Hazard of Contamination with Heavy Metals in Thymus serpyllum L. Herbs from Rural Areas
Abstract
1. Introduction
2. Materials and Methods
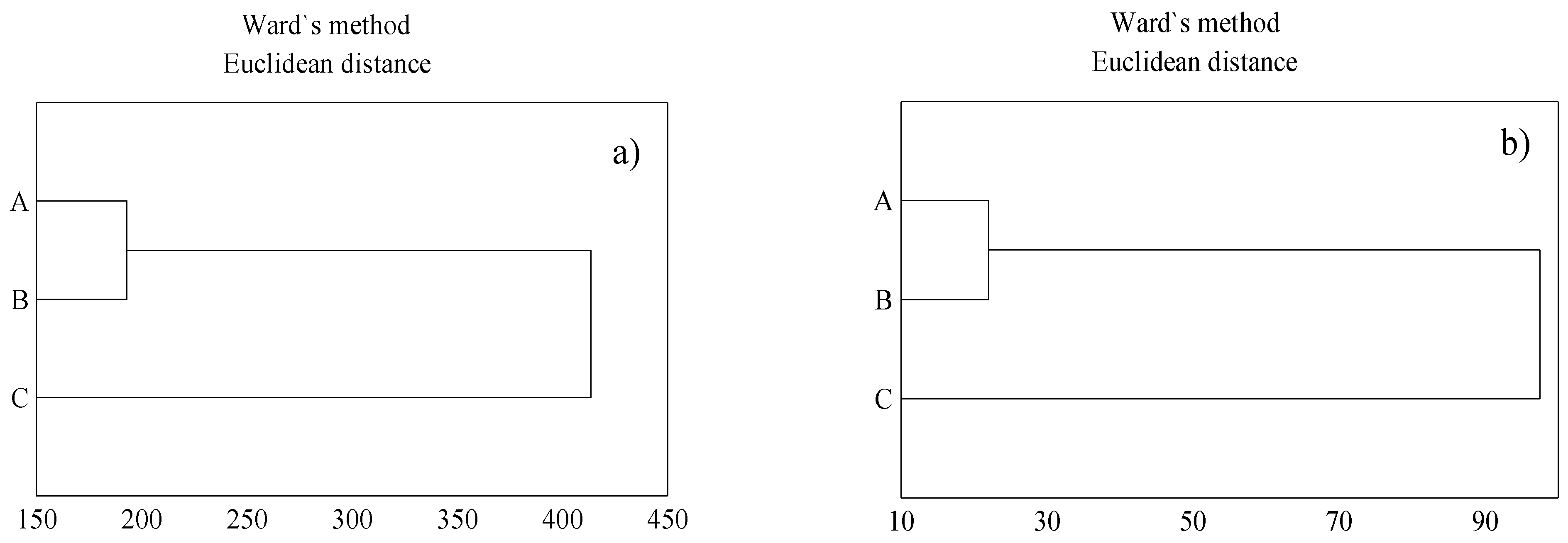
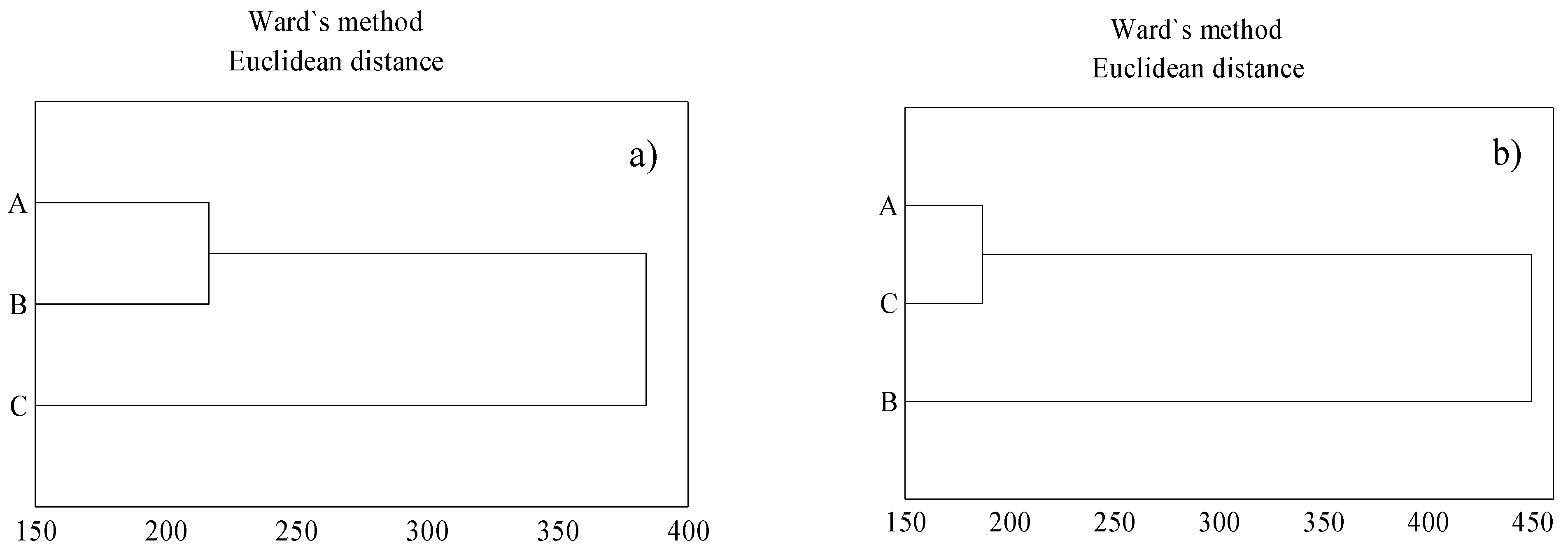
3. Results
3.1. Physical and Chemical Properties
3.2. Heavy Metal Content in Soil Samples
3.3. Content of Heavy Metal in T. serpyllum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsangd, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC: Boca Raton, FL, USA, 2001. [Google Scholar]
- Muchuweti, M.; Birkett, J.W.; Chinyanga, E.; Zvauya, R.; Scrimshaw, M.D.; Lester, J.N. Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Ecosyst. Environ. 2006, 112, 41–48. [Google Scholar] [CrossRef]
- Perrino, E.V.; Brunetti, G.; Farrag, K. Plant communities of multi-metal contaminated soils: A case study in the National Park of Alta Murgia (Apulia Region - southern Italy). J. Phytoremediat. 2014, 16, 871–888. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kita, A.; Janoska, P.; Połowniak, M.; Kozik, V. Multi-element analysis of mineral and trace elements in medicinal herbs and their infusions. Food Chem. 2012, 135, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Arceusz, A.; Wesolowski, M. Essential metals and phenolic acids in commercial herbs and spices. Multivariate analysis of correlations among them. Open Chem. 2015, 13, 1196–1208. [Google Scholar] [CrossRef]
- Ducat, G.; Torres, R.Y.; Santa, H.S.D.; Kaminski, C.I.; Kleinubing, S.A.; Stock, D.; Tussolini, L.; Justo, T.H.; Quinaia, S.P. Correlation among metallic ions, phenolic compounds and antimicrobial action in medicinal plants extracts. J. Food Qual. 2011, 34, 306–314. [Google Scholar] [CrossRef]
- Bolan, S.; Kunhikrishnan, A.; Seshadri, B.; Choppala, G.; Naidu, R.; Bolan, N.S.; Ok, Y.S.; Zhang, M.; Li, C.G.; Li, F.; et al. Sources, distribution, bioavailability, toxicity, and risk assessment of heavy metal(loid)s in complementary medicines. Environ. Int. 2017, 108, 103–118. [Google Scholar] [CrossRef]
- Farmakopea Europejska/European Pharmacopoeia 10.0; Komisja Farmakopei Europejskiej: Strasburg, France, 2020; Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (accessed on 1 January 2020).
- WHO—World Health Organization. Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; WHO Press, World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Farmakopea Polska/Polish Pharmacopoeia; X; Urząd Rejestracji Produktów Leczniczych: Warszawa, Poland, 2014; ISBN 978-83-63724-47-4.
