters of herbivorous species in three of five insect orders, i.e., Hemiptera, Diptera, and Lepidoptera. Quercetin had significantly more nonharmful effects on herbivores in the Coleoptera, which suggests that this flavonol has potential as a feeding stimulant or an attractant for herbivorous beetles. Quercetin had no significant effects (neither harmful nor nonharmful) on Orthoptera, perhaps due to a small sample size of published data. When the herbivore data were combined, quercetin had significantly more harmful than nonharmful effects. The most frequent specific harmful effects on herbivores were decreased survival and altered growth and development.
Chemical concentration (mg/mL) did not influence the outcome of the analysis of herbivore data. Note that not all concentration data were reported in mg/mL values, or was convertible to mg/mL, as indicated in Table 1, Table 2 and Table 3. Consequently, the lack of a significant relationship between concentration for data pooled across all herbivores and quercetin effects (harmful versus nonharmful) indicates that concentration, alone, cannot be used to predict an outcome. Other factors such as herbivore species, life stage examined, bioassay methods, and life history parameters tested, likely contributed to outcomes. Less data were available on the effects of quercetin on natural enemies and pollinators (see Table 2 and Table 3). Therefore, influence of chemical concentration on outcomes (harmful versus nonharmful effects) was not analyzed statistically for beneficials.
A comparison of harmful versus nonharmful effects of quercetin on natural enemies indicated nonharmful effects only. However, the data set was small; just two species had been subjected to quercetin in experiments in the published literature. Nevertheless, these results are encouraging because they provide evidence that quercetin could be used to manipulate the behavior of natural enemies. Stimulating oviposition behavior and manipulating oviposition site selection in predators and parasitoids has relevance to mass production and augmentative biological control.
The results of the analysis of the effects of quercetin on pollinators, i.e., A. mellifera was also encouraging. Although A. mellifera was the only species challenged with quercetin, based on reliable published data, the observation of significantly more nonharmful (than harmful) effects on workers suggests that quercetin, when applied against pests, is not expected to harm foraging bees.
Quercetin is a compound with relatively low volatility, high molecular weight, and very low vapor pressure [19]. Thus, detection of quercetin molecules must primarily involve physical contact with gustatory receptors and/or mechanoreceptors, rather than olfactory receptors, on the mouthparts or antennae of the insect. This fact could limit the applicability of a push-pull strategy to push pest herbivores away from crop plants and pull beneficial insects toward crop plants [66]. Insects must physically contact quercetin molecules on the plant surface before a change in behavior would ensue.
5.2. Conclusions
This study has highlighted evidence that quercetin affects the behavior and life history of herbivores, natural enemies, and pollinators. This study suggests that quercetin has utility in a modified push-pull strategy to deter pest herbivores, e.g., aphids, from crop plants and arrest natural enemies (ladybird beetles or aphid parasitoids) and pollinators (honeybees) on crop plants. Future research using more species and a multitrophic approach (the interaction of crop plant, pest, natural enemy, and pollinator) would be more informative than examining quercetin effects on a single trophic level. Additionally, testing the functionality of quercetin under semi-field (greenhouse, high tunnel, nursery) or open field conditions is necessary. Applying quercetin directly onto the leaf surface of non-engineered plants could boost the density of natural enemies (for aphid control) and pollinators (for pollination services) for a net benefit to the crop plant. Alternatively, crop plants could be metabolically engineered to produce and release greater quantities of quercetin. Research on flavonoid mass production via metabolic engineering of plants and microbes is underway [67,68,69]. Perhaps other common flavonoids, e.g., kaempferol [70], could be utilized as an alternative compound to alleviate insect resistance development arising from the overuse of quercetin. The utility of quercetin as a less-toxic natural product to manage herbivorous pests without disrupting the activity of natural enemies and pollinators on greenhouse, high tunnel, or nursery grown crops could become a reality.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Anonymous peer reviewers commented on an earlier version of this article. The US Government has the right to retain a nonexclusive, royalty free license in and to any copyright of this article. Mention of a commercial or proprietary product does not constitute an endorsement of the product by the US Department of Agriculture (USDA). The USDA, Agricultural Research Service is an equal opportunity provider and employer.
Conflicts of Interest
The author declares no conflict of interest.