- Szafer, W.; Kulczyński, S.; Pawłowski, B. Rośliny Polskie; PWN: Warszawa, Poland, 1953. [Google Scholar]
- Jalas, J. Thymus L. In Flora Europaea 3; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, S.M., Walters, A.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1972; pp. 172–182. [Google Scholar]
- Henslowa, M. Z badań nad wiedzą ludową o roślinach. II. Thymus serpyllum L.—macierzanka piaskowa. III. Ruta graveolens L.—Ruta zwyczajna. Slavia Antiq. 1977, 24, 163–212. [Google Scholar]
- Fiedoruk, Ł.; Mazik, M.; Pastwa, M. Encyklopedia Ziół; Wydawnictwo Dragon: Bielsko-Biała, Poland, 2012; pp. 124–125, 180–181. [Google Scholar]
- Rutkowski, L. Klucz Do Oznaczania Roślin Naczyniowych Polski Niżowej; PWN: Warszawa, Poland, 2019; pp. 397–398. [Google Scholar]
- Matuszkiewicz, W. Przewodnik Do Oznaczania Zbiorowisk Roślinnych Polski; PWN: Warszawa, Poland, 2011. [Google Scholar]
- Szafer, W.; Kulczyński, S.; Pawłowski, B. Rośliny Polskie. Opisy i Klucze Do Oznaczania Wszystkich Gatunków Roślin Naczyniowych Rosnących w Polsce Bądź Dziko, Bądź Też Zdziczałych Lub Częściej Hodowanych; PWN: Warszawa, Poland, 1969. [Google Scholar]
- Gortat, M. Macierzanka piaskowa (Thymus serpyllum L.) i tymianek pospolity (Thymus vulgaris L.)—źródło surowca dla przemysłu zielarskiego i właściwości lecznicze. Cz. I. In Nauka Dla Rozwoju Rolnictwa, Ekologii i Medycyny w Świetle Współczesnych Osiągnięć; Gortat, M., Ed.; Stowarzyszenie Studentów Nauk Przyrodniczych: Lublin, Poland, 2015; pp. 31–44. [Google Scholar]
- Prus, P. Sustainable Farming Production and its Impact on the Natural Environment—Case Study Based on a Selected Group of Farmers. In Proceedings of the 8th International Scientific Conference Rural Development, Aleksandras Stulginskis University, Lithuania, 23–24 November 2017; pp. 1280–1285. [Google Scholar] [CrossRef]
- Murawska, A.; Prus, P. The Progress of Sustainable Management of Ammonia Emissions from Agriculture in European Union States Including Poland—Variation, Trends, and Economic Conditions. Sustainability 2021, 13, 1035. [Google Scholar] [CrossRef]
- Tomaszewska-Sowa, M.; Kobierski, M.; Sawilska, A.K.; Figas, A. Assessment of phytoaccumulation of trace elements in medicinal plants from natural habitats. Herba Pol. 2018, 64, 11–19. [Google Scholar] [CrossRef]
- Bączek, K. Ekologiczny Zbiór Dziko Rosnących Roślin Leczniczych i ich Obróbka Pozbiorcza-Materiały Szkoleniowe; Instytut Nauk Ogrodniczych, SGGW: Warszawa, Poland, 2020. [Google Scholar]
- Łuczaj, Ł. Dzikie Rośliny Jadalne Polski—Przewodnik Survivalowy/Wild Edible Plants of Poland; Wydawnicwo Chemigrafia: Krosno, Poland, 2004. [Google Scholar]
- Łuczaj, Ł. Dziko rosnące rośliny jadalne użytkowane w Polsce od połowy XIX w. do czasów współczesnych. Pol. Ethnobiol. 2011, 1, 57–125. [Google Scholar]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss and Reut and Thymus vulgaris L. essential oils. Ind. Crop. Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Kirillov, V.; Stikhareva, T.; Mukanov, B.; Chebotko, N.; Ryazantsev, O.; Atazhanova, G.; Adekenov, S. Composition of the essential oil of Thymus serpyllum L. from Northern Kazakhstan. J. Essent. Oil Bear. Plants 2016, 19, 212–222. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regionsof Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Sokovic, M.; Marin, P.D.; Brkic, D.; van Griensven, L.J.L.D. Chemical composition and antibacterial activity of essential oils against human pathogenic bacteria. Food 2008, 1, 220–226. [Google Scholar]
- Sokovic, M.D.; Vukojevic, J.; Marin, P.D.; Brkic, D.D.; Vajs, V.; van Griensven, L.J.L.D. Chemical composition of essential oils of Thymus and Mentha speciesand their antifungal activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Salehi-Fathabadi, Z.; Maghsoudlou, Y.; Akhavan, H.; Moayedi, A.; Khorasani, S. The assessment of the effect of Aloe vera gel coating containing salicylic acid and thyme extract on the shelf life of fresh pistachios during storage. FSCT 2019, 16, 297–312. [Google Scholar]
- Jadczak, D.; Grzeszczuk, M. Tymianek. Panacea 2006, 3, 30–31. [Google Scholar]
- Aziz, S.; Rehman, H. Studies on the chemical constituents of Thymus serpyllum. Turk. J. Chem. 2008, 32, 605–614. [Google Scholar]
- Treben, M. Apteka Pana Boga; Wydawnictwo Ex Libris: Warszawa, Poland, 2013; p. 120. [Google Scholar]
- Gortat, M. Macierzanka piaskowa (Thymus serpyllum L.) i tymianek pospolity (Thymus vulgaris L.)—źródło surowca dla przemysłu zielarskiego i właściwości lecznicze. Cz. II. In Nauka Dla Rozwoju Rolnictwa, Ekologii i Medycyny w Świetle Współczesnych Osiągnięć; Gortat, M., Ed.; Stowarzyszenie Studentów Nauk Przyrodniczych: Lublin, Poland, 2015; pp. 45–54. [Google Scholar]