References
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Diaz Napal, G.N.; Palacios, S.M. Bioinsecticidal effect of the flavonoids pinocembrin and quercetin against Spodoptera frugiperda. J. Pest Sci. 2015, 88, 629–635. [Google Scholar] [CrossRef]
- Bentivenha, J.P.F.; Canassa, V.F.; Baldin, E.L.L.; Borguini, M.G.; Lima, G.P.P.; Lourenção, A.L. Role of the rutin and genistein flavonoids in soybean resistance to Piezodorus guildinii (Hemiptera: Pentatomidae). Arthropod. Plant Interact. 2018, 12, 311–320. [Google Scholar] [CrossRef]
- Kariyat, R.R.; Gaffoor, I.; Sattar, S.; Dixon, C.W.; Frock, N.; Moen, J.; De Moraes, C.M.; Mescher, M.C.; Thompson, G.A.; Chopra, S. Sorghum 3-deoxyanthocyanidin flavonoids confer resistance against corn leaf aphid. J. Chem. Ecol. 2019, 45, 502–514. [Google Scholar] [CrossRef]
- Harborne, J.B. Biochemistry of plant pollination. In Introduction to Ecological Biochemistry; Academic Press Inc.: London, UK, 1977; Chapter 2; pp. 28–57. [Google Scholar]
- Haribal, M.; Renwick, J.A.A. Oviposition stimulants for the monarch butterfly: Flavonol glycosides from Asclepias curassavica. Phytochemistry 1996, 41, 139–144. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Simmonds, M.S.J. Importance of flavonoids in insect-plant interactions: Feeding and oviposition. Phytochemistry 2001, 56, 245–252. [Google Scholar] [CrossRef]
- Simmonds, M.S.J. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Goławska, S.; Sprawka, I.; Łukasik, I.; Goławski, A. Are naringenin and quercetin useful chemicals in pest-management strategies? J. Pest Sci. 2014, 87, 173–180. [Google Scholar] [CrossRef]
- Perić-Mataruga, V.; Hackenberger, B.K.; Vlahović, M.; Ilijin, L.; Mrdaković, M. Potential improvement of Lymantria dispar L. management by quercetin. Arch. Biol. Sci. 2014, 66, 1125–1129. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Wiedenmann, R.N. Nutritional suitability of corn pollen for the predator Coleomegilla maculata (Coleoptera: Coccinellidae). J. Ins. Physiol. 2004, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Aljuhaimi, F.; Babiker, E.E.; Uslu, N.; Ceylan, D.A.; Ghafoor, K.; Özcan, M.M.; Dursun, N.; Ahmed, I.M.; Jamiu, F.G.; et al. Determination of antioxidant activity, phenolic compound, mineral contents and fatty acid compositions of bee pollen grains collected from different locations. J. Apicul. Sci. 2019, 63, 69–79. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; Wang, K.; Yang, Y.; Su, N.; Huang, W.; Wu, Y. Chemical and cytological evaluation of honeybee pollen antioxidant ability. J. Food Sci. 2020, 85, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Diaz Napal, G.N.; Defagó, M.T.; Valladares, G.R.; Palacios, S.M. Response of Epilachna paenulata to two flavonoids, pinocembrin and quercetin, in a comparative study. J. Chem. Ecol. 2010, 36, 898–904. [Google Scholar] [CrossRef]
- Riddick, E.W.; Wu, Z.; Eller, F.J.; Berhow, M.A. Do bioflavonoids in Juniperus virginiana heartwood stimulate oviposition in the ladybird Coleomegilla maculata? Int. J. Insect. Sci. 2018, 10, 1–13. [Google Scholar] [CrossRef]
- Riddick, E.W.; Wu, Z.; Eller, F.J.; Berhow, M.A. Utilization of quercetin as an oviposition stimulant by lab-cultured Coleomegilla maculata in the presence of conspecifics and a tissue substrate. Insects 2018, 9, 77. [Google Scholar] [CrossRef]
- Riddick, E.W. Volatile and non-volatile organic compounds stimulate oviposition by aphidophagous predators. Insects 2020, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.Y.; Huang, X.B.; Li, S.; Hao, K.; Chang, B.H.; Tu, X.B.; Pang, B.P.; Zhang, Z.H. Quercetin affects the growth and development of the grasshopper Oedaleus asiaticus (Orthoptera: Acrididae). J. Econ. Entomol. 2019, 112, 1175–1182. [Google Scholar] [CrossRef]