- Wesołowska, A.; Grzeszczuk, M.; Jadczak, D.; Nawrotek, P.; Struk, M. Comparison of the chemical composition and antimicrobial activity of Thymus serpyllum essential oils. Not. Bot. Horti Agrobot. 2015, 43, 432–438. [Google Scholar] [CrossRef]
- Rehman, A.; Mannan, A.; Inayatullah, S.; Akhtar, M.Z.; Qayyum, M.; Mirza, B. Biological evaluation of wild thyme (Thymus serpyllum). Pharm. Biol. 2009, 47, 628–633. [Google Scholar] [CrossRef]
- Djukic-Cosic, D.; Curcic, M.; Cmiljanovic, M.; Vasovic, I.; Matovic, V. Heavy metal contents in samples of Hypericum and Thymus spec. collected from different mountain areas in Serbia. Planta Med. 2007, 587. [Google Scholar] [CrossRef]
- Cegielska, W.; Michalska-Kacymirow, M.; Wierzbicka, M. Metale ciężkie w środowisku. In Ekotoksykologia. Rośliny, Gleby, Metale; Wierzbicka, M., Ed.; Wydawnictwa UW: Warszawa, Poland, 2015; pp. 22–51. [Google Scholar]
- Perrino, E.V.; Valerio, F.; Gannouchi, A.; Trani, A.; Mezzapesa, G. Ecological and Plant Community Implication on Essential Oils Composition in Useful Wild Officinal Species: A Pilot Case Study in Apulia (Italy). Plants 2021, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, Z.; Ebrahimi, M.; Farajpour, M.; Mirza, M.; Ramshini, H. Compositions and yield variation of essential oils among and within nine Salvia species from various areas of Iran. Crop Prod. 2014, 61, 233–239. [Google Scholar] [CrossRef]
- Musielińska, R.; Kowol, J.; Kwapuliński, J.; Rochel, R. Antagonism between lead and zinc ions in plants. Arch. Environ. Prot. 2016, 42, 78–91. [Google Scholar] [CrossRef]
- Visioli, G.; Marmiroli, N. The proteomics of heavy metal hyperaccumulation by plants. J. Proteom. 2013, 79, 133–145. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Ociepa-Kubicka, A.; Ociepa, E. Toksyczne oddziaływanie metali ciężkich na rośliny, zwierzęta i ludzi. Inżynieria Ochr. Śr. 2012, 15, 169–180. [Google Scholar]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.M.S.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Sawilska, A.K.; Jendrzejczak, E.; Kieliszewska-Rokicka, B. Influence of mycorrhiza on the growth and flowering in cultivated plants of Helichrysum arenarium (L.) Moench (Asteraceae). Pol. J. Ecol. 2010, 58, 767–774. [Google Scholar]
- Turnau, K.; Ryszka, P.; Wojtczak, G. Metal tolerant mycorrhizal plants: A review from the perspective on industrial waste in temperate region. In Arbuscular Mycorrhizas: Physiology And Function; Koltai, H., Kapulnik, Y., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 257–276. [Google Scholar]
- Blackwell, M. Terrestrial life: Fungal from the start? Science 2000, 289, 1884–1885. [Google Scholar] [CrossRef] [PubMed]
- Koide, R. The Effect of VA mycorrhizal infection and phosphorus status on sunflower hydraulic and stomatal properties. J. Exp. Bot. 1985, 36, 1087–1098. [Google Scholar] [CrossRef]
- Fitter, A.H. Water relations of red clover Trifolium pratense L. as affected by VA mycorrhizal infection and phosphorus supply before and during drought. J. Exp. Bot. 1988, 39, 595–603. [Google Scholar] [CrossRef]
- Salt, D.E.; Prince, R.C.; Pickering, I.J.; Raskin, I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol. 1995, 109, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Przedpełska-Wąsowicz, E.M. Hiperakumulatory metali ciężkich. In Ecotoxicology: Plants, Soils, Metals; Wierzbicka, M., Ed.; Wydawnictwa UW: Warszawa, Poland, 2015; pp. 428–449. [Google Scholar]
- Branquinho, C.; Serrano, H.C.; Pinto, M.J.; Martins-Loução, M.A. Revisiting the plant hyperaccumulation criteria to rare plants and earth abundant elements. Environ. Pollut. 2007, 146, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Kobierski, M.; Tomaszewska-Sowa, M.; Figas, A.; Gatz, A.; Sawilska, A.K. Bioaccumulation of heavy metals in herbal plants from areas not exposed to heavy anthropopressure. Pol. J. Soil Sci. 2017, 50, 41–53. [Google Scholar] [CrossRef]
- Figas, A.; Tomaszewska-Sowa, M.; Sawilska, A.K.; Kobierski, M. Assessment the Phytoaccumulation of Trace Elements in Plants of Evening Primrose Oenothera biennis L. from Kuyavia-Pomerania Provinces (Poland). In Infrastructure and Environment; Springer: Cham, UK, 2019; pp. 252–259. [Google Scholar] [CrossRef]
- ISO 10390:2005. Soil Quality–Determination of pH. International Organization for Standardization; Geneva, Switzerland. 2005. Available online: https://www.iso.org/standard/40879.html (accessed on 1 February 2005).
- Crock, J.G.; Severson, R. Four reference soil and rock samples for measuring element availability in the western energy regions. U.S. Geol. Surv. Circ. 1980, 841, 16. [Google Scholar]
- Gediga, K.; Spiak, Z.; Piszcz, U.; Bielecki, K. Suitability of different soil extractants for determination of available Cu and Mn contents in Polish soils. Commun. Soil Sci. Plant Anal. 2015, 46, 81–93. [Google Scholar] [CrossRef]
- Gąsior, J.; Kaniuczak, J.; Hajduk, E.; Właśniewski, S.; Nazarkiewicz, M. Analytical methods for physico-chemical soil properties. Acta Carpathica 2014, 14, 1–51. [Google Scholar]
- Smoleń, S.; Sady, W.; Ledwożyw-Smoleń, I. Quantitative relations between the content of selected trace elements in soil extracted with 0.03 M CH3COOH or 1 M HCl and its total concentration in lettuce and spinach. Acta. Sci. Hortorum Cultus 2010, 9, 13–23. [Google Scholar]
- Korzeniowska, J.; Stanisławska-Glubiak, E. Comparison of 1 M HCl and Mehlich 3 for assessment of the micronutrient status of polish soils in the context of winter wheat nutritional demands. Commun. Soil Sci. Plant Anal. 2015, 46, 1263–1277. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanisławska-Glubiak, E.; Lipiński, W. New limit values of micronutrient deficiency in soil determined using 1 M HCl extractant for wheat and rapeseed. Soil Sci. Annual. 2020, 71, 205–214. [Google Scholar] [CrossRef]
- StatSoft Inc. OK, USA, STATISTICA, Version 12.0. Data Analysis Software System. 2012. Available online: https://statisticasoftware.wordpress.com/2013/05/15/statsoft-releases-version-12-of-statistica-software (accessed on 7 May 2013).
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Wilding, L.P. Spatial variability: Its documentation, accommodation and implication to soil surveys. In Soil Spatial Variability; Nielsen, D.R., Bouma, J., Eds.; Pudoc: Wageningen, The Netherlands, 1985; pp. 166–194. [Google Scholar]
- Martin, J.M.; Meybeck, M. Elemental mass-balance of material carried by major world rivers. Mar. Chem. 1979, 7, 173–206. [Google Scholar] [CrossRef]
- Sutherland, R.A.; Tolosa, C.A.; Tack, F.M.G.; Verloo, M.G. Characterization of Selected Element Concentration and Enrichment Ratios in Background and Anthropogenically Impacted Roadside Areas. AECT 2000, 38, 428–438. [Google Scholar] [CrossRef]
- USDA (United States Department of Agriculture). Soil Mechanics Level I-Module 3: USDA Textural Classification Study Guide; National Employee Development Staff; Soil Conservation Service: Washington, DC, USA, 1987. [Google Scholar]
- Journal of Laws, Item 1395. Regulation of the Minister of the Environment of 1 September 2016 on the Method for Assessment of Land Surface Contamination. 2016. Available online: www.gdos.gov.pl (accessed on 5 September 2016).