- Zahedi, A.; Razmjou, J.; Raflee-Dastjerdi, H.; Leppla, N.C.; Golizadeh, A.; Hassanpour, M.; Ebadollahi, A. Tritrophic interactions of cucumber cultivar, Aphis gossypii (Hemiptera: Aphididae), and its predator Hippodamia variegata (Coleoptera: Coccinellidae). J. Econ. Entomol. 2019, 112, 1774–1779. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, G.Y.; Yu, Y.S.; Liu, F.L. High concentration of nectar quercetin enhances worker resistance to queen’s signals in bees. J. Chem. Ecol. 2010, 36, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Wu, W.; Berenbaum, M.R. Impacts of dietary phytochemicals in the presence and absence of pesticides on longevity of honey bees (Apis mellifera). Insects 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Wu, W.; Berenbaum, M.R. Behavioral responses of honey bees (Apis mellifera) to natural and synthetic xenobiotics in food. Sci. Rep. 2017, 7, 15924. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Dharma, K.; Kumar, N.R. Insecticidal effects of aqueous extracts of wild pomegranate peel and seed (Punica granatum L.) against rose aphids, Macrosiphum rosaeformis. J. Appl. Nat. Sci. 2017, 9, 1397–1405. [Google Scholar] [CrossRef]
- Li, X.-Q.; Guo, X.-R.; Li, K.-B.; Yin, J.; Cao, Y.-Z. Resistance of wheat varieties (lines) to Sitobion miscanthi (Takahashi) (Aphidoidea: Aphididae). Acta Entomol. Sin. 2006, 49, 963–968. [Google Scholar]
- Roda, A.L.; Oldham, N.J.; Svatos, A.; Baldwin, I.T. Allometric analysis of the induced flavonols on the leaf surface of wild tobacco (Nicotiana attenuata). Phytochemistry 2003, 62, 527–536. [Google Scholar] [CrossRef]
- Salunke, B.K.; Kotkar, H.M.; Mendki, P.S.; Upasani, S.M.; Maheshwari, V.L. Efficacy of flavonoids in controlling Callosobruchus chinensis (L.) (Coleoptera: Bruchidae), a post-harvest pest of grain legumes. Crop. Prot. 2005, 24, 888–893. [Google Scholar] [CrossRef]
- Matsumoto, H.; Tebayashi, S.; Kuwahara, Y.; Matsuyama, S.; Suzuki, T.; Fujii, K. Identification of taxifolin present in the Azuki bean as an oviposition stimulant of the Azuki bean weevil. J. Pest Sci. 1994, 19, 181–186. [Google Scholar] [CrossRef][Green Version]
- Adeyemi, M.M.; Agbaji, A.S.; Adebote, D.A.; Amupitan, J.O.; Oyewale, A.O. Antifeedant activity of quercetin isolated from the stem bark of Bobgunnia madagascariensis (Desv.) J.H.Kirkbr & Wiersema (Caesalpiniaceae). Aust. J. Basic. Appl. Sci. 2010, 4, 3342–3346. [Google Scholar]
- Skrzecz, I.; Sowińska, A.; Janiszewski, W. Effects of botanical antifeedants on Melolontha melolontha grub feeding on Scots pine roots. Folia For. Pol. Series A 2014, 56, 135–140. [Google Scholar] [CrossRef]
- Fulcher, A.F.; Ranney, T.G.; Burton, J.D.; Walgenbach, J.F.; Danehower, D.A. Role of foliar phenolics in host plant resistance of Malus taxa to adult Japanese beetles. Hortscience 1998, 33, 862–865. [Google Scholar] [CrossRef]
- Patton, C.A.; Ranney, T.G.; Burton, J.D.; Walgenbach, J.F. Feeding responses of Japanese beetle to naturally occurring metabolites found in rosaceous plants. J. Environ. Hort. 1997, 15, 222–227. [Google Scholar] [CrossRef]
- Dowd, P.F. Responses of Carpophilus hemipterus larvae and adults to selected secondary metabolites of maize. Entomol. Exp. Appl. 1990, 54, 29–36. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Cheng, X.; Liu, S.; Wei, Q.; Scott, I.M. Conifer flavonoid compounds inhibit detoxification enzymes and synergize insecticides. Pest Biochem. Physiol. 2016, 127, 1–7. [Google Scholar] [CrossRef]
- Matsuda, K. Feeding stimulation of flavonoids for various leaf beetles (Coleoptera: Chrysomelidae). Appl. Entomol. Zool. 1978, 13, 228–230. [Google Scholar] [CrossRef][Green Version]