- Kobierski, M.; Dąbkowska-Naskręt, H. Local background concentration of heavy metals in various soil types formed from glacial till of the Inowrocławska Plain. J. Elem. 2012, 17, 559–585. [Google Scholar] [CrossRef]
- Vega, F.A.; Covelo, E.F.; Cerqueira, B.; Andrade, M.L. Enrichment of marsh soils with heavy metals by effect of anthropic pollution. J. Hazard. Mater. 2009, 170, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M. The Importance of Enrichment Factor (EF) and Geoaccumulation Index (Igeo) to Evaluate the Soil Contamination. J. Geol. Geophys. 2016, 5, 237. [Google Scholar] [CrossRef]
- Mercik, S. Chemia Rolna; Wydawnictwo SGGW: Warszawa, Poland, 2004. [Google Scholar]
- Ociepa, E. The effect of fertilization on yielding and heavy metals uptake by maize and virgina fanpetals (Sida Hermaphrodita). Arch. Environ. Prot. 2011, 37, 123–129. [Google Scholar]
- Ali, A.; Badshah, L.; Hussain, F. Screening of five plant species for macro/micro nutrients and heavy metals at various phenological stages. Pak. J. Bot. 2018, 50, 1941–1949. [Google Scholar]
- Rostański, A.; Nowak, T.; Jędrzejczyk-Korycińska, M. Metalolubne gatunki roślin naczyniowych we florze Polski. In Ecotoxicology: Plants, Soils, Metals; Wierzbicka, M., Ed.; Wydawnictwa UW: Warszawa, Poland, 2015; pp. 297–322. [Google Scholar]
- Lucassen, E.C.H.E.T.; Eygensteyn, J.; Bobbink, R.; van de Riet, B.P.; Smolders, A.J.P.; Kuijpers, D.J.C.; Roelofs, J.G.M. The decline of metallophyte vegetation in floodplain grasslands: Implications for conservation and restoration. Appl. Veget. Sci. 2009, 12, 69–80. [Google Scholar] [CrossRef]
- Lucassen, E.C.H.E.T.; van Kempen, M.M.L.; Roelofs, J.G.M.; van der Velde, G. Decline in metallophytes in tertiary polluted floodplain grasslands in the Netherlands: Experimental evidence for metal and nutritional changes in soil as driver factors. Chem. Ecol. 2010, 26, 273–287. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Zinc. Environmental Health Criteria; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Biogeochemia Pierwiastków Śladowych; PWN: Warszawa, Poland, 1999; pp. 329–336. [Google Scholar]
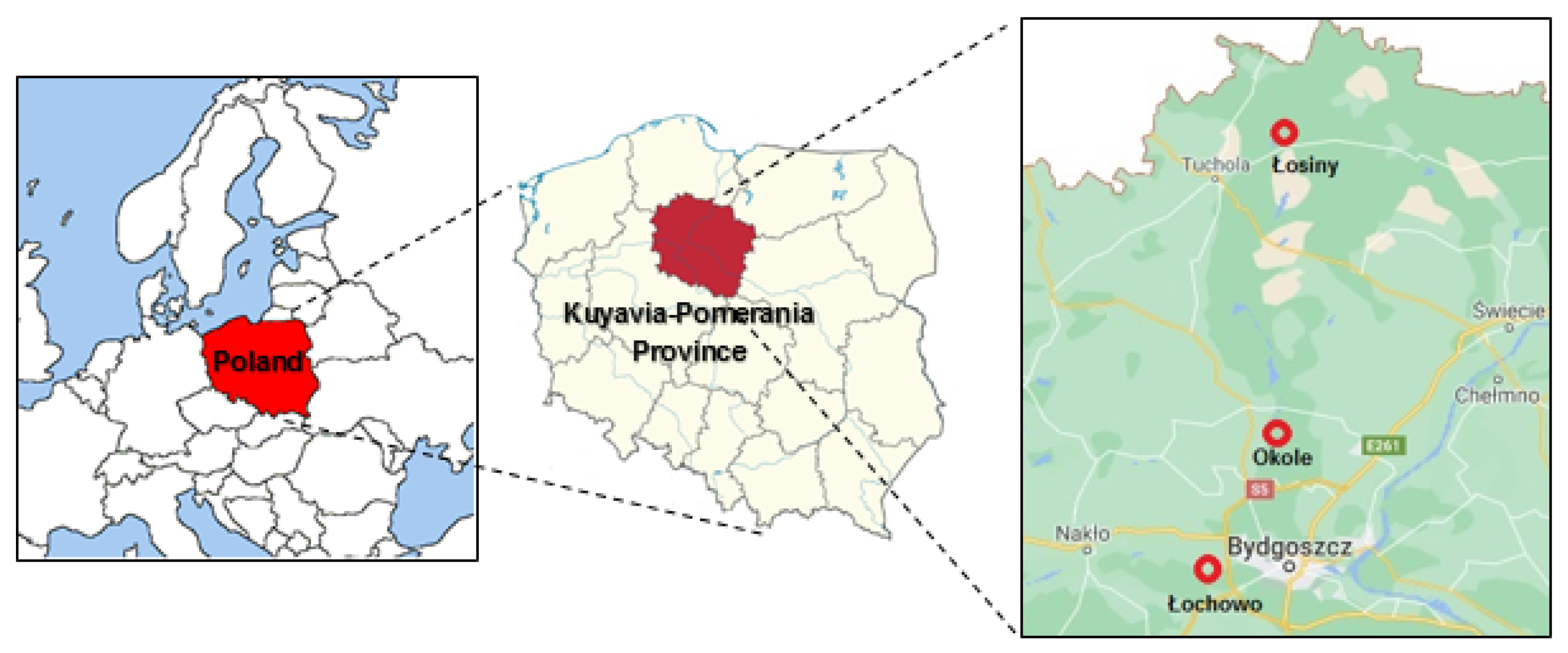
| Sampling Location * | pH H2Odest | pH 1M KCl | TOC g kg−1 | Grain Size Composition [%] | Textural Class USDA | ||
|---|---|---|---|---|---|---|---|
| Sand 2.0–0.05 mm | Silt 0.05–0.002 mm | Clay < 0.002 mm | |||||
| A | 4.97 ± 0.05 | 4.34 ± 0.10 | 8.51 ± 0.22 | 87.4 | 11.7 | 0.9 | ** S |
| B | 5.56 ± 0.03 | 4.59 ± 0.04 | 13.8 ± 0.31 | 79.1 | 19.6 | 1.3 | LS |
| C | 6.94 ± 0.03 | 6.39 ± 0.06 | 9.31 ± 0.21 | 77.9 | 20.7 | 1.4 | LS |
| Sampling Location * | Znt | Cut | Mnt | Pbt | Fet |
|---|---|---|---|---|---|
| mg kg−1 | g kg−1 | ||||
| A | 25.5 ± 1.25 | 4.0 ± 0.08 | 87.6 ± 4.11 | 48.9 ± 2.05 | 4.7 ± 0.09 |
| B | 58.6 ± 2.20 | 9.1 ± 0.44 | 277.7 ± 11.12 | 54.1 ± 2.84 | 6.1 ± 0.13 |
| C | 25.6 ± 1.74 | 5.5 ± 0.11 | 536.6 ± 23.34 | 45.0 ± 1.76 | 6.2 ± 0.16 |
| Mean | 36.60 | 6.20 | 300.63 | 49.33 | 5.67 |
| Sd | 19.05 | 2.62 | 225.38 | 4.57 | 0.84 |
| CV(%) | 52.05 | 42.26 | 74.97 | 9.27 | 14.74 |
| Sampling Location * | Znt | Cut | Mnt | Pbt | Fet |
|---|---|---|---|---|---|
| mg kg−1 | g kg−1 | ||||
| A | 30.0 ± 1.65 | 5.10 ± 0.25 | 414.0 ± 22.25 | 15.0 ± 0.43 | 9.4 ± 0.34 |
| B | 35.2 ± 1.44 | 7.31 ± 0.31 | 435.1 ± 24.10 | 17.2 ± 0.51 | 10.2 ± 0.54 |
| C | 29.4 ± 1.12 | 6.10 ± 0.20 | 503.0 ± 26.21 | 15.5 ± 0.52 | 7.8 ± 0.40 |
| Mean | 31.50 | 6.17 | 450.7 | 15.90 | 9.13 |
| Sd | 3.19 | 1.11 | 46.61 | 1.15 | 1.22 |
| CV(%) | 10.1 | 18.0 | 10.3 | 7.2 | 13.4 |
| Sampling Location * | Zna | Cua | Mna | Fea | Pba *** | ||||
|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | Content Rating ** | mg kg−1 | Content Rating ** | mg kg−1 | Content Rating ** | g kg−1 | Content Rating ** | mg kg−1 | |
| A | 7.9 ± 0.19 | high | 1.7 ± 0.07 | average | 56.8 ± 0.34 | average | 0.85 ± 0.03 | average | 0.8 ± 0.05 |
| B | 14.2 ± 0.61 | high | 4.3 ± 0.16 | high | 32.9 ± 1.27 | average | 0.97 ± 0.04 | average | 1.1 ± 0.05 |
| C | 6.7 ± 0.34 | high | 2.0 ± 0.13 | average | 65.7 ± 1.44 | average | 1.05 ± 0.04 | average | 0.7 ± 0.03 |
| Sampling Location * | Zn (EF) | Cu (EF) | Mn (EF) | Pb (EF) |
|---|---|---|---|---|
| A | 1.58 | 1.25 | 0.38 | 5.98 |
| B | 2.78 | 2.19 | 0.92 | 5.10 |
| C | 1.20 | 1.30 | 1.75 | 4.17 |
Sampling Location * | Metal | Thymus serpyllum L. | ||
|---|---|---|---|---|
| Inflorescences | Leaves + Stems | Roots | ||
| A | Zn | 106.2 ± 6.90 CV = 6.50% | 116.9 ± 5.40 CV = 4.62% | 68.55 ± 12.94 CV = 18.88% |
| Cu | 23.50 ± 1.62 CV = 6.89% | 7.80 ± 0.28 CV = 3.59% | 28.20 ± 1.30 CV = 4.61% | |
| Pb | 6.80 ± 0.60 CV = 8.82% | 17.90 ± 5.23 CV = 29.22% | 5.00 ± 3.53 CV = 70.60% | |
| Mn | 338.6 ± 18.20 CV = 5.38% | 289.1 ± 20.93 CV = 7.23% | 138.6 ± 47.16 CV = 34.01% | |