- Maxwell, F.G.; Jenkins, J.N.; Parrott, W.L. Influence of constituents of the cotton plant on feeding, oviposition, and development of the boll weevil. J. Econ. Entomol. 1967, 60, 1294–1297. [Google Scholar] [CrossRef]
- Hedin, P.A.; Miles, L.R.; Thompson, A.C.; Minyard, J.P. Constituents of a cotton bud formulation of a boll weevil feeding stimulant mixture. J. Agric. Food. Chem. 1968, 16, 505–513. [Google Scholar] [CrossRef]
- Chen, C.; Yan, W.; Wang, S.; Shi, X.; Gao, X.; Han, P.; Zhou, X.; Desneux, N. Uptake of quercetin reduces larval sensitivity to lambda-cyhalothrin in Helicoverpa armigera. J. Pest Sci. 2018, 91, 919–926. [Google Scholar] [CrossRef]
- Selin-Rani, S.; Senthil-Nathan, S.; Thanigaivel, A.; Vasantha-Srinivasan, P.; Edwin, E.-S.; Ponsankar, A.; Lija-Escaline, J.; Kalaivani, K.; Abdel-Megeed, A.; Hunter, W.B.; et al. Toxicity and physiological effect of quercetin on generalist herbivore, Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Chemosphere 2016, 165, 257–267. [Google Scholar] [CrossRef]
- Li, Z.; Guan, X.; Zhang, Q.; Liu, X.; Michaud, J.P. Quercetin interacts with Cry1Ac protein to affect larval growth and survival of Helicoverpa armigera. Pest Manag. Sci. 2016, 72, 1359–1365. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, Y.; Li, M.; Qiu, X. Effects of dietary quercetin on performance and cytochrome P450 expression of the cotton bollworm, Helicoverpa armigera. Bull Entomol. Res. 2015, 105, 771–777. [Google Scholar] [CrossRef]
- Chacón-Fuentes, M.; Parra, L.; Rodriguez-Saona, C.; Seguel, I.; Ceballos, R.; Quiroz, A. Domestication in Murtilla (Ugni molinae) reduced defensive flavonol levels but increased resistance against a native herbivorous insect. Environ. Entomol. 2015, 44, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-E.; Ma, H.-J.; Feng, D.-D.; Lai, X.-F.; Chen, Z.-M.; Xu, M.-Y.; Yu, Q.-Y.; Zhang, Z. Induction of detoxification enzymes by quercetin in the silkworm. J. Econ. Entomol. 2012, 105, 1024–1042. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zaid, M.M.; Beninger, C.W.; Arnason, J.T.; Nozzolillo, C. The effect of one flavone, two catechins and four flavonols on mortality and growth of the European corn borer (Ostrinia nubilalis Hubner). Biochem. Syst. Ecol. 1993, 21, 415–420. [Google Scholar] [CrossRef]
- Gould, F. Stress specificity of maternal effects in Heliothis virescens (Boddie) (Lepidoptera, Noctuidae) larvae. Mem. Entomol. Soc. Canada 1988, 146, 191–197. [Google Scholar] [CrossRef]
- Shaver, T.N.; Lukefahr, M.J.; Garcia, J. Food utilisation, ingestion, and growth of larvae of the bollworm and tobacco budworm on diets containing gossypol. J. Econ. Entomol. 1970, 63, 1544–1546. [Google Scholar] [CrossRef]
- Shaver, T.N.; Lukefahr, M.J. Effect of flavonoid pigments and gossypol on growth and development of the bollworm, tobacco budworm, and pink bollworm. J. Econ. Entomol. 1969, 62, 643–646. [Google Scholar] [CrossRef]
- Lukefahr, M.J.; Martin, D.F. Cotton-plant pigments as a source of resistance to the bollworm and tobacco budworm. J. Econ. Entomol. 1966, 59, 176–179. [Google Scholar] [CrossRef]
- Sharma, R.; Sohal, S.K. Oviposition response of melon fruit fly, Bactrocera cucurbitae (Coquillett) to different phenolic compounds. J. Biopest. 2016, 9, 46–51. [Google Scholar]
- Sharma, R.; Sohal, S.K. Bioefficacy of quercetin against melon fruit fly. Bull Insectol. 2013, 66, 79–83. [Google Scholar]
- Pree, D.J. Resistance to development of larvae of the apple maggot in crab apples. J. Econ. Entomol. 1977, 70, 611–614. [Google Scholar] [CrossRef]
- Saric, A.; Kalafatic, M.; Rusak, G.; Kovacevic, G.; Franjevic, D.; Gutzeit, H.O. Postembryonic development of Drosophila melanogaster Meigen, 1830 under the influence of quercetin. Entomol. News 2007, 118, 235–240. [Google Scholar] [CrossRef]