| Fe | 605.0 ± 62.21 CV = 10.28% | 207.0 ± 9.89 CV = 4.77% | 303.5 ± 146,00 CV = 48.1% | |
| B | Zn | 77.60 ± 10.47 CV = 6.13.49% | 97.80 ± 6.22 CV = 6.36% | 109.4 ± 14,9 CV = 13.61% |
| Cu | 21.75 ± 1.06 CV = 4.87% | 11.35 ± 0.65 CV = 5.73% | 22.00 ± 4.58 CV = 20.82% | |
| Pb | 6.75 ± 6.29 CV = 93.18% | 16.30 ± 0.88 CV = 5.39% | 20.80 ± 2.40 CV = 11.54% | |
| Mn | 124.3 ± 9.47 CV = 7.62% | 128.0 ± 6.89 CV = 5.38% | 264.6 ± 67.30 CV = 25.42% | |
| Fe | 619.0 ± 131.52 CV = 21.24% | 878.0 ± 70.71 CV = 8.05% | 852.5 ± 243.40 CV = 28.55% | |
| C | Zn | 64.75 ± 12.02 CV = 18.56% | 41.95 ± 2.89 CV = 6.89% | 34.05 ± 0.35 CV = 1.03% |
| Cu | 30.85 ± 2.47 CV = 8.00% | 16.20 ± 1.04 CV = 6,42% | 25.00 ± 4.24 CV = 16.96% | |
| Pb | 4.1 ± 5.37 CV = 130.9% | 12.00 ± 0.42 CV = 3.50% | 10.45 ± 5.66 CV = 54.16% | |
| Mn | 145.8 ± 4.17 CV = 2.86% | 146.5 ± 7.96 CV = 5.43% | 119.9 ± 9.05 CV = 7.55% | |
| Fe | 299.5 ± 98.29 CV = 32.81% | 326.5 ± 7.77 CV = 2.37% | 265.0 ± 31.11 CV = 11.74% | |
| Variables | Regression Equation | R | R2 | |
|---|---|---|---|---|
| Dependent * | Independent * | |||
| Zn L+S | pH KCl | y = 307.99 − 38.22x | −0.998 | 0.996 |
| Pb L+S | Zn L+S | y = 8.6693 + 0.07889x | 0.999 | 0.998 |
| Pb L+S | pH KCl | y = 32.995 − 3.020x | −0.999 | 0.998 |
| Mn I | Mn L+S | y = −48.56 + 1.3385x | 0.999 | 0.998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figas, A.; Tomaszewska-Sowa, M.; Kobierski, M.; Sawilska, A.K.; Klimkowska, K. Hazard of Contamination with Heavy Metals in Thymus serpyllum L. Herbs from Rural Areas. Agriculture 2021, 11, 375. https://doi.org/10.3390/agriculture11040375
Figas A, Tomaszewska-Sowa M, Kobierski M, Sawilska AK, Klimkowska K. Hazard of Contamination with Heavy Metals in Thymus serpyllum L. Herbs from Rural Areas. Agriculture. 2021; 11(4):375. https://doi.org/10.3390/agriculture11040375
Chicago/Turabian StyleFigas, Anna, Magdalena Tomaszewska-Sowa, Mirosław Kobierski, Anna Katarzyna Sawilska, and Katarzyna Klimkowska. 2021. "Hazard of Contamination with Heavy Metals in Thymus serpyllum L. Herbs from Rural Areas" Agriculture 11, no. 4: 375. https://doi.org/10.3390/agriculture11040375
APA StyleFigas, A., Tomaszewska-Sowa, M., Kobierski, M., Sawilska, A. K., & Klimkowska, K. (2021). Hazard of Contamination with Heavy Metals in Thymus serpyllum L. Herbs from Rural Areas. Agriculture, 11(4), 375. https://doi.org/10.3390/agriculture11040375