- Schramm, D.D.; Collins, H.E.; Hawley, R.S.; German, J.B. Unaltered meiotic chromosome segregation in Drosophila melanogaster raised on a 5% quercetin diet. Food Chem. Toxicol. 1998, 36, 585–589. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Y.; Liu, X.; Yuan, F.; Su, N.; Chen, Y.; Wu, Y.; Zhang, Q. Effects of CryIAc and secondary metabolites in Bt transgenic cottonseed on Lycoriella pleuroti Yang et Zhang (Diptera: Sciaridae). Environ. Entomol. 2006, 35, 807–810. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Chang, B.H.; Zhang, Z. Growth performance and enzymatic response of the grasshopper, Calliptamus abbreviatus (Orthoptera: Acrididae), to six plant-derived compounds. J. Insect Sci. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Westcott, N.D.; Hinks, C.F.; Olfert, O. Dietary effects of secondary plant compounds on nymphs of Melanoplus sanguinipes (Orthoptera: Acrididae). Ann. Entomol. Soc. Am. 1992, 85, 304–309. [Google Scholar] [CrossRef]
- Rani, P.U.; Sambangi, P.; Sandhyarani, K. Impact of plant phenolics as semiochemicals on the performance of Trichogramma chilonis Ishii. J. Insect. Behav. 2017, 30, 16–31. [Google Scholar] [CrossRef]
- Ardalani, H.; Vidkjær, N.H.; Laursen, B.B.; Kryger, P.; Fomsgaard, I.S. Dietary quercetin impacts the concentration of pesticides in honey bees. Chemosphere 2021, 262, 1–8. [Google Scholar] [CrossRef]
- Liao, L.-H.; Pearlstein, D.J.; Wu, W.-Y.; Kelley, A.G.; Montag, W.M.; Hsieh, E.M.; Berenbaum, M.R. Increase in longevity and amelioration of pesticide toxicity by natural levels of dietary phytochemicals in the honey bee, Apis mellifera. PLoS ONE 2020, 15, e0243364. [Google Scholar] [CrossRef]
- Liao, L.-H.; Wu, W.-Y.; Dad, A.; Berenbaum, M.R. Fungicide suppression of flight performance in the honeybee (Apis mellifera) and its amelioration by quercetin. Proc. R. Soc. B 2019, 286, 20192041. [Google Scholar] [CrossRef]
- Wong, M.J.; Liao, L.-H.; Berenbaum, M.R. Biphasic concentration-dependent interaction between imidacloprid and dietary phytochemicals in honey bees (Apis mellifera). PLoS ONE 2018, 13, e0206625. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Disruption of quercetin metabolism by fungicide affects energy production in honey bees (Apis mellifera). Proc. Nat. Acad. Sci. USA 2017, 114, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- Guseman, A.J.; Miller, K.; Kunkle, G.; Dively, G.P.; Pettis, J.S.; Evans, J.D.; van Engelsdorp, D.; Hawthorne, D.J. Multi-drug resistance transporters and a mechanism-based strategy for assessing risks of pesticide combinations to honey bees. PLoS ONE 2016, 11, e0148242. [Google Scholar] [CrossRef]
- Johnson, R.M.; Mao, W.F.; Pollock, H.S.; Niu, G.D.; Schuler, M.A.; Berenbaum, M.R. Ecologically appropriate xenobiotics induce cytochrome P450s in Apis mellifera. PLoS ONE. 2012, 7, e31051. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Yu, O. Metabolic engineering of flavonoids in plants and microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949–956. [Google Scholar] [CrossRef]
- Yoon, J.-A.; Kim, B.-G.; Lee, W.J.; Lim, Y.; Chong, Y.; Ahn, J.-H. Production of a novel quercetin glycoside through metabolic engineering of Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 4256–4262. [Google Scholar] [CrossRef]
- Sheng, H.; Sun, X.; Yan, Y.; Yuan, Q.; Wang, J.; Shen, X. Metabolic engineering of microorganisms for the production of flavonoids. Front. Bioeng. Biotechnol. 2020, 8, 589069. [Google Scholar] [CrossRef]
- Bernklau, E.; Bjostad, L.; Hogeboom, A.; Carlisle, A.; Arathi, H.S. Dietary phytochemicals, honey bee longevity and pathogen tolerance. Insects 2019, 10, 14. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).